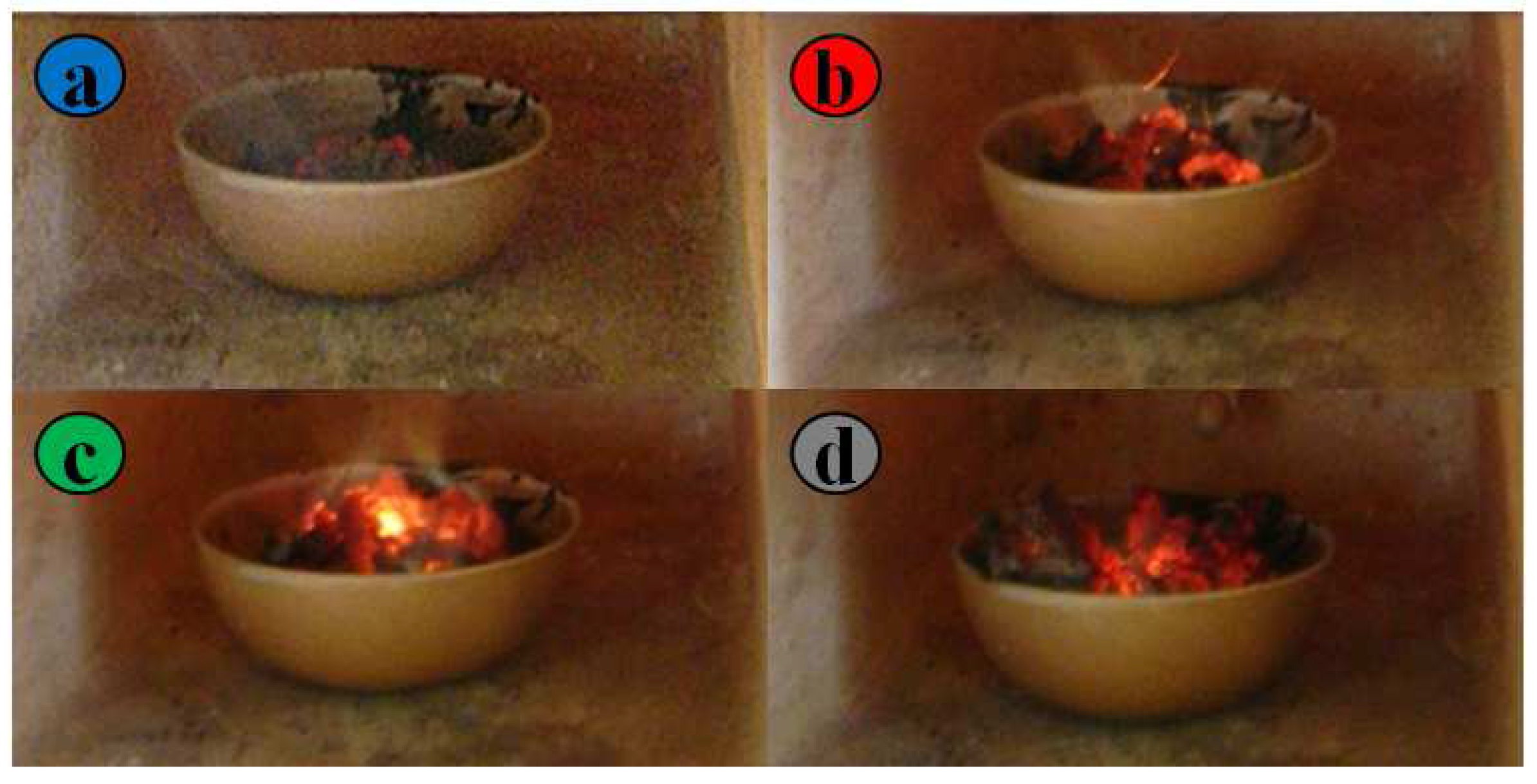
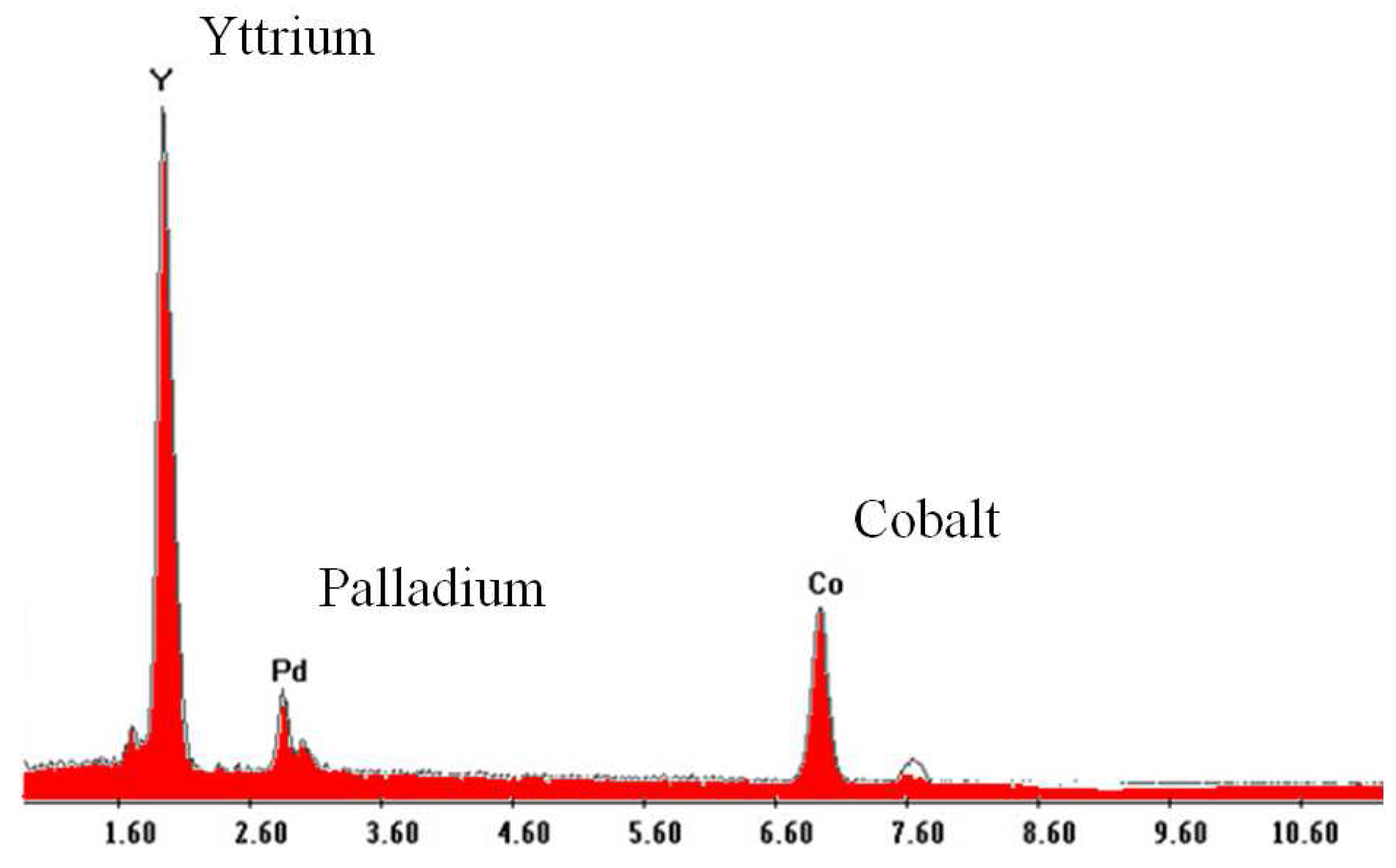
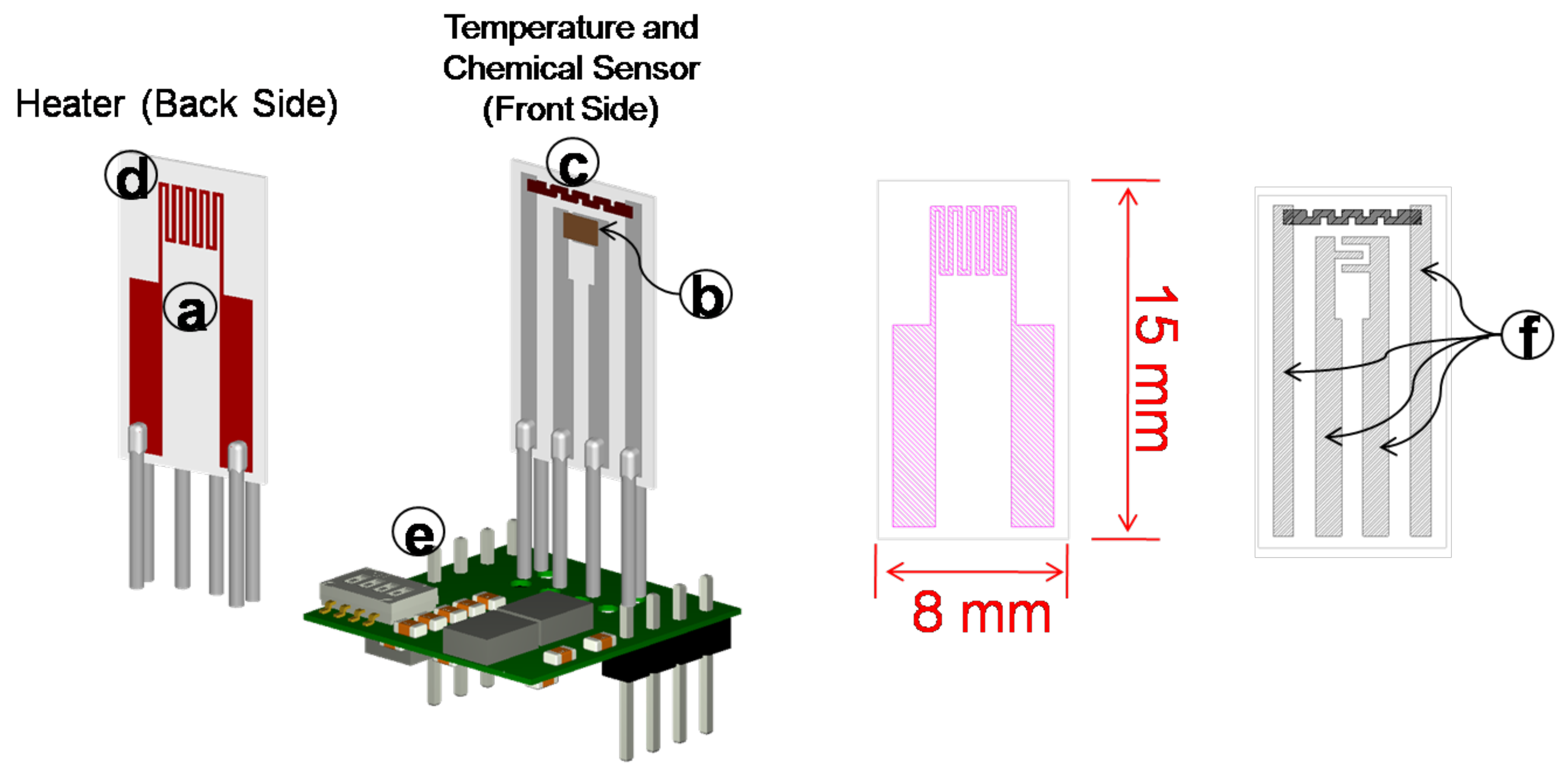
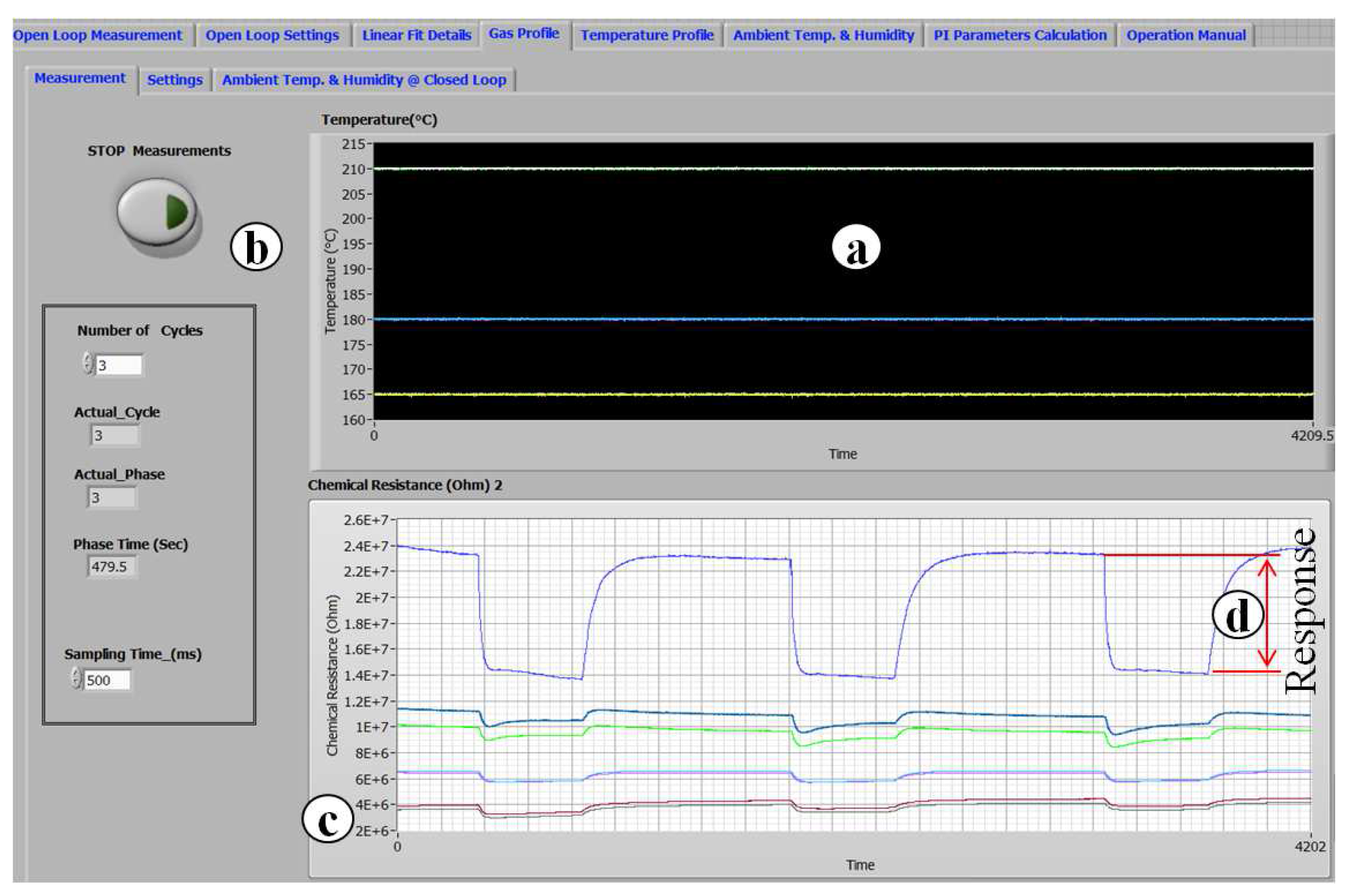
1. Introduction
Perovskite metal oxides, with general formula ABO
, where the A-site ion is usually an alkaline earth or rare earth element and the B site ions could be 3d, 4d and 5d transition metal elements, have received great attention in the last few years, due to the their properties that make them suitable for many technological applications, such as oxygen membranes, heterogeneous catalysts, capacitors, gas sensors, etc. [
1]. YCoO
is a perovskite, studied by the authors, that proved to be sensitive to changes of the chemical composition of the surrounding atmosphere [
2,
3]. Stoichiometric, as well as defective or doped YCoO
proved to be p-type semiconductors that change their conductivity as a function of oxidizing or reducing gas concentrations. This paper addresses the application of YCoO
-based nano-structured materials as low temperature conductometric sensors for toxic gas detection. For this kind of sensor, the working principle is based on reversible gas adsorption on the oxide surface that involves the exchange of charge between the oxide and the adsorbates, which modifies the oxide electronic conductivity either by creating depleted regions at the grain boundaries and/or by trapping/releasing free carriers. The mechanism of interaction of these materials with oxidizing and reducing gases, such as CO and NO
, is still under study, and further research is needed in order to gain the knowledge required for the full exploitation of their gas sensing properties.
In general, it is well known that many material characteristics and physical or chemical quantities influence the gas response [
4,
5]. For instance, the gas sensing behavior depends dramatically on the surface temperature, but also on the humidity and on the presence of interfering gas. As far as the material characteristics are concerned, it was shown that the bulk electronic properties, together with the bulk defect population, are relevant, but also that the surface type and the microstructure of the sensing film are very important. For example, it was pointed out that the use of a nano-structured material is usually related to a large gas response, mainly due to its large surface/volume ratio. This fact triggered a growing interest in the application of nano-structured materials for the detection of gases [
6,
7], and for this reason, nanoscale metal oxides, such as nanoparticles, nanospheres, nanotubes, nanowires and nanoporous materials, are routinely synthesized for the development of solid-state gas sensors with improved sensing properties [
8,
9]. Furthermore, the main problems of semiconductor conductometric gas sensors are the reproducibility, the stability and the reliability [
10]. YCoO
-based perovskites are stable in long-term operation at fixed working temperature and gas concentration [
11]. Therefore, the aging process of the chemical film can be limited using a low working temperature.
Concluding, the sensor response of conductometric gas sensors is a complex function of many quantities and parameters, that are only partially known. This paper proposes an approach to search the optimal working conditions of YCoO
sensors by applying statistical methods, in particular the design of experiment and the Response Surface Methodology (RSM); in this direction, see also [
12]. The proposed approach involves an ad hoc planning of a split-plot design as an RSM second order experimental design and the application of RS models with random effects, e.g., mixed RS models, in order to expound and to improve the contribution achieved through a previous study [
13].
Since 1990s, RSM and experimental designs have played a relevant role aimed at improving some specific issues, such as modeling [
14] and process optimization [
15]. By considering the modeling features, many approaches are introduced in order to extend: (i) the concept of the fixed effect especially when evaluating blocks or noise variables; (ii) the relevance of the heteroscedasticity for the error variance.
Undoubtedly, when considering the sources of variabilities, the connection of RSM with the inclusion of random effects is a notable improvement in the study of those components, e.g., variance components [
16], which may influence the main and operative variables of the production process [
17,
18]. More specifically, when considering the search for an optimal solution for the experimental variables in order to set a robust design, the inclusion of random effects within the fitted mixed response surface allows for evaluating the error components linked to noises and/or sub-experimental factors. To this end, a further improvement in this research is the application of a split-plot design [
19,
20], which is a notable experimental design when considering specific issues of noise variables studied through mixed effects. Moreover optimization through split-plot design has been recently involved in a Bayesian context, such as in [
21]. Furthermore, we may also evaluate the context in which the experimental design is applied for optimizing products and/or process. In this direction, wireless communications and transmission are studied through a full factorial design in [
22], where the received signal strength is analyzed. Similarly, in [
23], an Analog to Digital Converter (ADC) channel optimization is carried out by considering random noise effects and the multi-response case; in [
24], a statistical analysis is applied in order to study and to remove noises by the spectral domain. When considering the application of the well-known full factorial experimental design, as in [
25], it must be noted that this design is an efficient statistical tool when fractionated and applied in an RSM context; and also, as basic design for the split-plot, as in [
26]. Moreover, the application of an experimental design leads to the subsequent statistical modeling approach and, then, to the process optimization. The process optimization allows for achieving the optimal setting of factors involved in the experimental design, also including noises and the concept of robust design. In this way, process optimization could be performed by applying alternative models and algorithms, such as in [
27], where the Jacobian matrix is calculated on the feasible region in order to obtain an optimal rehabilitation robot, or in [
28], where the lassoregression and the TREFEXalgorithm are applied for optimizing gas sensors in an open system.
In our case-study, we applied the split-plot design and modeling for optimizing gas-sensor materials. The optimization step is carried out through an analytical approach involving an objective function based on a dual approach, in which the target (desirable) value is achieved with minimum process variability and also taking random effects into account.
Statistical results are satisfactory both considering modeling issues and optimization performance. Modeling results confirm the hypotheses assumed during the design planning, especially for gas concentration and temperatures; the optimization results are obtained by considering each target gas and the corresponding gas sensing material, conditioned to temperature range values.
This paper is organized as follows: the sensor samples and the measurement system are described in
Section 2. The split-plot design is illustrated in
Section 3, where the experimental planning has been detailed. The model and the optimization results are presented in
Section 4. The discussion and final remarks are reported in
Section 5 and
Section 6, which conclude this paper.
3. The Split-Plot Design
In the last two decades, split-plot design [
40] has received great attention as a valid plan in the technological field and for a robust design approach, following the seminal contributions by [
19,
20,
26,
41,
42]. In [
19], this experimental design has been developed by considering its specific framework, in which the main role is played by the distinction between Whole-Plot (WP) and Sub-Plot (SP) factors. The bi-randomization of the split-plot design allows us to study noise and block variables as WP factors, also considering random effects; following, the experimental (main) variables, e.g., SP factors, are randomized within the WP units. Furthermore, the inclusion of experimental variables as SP factors guarantees a more accuracy estimation for these effects. The inclusion of the split-plot design as a crossed bi-randomized design implies that this design may be also considered as a second order design in a Response Surface Methodology (RSM) context [
43]. Further theoretical developments contributed to expound the relevance of the split-plot design: in [
20] is established the equivalence between Ordinary Least Squares (OLS) and Generalized Least Squares (GLS) estimation methods for fixed effects, if the split-plot design is planned according to specific structure.
In our case study, the split-plot design is planned for studying and optimizing the performance of the characterized gas sensing materials. We distinguish between two types of factors: the sub-experimental factors, such as block factors and noises, which are included in the whole plots; the experimental factors, e.g., process variables, which are the main object of interest and are studied as sub-plot factors.
3.1. Planning the Split-Plot
The step of planning for the split-plot design is started by considering all of the sources of variability involved in the process, independently from the role played by each source, e.g., experimental or sub-experimental role. For example, we distinguish between two temperatures: (i) the temperature of the measurement chamber; (ii) the temperature measured by the RTD on the sensor, also called the working temperature. The latter plays a relevant role in the final experimental design, while the first one is evaluated as an external variable.
More precisely, we planned a split-plot design with the block variable, target gas, at two-levels: two gases, NO
and CO, coded as 1 and 2 in
Table 2 respectively. For each target gas, three chambers are studied; eight different sensors are studied within each chamber, each sensor relating to a gas sensing material. Each chamber, within a target gas, is considered as a replicate with a specified gas concentration level. Therefore, the gas concentration is studied as a random variable, and it is included in the experimental design as a factor at three levels for each target gas, each level corresponding to a chamber. Further, a noise variable is included as the WP factor: the humidity, at two levels (wet and dry), corresponding to
and
; the humidity is induced through an a priori setting of the thermostatic bath. The gas sensing materials (eight types of perovskites) and the working temperature are considered as SP factors. The material type is then evaluated as the categorical variable at eight levels, while the temperature is studied by considering a different range of values (
C) by the target gas. In order to better evaluate the working temperature, we studied four temperature intervals for each target gas. The response variable is illustrated in Formula (
1).
Therefore, for each target gas, we applied a split-plot design with two WP factors (humidity and gas concentration) and two SP factors (material type and working temperature). Each replicate is formed by a chamber obtained by a given sensors array and performed at a specific gas concentration level; within each replicate, humidity is studied at two levels, and at the SP level, the eight material types are studied according to the four levels of the working temperature. The total amount of experimental observations is 48: therefore, we have 24 observations for each target gas. The environmental temperature for each chamber has been measured during the experimentation step, and it has been involved in the statistical model as a random variable; but, it did not result in being relevant for the whole process, not even as an external variable. Other sources of variability were considered during the planning and the subsequent measurement process: (i) the difference in the heating during sensors’ fabrication, for each type of perovskite, which is included in the analysis through the material type; (ii) gas-flow, which is kept constant at 300 mL/min.
In
Table 2, the detailed description of the experimental variables is reported.
3.2. The Split-Plot Model
Let us define the set (
Z) of
I whole-plot factors (noises and block) and the set (
X) of
J sub-plot factors. In addition, we define with WP
the combinations of levels for the
I Whole-Plot (WP) factors; while we define with SP
the combinations of levels of the
J Sub-Plot (SP) factors, in the replicate (block)
k. Then, within each replicate
k, we have
= WP
× SP
combinations, and therefore, the total number of trials is
. Furthermore, we define
as the generic vector, related to the
i-th WP factor,
; while
is the generic vector related to the
j-th SP factor,
. Therefore, the general second order split-plot model in an RSM setting, defined for a single observation
, a single replicate (K = 1) and a single response variable
Y, is the following:
note that
and
are the coefficients related to the interaction effects of WP and SP factors, respectively;
and
are the coefficients of the quadratic effects of WP and SP factors, respectively; while
are the coefficients related to the interaction terms between WP and SP factors. These last terms play a relevant role in the robust design approach, since they contain the parameters of the control ☆ noise interaction effects. The error terms are represented by
(whole-plot error) and
(sub-plot error). We suppose that
and
in addition, we suppose that the error terms are uncorrelated. Note also that the first error term is formed by the interactions between the replicate effect and the sub-experimental factors; the WP effects are tested against this error component. The second error term is the residual error of the model. It must be noted that in a split-plot design, a single replicate is identified by one block. In our study, each target gas is analyzed through three replicates; each replicate is identified by a chamber and a pre-specified gas concentration level.
3.3. The Optimization Measure
The optimization step is carried out by applying the method explained and applied firstly in [
44] and subsequently extended and improved in [
45]. In the literature, many authors (among others, [
46,
47]) suggest different measures of optimization in order to set an optimal solution, according to the different kinds of experimental situations involved. When considering the dual response approach [
14], two surfaces are estimated, one for the mean and one for the dispersion effect; in addition, weights could be introduced in the objective function; for example, in the cited literature, two global weights, one for the mean and one for dispersion, are defined. Recent and further developments could be also found in [
15].
The method proposed by [
44] takes the estimated model and the target value into account. In general, we may define the following distance between the estimated surface
, considered as a function of the two sets of variables, process (
X) and noise and/or random (
Z) variables and the target value
:
S can be viewed as a crude measure of variability as a basis for the dual approach, where the adjustment to the target value is performed. The second aim is the minimization of Formula (
3) on the coded experimental region:
It must be noted that our aim is to find the best solution for the set of factors
by minimizing Formula (
4), defined here for only one response variable, and simultaneously, to achieve the desired value for the target. In our case study, the target value for the variation of the electrical resistance of the sensor response, Formula (
1), is maximized as in a larger-the-better situation also by conditioning to the working temperature.
Moreover,
is the surface to be optimized, and in this case, it corresponds to Model (
2), estimated for each material type within a target gas, and by including fixed and random effects. The optimization measure applied in this study, even though extended to a split-plot experimental situation, is conceptually similar to the optimization measure applied in [
13].
Our contribution can be summarized as follows:
Therefore, by applying Formula (
3), the optimization is carried out by considering Model (
2) applied within each target gas and material type and involving random and fixed effects. Each random effect (Model (
2)) is included in Formula (
3) through its estimated coefficient, with lower and upper bounds (confidence interval at the
confidence level).
The relevance must be stressed of the application of the dual response approach, e.g., optimization according to mean and dispersion of the response variable, through the objective measure (
4), which evaluates only one estimated RS mixed model for a split-plot design, and involving fixed, as well as random effects. The process optimization is carried out by applying the NLP procedure (SAS Software; Version 9.2, Windows Platform).
4. Model and Optimization Results
The statistical modeling is carried out by applying the MIXED procedure of SAS software (Version 9.2, Windows Platform) on the experimental data (N = 48) described in
Section 3.1.
At the beginning, a single RS mixed model is applied for both the target gases jointly; even though the results are satisfactory, nevertheless, the gases show a different behavior by random effects, in particular error components split by replicates (chambers). However, it must be noted that the fixed part of the model, related to the main experimental and sub-experimental variables, is the same for both target gases. Therefore, in order to achieve a better optimization for each target gas and each material type, we further apply two distinct RS mixed models, one for each target gas; each applied model differentiates by considering the random part.
All of the experimental quantitative variables are standardized; nevertheless, the optimization results are reported in the original scale.
4.1. Model Results on NO
A mixed response surface model is estimated for NO
by taking into account the sensing films all together, also named the material type (Mtx). Here, we have 24 experimental observations, e.g., three chambers (replicates), each chamber containing eight sensing films. More precisely, the fixed part of the model involves: the sensing films as categorical variables at eight levels (see
Table 1), the working temperature, in the linear and quadratic effects, and the first order interaction between the temperature and the sensing films. The random effects, also included in the mixed response surface model, are the gas concentration and the first order interaction between temperature and the three chambers. Therefore, four random coefficients are estimated. It must be noted that for this target gas, NO
, humidity and environmental temperature are not relevant in the final model. Furthermore, the estimation of the fixed part of Model (
5) is conducted via the consolidated estimation method of Generalized Least Squares (GLS), which enlarges the Ordinary Least Squares (OLS) method in order to consider the variance-covariance matrix for the response variable [
16]; moreover, the denominator degrees of freedom are calculated according to the Kenward–Roger method, which works well for the small sample size [
18]. Variance components are estimated through the REML-Restricted Maximum Likelihood estimation method, which performs residual (restricted) maximum likelihood; for details, see also [
16].
The specific mixed RS model, estimated for NO
, is the following:
In Formula (
5),
is the coefficient for the linear effect of temperature, while
is related to the estimation of the quadratic effect for this SP factor;
are the coefficients related to the material type, which is a categorical variable at eight levels and denoted here as
for the generic
i level;
are the eight estimated coefficients relating to the interaction term between these two fixed effects. It must be noted that for the categorical variable material type and for the interaction effect, the highest level is used as reference level in model estimation, i.e.,
and
, respectively. By considering the random effects,
are the three coefficients related to the evaluation of the interaction between the three chambers and the working temperature. The other random component of the model is the gas concentration
.
In
Table 3, the estimated coefficients for fixed effects of Model (
5) are shown; it must be noted that the linear effect of material type is also relevant for setting the working temperature, through the first order interaction effect. Temperature is very relevant as a linear effect, as well as a quadratic effect. Furthermore, the first order interaction between material type and temperature plays a very relevant role for the subsequent optimization process (
Section 4.3).
Table 4 shows the REML estimates related to the random effects included in Model (
5).
Furthermore, we also illustrate the fixed effects by the amount of variability explained, through the Type III test, as shown in
Table 5. The F-test explains the global relevance for each fixed effect involved in Model (
5); it must be noted that the main effect of material type is globally highly significant; while the interaction between material type and temperature is nearly significant at
.
4.2. Model Results on CO
Analogously to the mixed response surface model estimated for NO
, in this subsection, we show the modeling results related to CO, by taking the sensing films (Mtx) all together into account. In this case, the 24 experimental observations, related to the three chambers (replicates), are also used to better evaluate the error variance component; in fact, the error is split according to each chamber. By considering the fixed part of the model, we evaluate: the sensing films as categorical variables at eight levels (see
Table 1), the working temperature, in the linear and quadratic effects, and the first order interaction between the temperature and the sensing films. For this type of gas, the WP factor humidity is relevant for a better fitting of the model, and thus, it is included as a fixed effect. The random part of the model differentiates with respect to Model (
5) in the evaluation of the error component, while only one random effect is included: the gas concentration. Therefore, we estimate a single random coefficient for gas concentration and four variance components, related to the gas concentration and the three components of the error variance split by the three chambers. It must be noted that also for the target gas CO, the environmental temperature is not relevant in the final selected model.
The specific mixed RS model, estimated for CO, is the following:
In Formula (
6),
is the coefficient for the linear effect of temperature, while
is related to the estimation of the quadratic effect for this SP factor;
are the coefficients related to the material type, which is a categorical variable at eight levels and denoted here as
for the generic
i level;
are the eight estimated coefficients relating to the interaction term between these two fixed effects. A further fixed coefficient
is related to the linear effect of the WP factor humidity.
By considering the random part of the model, are the coefficients related to the evaluation of random error component split by chambers. The random effect of the model is the gas concentration .
In
Table 6, the estimated coefficients for the fixed effects of Model (
6) are shown; it must be noted that the linear effect of material type is more relevant for this type of gas; the working temperature is relevant both in the linear and quadratic effects. Furthermore, the first order interaction between material type and temperature shows a better fitting through the estimated coefficients, also due to the standard errors, which are lower than the corresponding standard errors estimated in Model (
5) (
Table 3).
Table 7 shows the REML estimates related to the variance components included in Model (
6).
In
Table 8, the fixed effects by the amount of variability explained, through the Type III test, are reported. The F-values explain the global relevance for each fixed effect involved in Model (
6); the highly significant p-value must be noted for the material type effect and also for the linear effect of the working temperature. The interaction between temperature and material type is also significant (
), while the quadratic effect of the temperature shows a low importance.
4.3. Optimization Results
The process optimization is carried out by applying Formulas (
3) and (
4) reported in
Section 3.3. Moreover, the two estimated models, one for each target gas (Formulas (
5) and (
6)), are then used to compute Formula (
3), by considering the specific features for this process and the peculiar characteristics of the experimental variables involved therein. Therefore, during the optimization step, a further distinction is performed by considering the type of effect, fixed or random, and the nature of variables. All of the procedures are carried out on the coded experimental region, even though the results are reported on the original scale.
Furthermore, in the case study, the target value
for the sensor response values
is maximized for each material type within the target gas, and simultaneously, a minimum for the working temperature has been investigated, within constrained and specified intervals, as shown in
Table 2, e.g.,
C and
C for NO
and CO, respectively. The work in [
11] shows that the aging process of the chemical film can be limited using a low working temperature by providing savings in terms of electrical power consumption. The achieved optimal solution, related to the maximization of the sensor response, may be viewed as a global performance measure for each material type, by considering the related gas concentration, the working temperature and the other effects involved in the models, also considering first order interactions.
Note that each sensor film is optimized separately within a target gas, through the estimated RS model, because the eight sensing films are included in the split-plot design as a categorical variable at eight levels. For each film, the variability of the estimated coefficient is evaluated by including the confidence interval (lower and upper bounds) in the optimization code. The other fixed effect of the model, temperature, is also evaluated through the estimated coefficient and the range on the coded experimental region. When considering the interaction between sensor film and temperature, this effect is involved in the optimization step by the estimated coefficients, one for each material type, but also including a control on coefficient variability through the related confidence interval. Random effects, e.g., gas concentration, the related first order interaction (Model (
5)) and error components (Model (
6)) are included in the objective function
by confidence intervals.
The optimal solutions are reported at the original level, for each target gas (
Table 9 and
Table 10); more precisely, for NO
(
Table 9), we illustrate the achieved results for material type Nos. 1, 3, 8; for
(
Table 10), we report the results obtained for material type Nos. 1, 5. When observing
Table 9, for each material type (Nos. 1, 3, 8), the achieved optimal response value (
) and the working temperature (
) are shown. Moreover, these results are reached by also involving the gas concentration as a random effect (
) through the REML estimation (
Table 4) and the corresponding lower and upper values of the confidence interval. In addition, the interaction between each chamber and the working temperature (
Table 4) is evaluated, and these three variance components are useful for a better achievement of the optimal solutions, even though they are not significant. As regards the second target gas CO, in
Table 10, optimization results are reported for the material types No. 1 and No. 5. Analogously, optimal achieved values (
) for the response conditional to the working temperatures are reported for both materials. In this case, the REML estimate (
) for gas concentration is reported in
Table 7, and it is also included in the optimization step with the corresponding lower and upper limits (confidence interval). It must be noted that for CO, the variance components for the residual error are split by the three chambers, in order to better evaluate the variability of each replicate; these variance components are also included in the optimization step for better controlling the achievement of the optimal response conditional to the temperature values.
Optimization results are checked through the objective function value , the gradient estimates (maximum absolute gradient value) and the determinant of the Hessian matrix A last remark relates to the attention paid to avoid constrained and unstable optimal solutions; a further check is the specified limit equal to an upper-bound for the step length () of the line search algorithm, during the first n iterations.
5. Discussion
The designed and implemented MOX chemical sensors appear complex; therefore, this research allowed us to identify the main effects and their associations among the experimental and sub-experimental factors involved in the planned split-plot design, by also including the related variability, in order to assess the response characteristics of perovskite materials under CO and NO
atmospheres. The applied split-plot design displayed an excellent performance for searching the optimum point of the response conditionally to the working temperature. The obtained response measurements for the eight analyzed materials appear comparable with the well-known YCO
background [
2,
37]. Furthermore, the experimental design is planned by considering a very small amount of trials (
N = 48), 24 observations for each target gas. The experimental data are then modeled through mixed response surface models for taking into account fixed, as well as random effects, specifically noises, e.g., humidity, and replicates, identified by chambers (three ones for each target gas); each chamber settled at a specific level of gas concentration. The choice of planning a split-plot design is related to the peculiar characteristics of this design when considering the distinction between WP factors and SP factors. The process optimization is carried out by involving: (i) the specific design variables, e.g., experimental and sub-experimental; (ii) the role played by each variable in this study. By considering the obtained optimization results, we make a distinction between the two target gases.
As for TG.1—Target Gas.1 (
Table 9), e.g., NO
(16 ppm, from design), Mt1 achieved several acceptable solutions during the optimization step; however, the best final solution is reached for the response value at
. Similarly, the material type Mt8, YCo
O
Pd, achieved the best solution with the response value optimized at
with the working temperature at
C, whereas for Mt3, YCo
Pd
O
, the best achieved response value is equal to
with temperature equal to
C. Unfortunately, the other material types gave less acceptable solutions from the engineering point of view, even though they satisfied all of the statistical requirements.
As regards TG.2 (
Table 10), e.g., CO, better results are obtained from the statistical point of view, especially by considering all of the diagnostic measures (convergence criteria and objective function values). Excellent results are achieved during the optimization procedure for Mt1. In particular, YCo
Pd
O
is useful and feasible for the detection of both gases, with optimal response equal to +10.23% and working temperature at
C for CO (284 ppm, from design) and response equal to −14.17% at
C for NO
(16 ppm, from design). In this context, the second sensing film, Mt5, YCoO
, is optimized at a good response value equal to +9.86% with temperature equal to
C. For this specific gas, CO, the other six sensing films show good performances from the statistical point of view; nevertheless, the engineering interpretation requires further studies.
When considering the gas concentration, the estimated random effects, reported in
Table 4 and
Table 7, allow for setting the optimization results for this variable into a range of variability equal to [16, 21 ppm] for TG.1 and equal to [15, 17.1 ppm] for TG.2.
The statistical study here applied reinforces the technical aims, also for providing the optimization of gas sensing materials conditioning to temperatures.
In this context, the proposed materials can be used for on-site detection of combustion of by-products (CO and NO
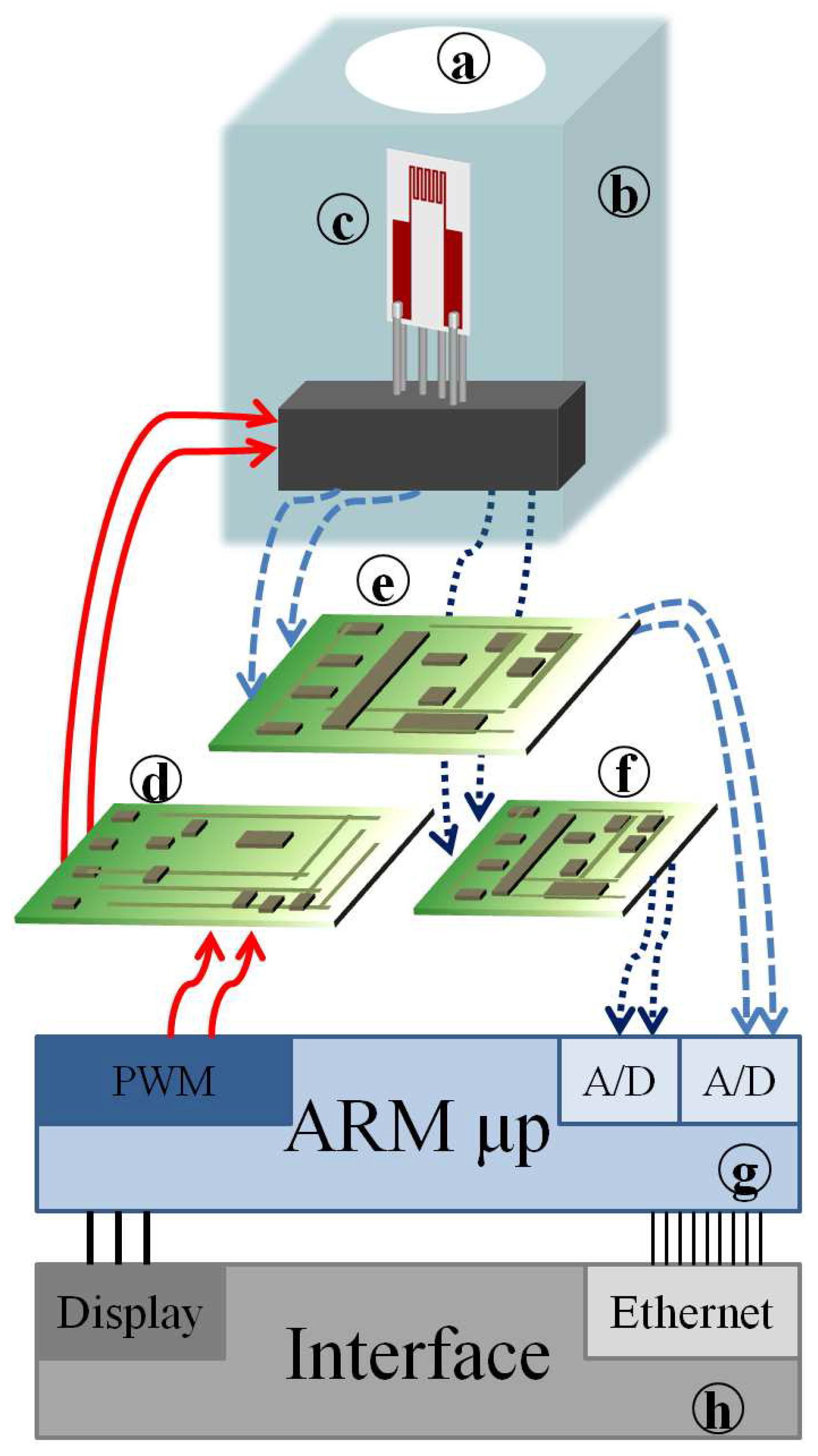
) in industrial applications where a complete detection system is based on the architecture shown in
Figure 7. This system allows for programming the operating temperatures of the sensors and hence for optimizing the response to the target gases both for CO and NO
.
The portable system (
Figure 7) is based on an ad hoc substrate (c) hosting both the sensing film (i.e., Mt1, Mt2, Mt5 and Mt8), the heater and RTD temperature sensors, and it allows for implementing a simple, fast and accurate working temperature control system. The front-end is designed in order to grant a high accuracy of temperature measurement and control (e) and a very large measurement range for the resistance of chemical film (f). Moreover, the overall design of the sensor aims at reducing the power consumption (d). A low-cost microprocessor, ARM (g), with a Reduced Instruction Set Computing (RISC) architecture, controls the system, acquires and processes the measurement data. This architecture provides all of the requirements for stand-alone devices. An Ethernet interface allows for easy connectivity (h). By regarding the contribution of this work in conjunction with the previous study [
13], a significant improvement in the detection of dangerous gases has been provided. Moreover, the significant improvement can also be acknowledged by considering the methodological approach, through the planning and optimization of the split-plot with the inclusion of environmental noise effects, such as humidity.