Experimental Study on the Icing Dielectric Constant for the Capacitive Icing Sensor
Abstract
:1. Introduction
2. The Background of the Capacitive Sensing Technique
2.1. The Complex Dielectric Constant
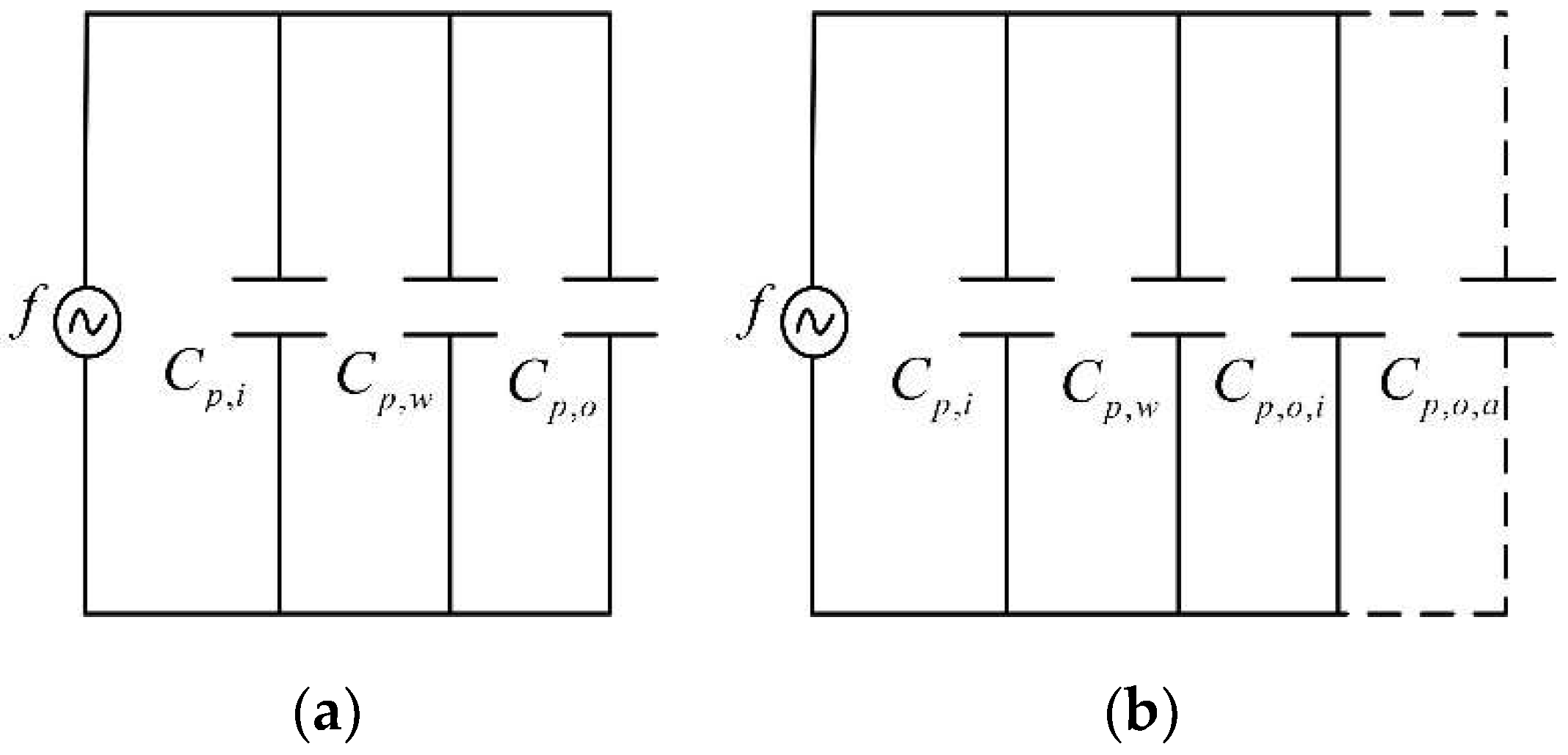
2.2. Mathematical Background for the Experiments on Icing Dielectric Constant
2.3. The Relative Dielectric Constant for the Two Mixture Materials
3. Experiments
3.1. Artificial Icing Laboratory
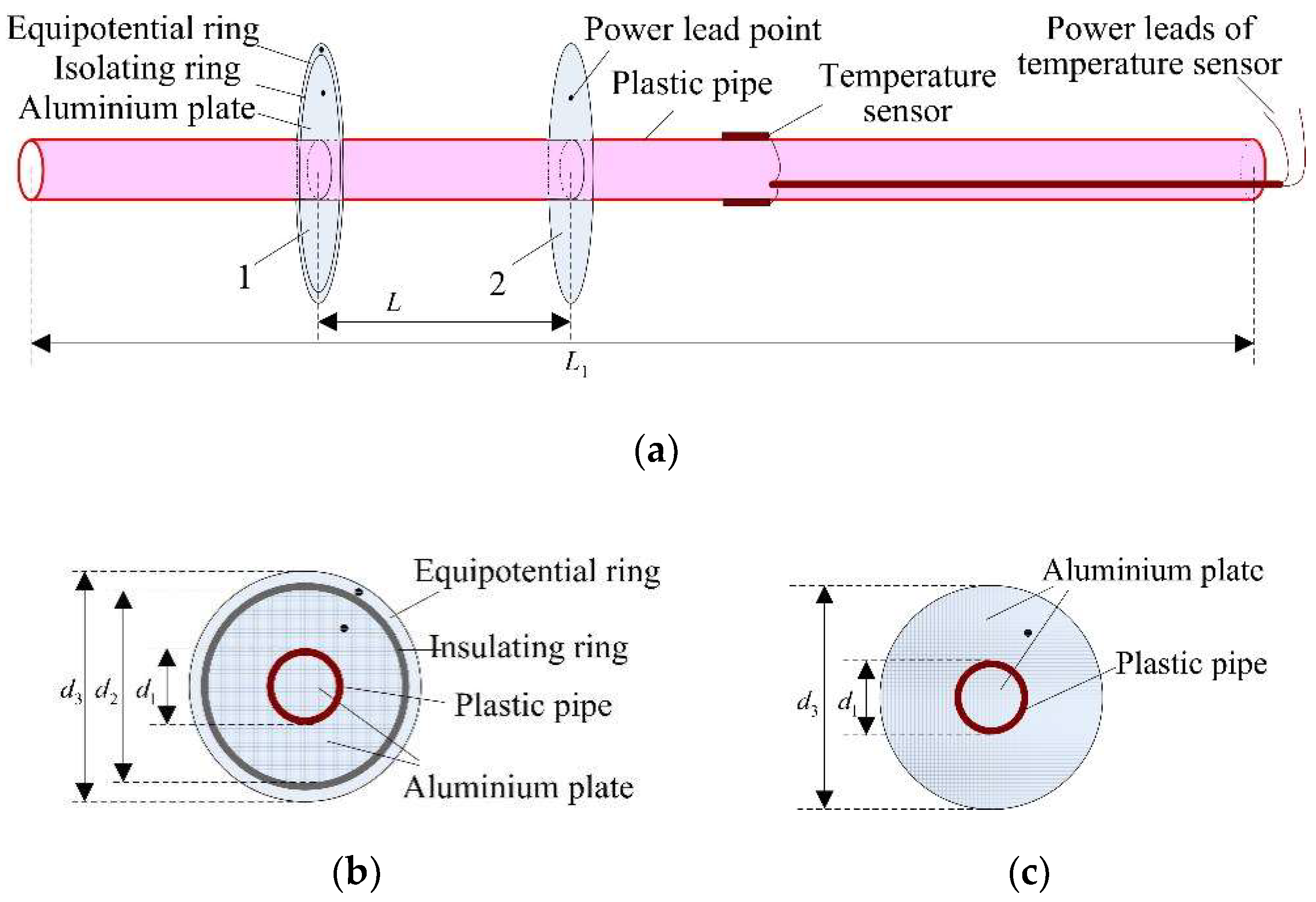
3.2. Experimental Apparatus
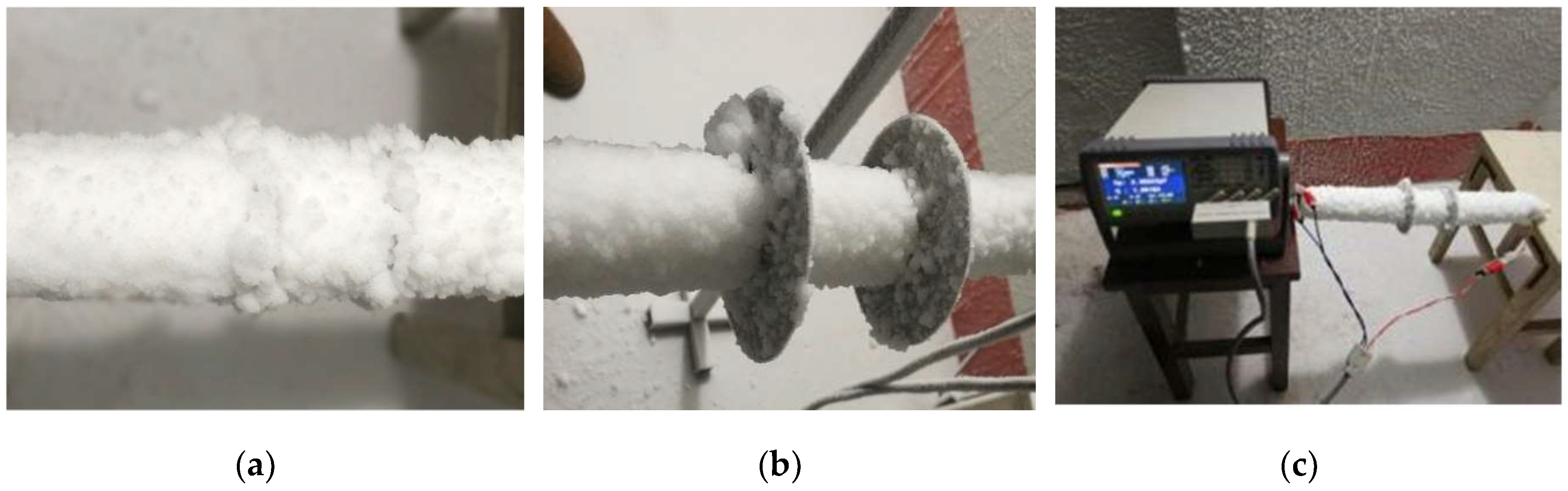
3.3. Experimental Procedures
4. Experimental Results
4.1. Icing Crystal Accretion on the Simulation Conductors
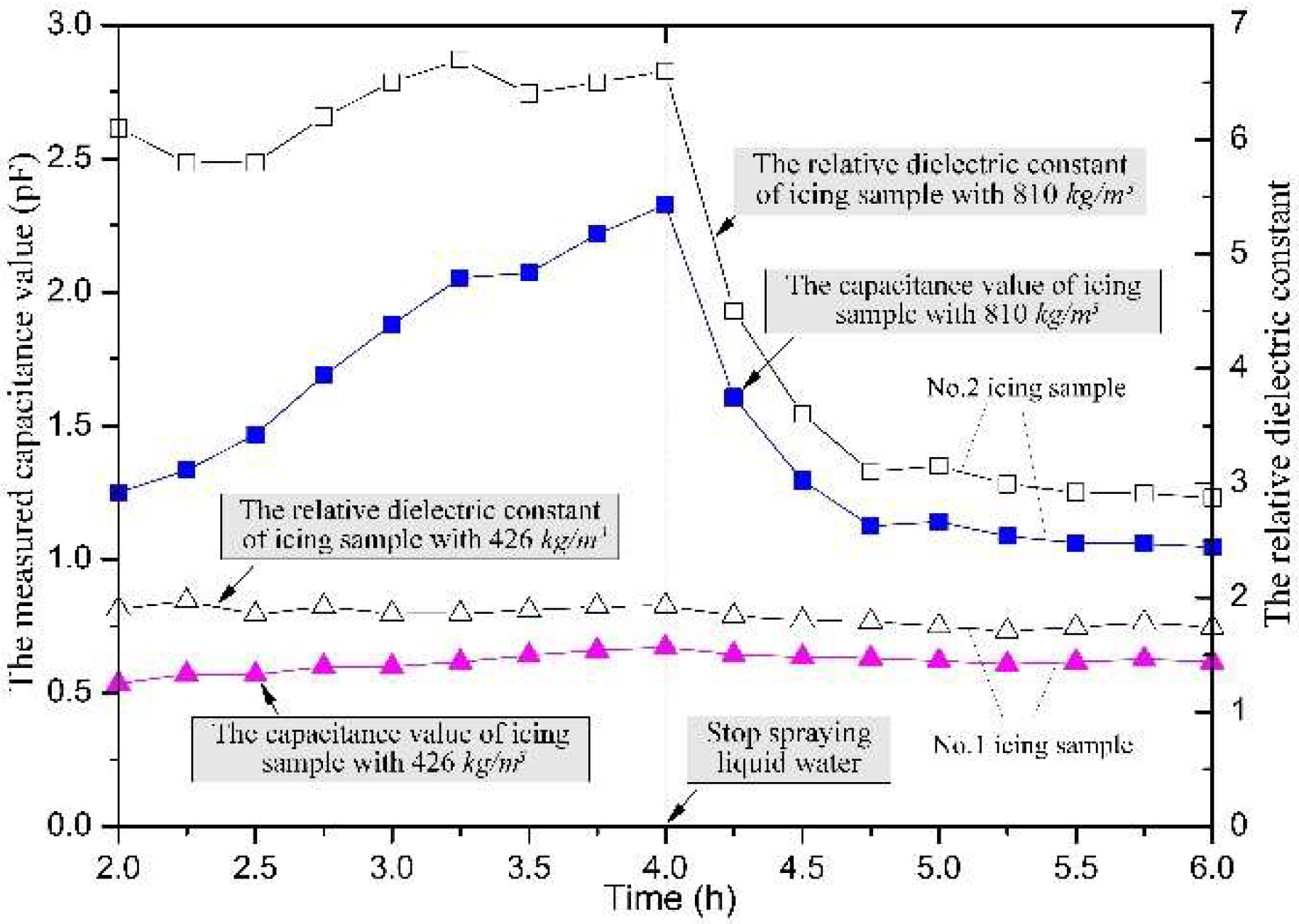
4.2. Effects of Exposure Time on the Relative Dielectric Constant of the Icing Sample
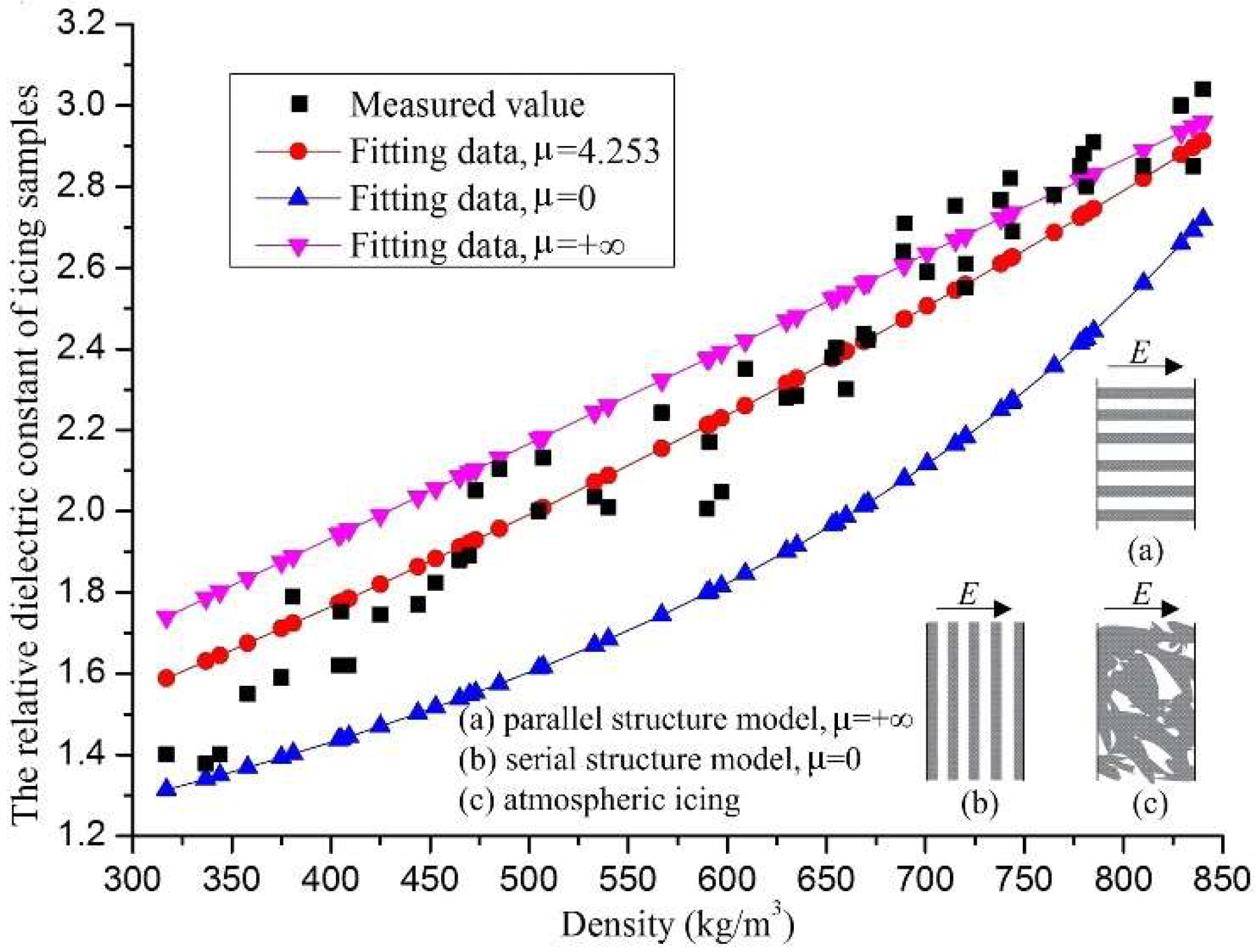
4.3. Effects of Icing Density on Its Relative Dielectric Constant
4.4. Effects of the Icing Temperature on the Relative Dielectric Constant
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Farzaneh, M. Modern Meteorology and Atmospheric Icing. In Atmospheric Icing of Power Networks; Springer Netherlands: Berlin, Germany, 2008; pp. 1–10. ISBN 978-1-4020-8530-7. [Google Scholar]
- Makkonen, L. Models for the growth of rime, glaze, icicles and wet snow on structures. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2000, 1776, 2913–2939. [Google Scholar] [CrossRef]
- Lozowski, E.P.; Szilder, K.; Makkonen, L. Computer simulation of marine ice accretion. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2000, 1776, 2811–2845. [Google Scholar] [CrossRef]
- Homola, M.C.; Nicklasson, P.J.; Sundsbø, P.A. Ice sensors for wind turbines. Cold Reg. Sci. Technol. 2006, 2, 125–131. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Huang, X.B.; Jia, J.Y. Experimental Study on the Thermal Conductivity for Transmission Line Icing. Cold Reg. Sci. Technol. 2016, 129, 96–103. [Google Scholar] [CrossRef]
- Hu, Y. Analysis and countermeasures discussion for large area icing accident on power grid. High Volt. Eng. 2008, 2, 215–219. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, X.L.; Fan, S.H.; Zhang, Z.J. Simulation and experimental investigation of DC ice-melting process on an iced conductor. IEEE Trans. Power Deliv. 2010, 2, 919–929. [Google Scholar] [CrossRef]
- Makkonen, L. Modeling of ice accretion on wires. J. Clim. Appl. Meteorol. 1984, 6, 929–939. [Google Scholar] [CrossRef]
- Huneault, M.; Langheit, C.; Caron, J. Combined models for glaze ice accretion and de-icing of current-carrying electrical conductors. IEEE Trans. Power Deliv. 2005, 2, 1611–1616. [Google Scholar] [CrossRef]
- Péter, Z.; Volat, C.; Farzaneh, M. Numerical investigations of a new thermal de-icing method for overhead conductors based on high current impulses. IET Gener. Transmiss. Distrib. 2008, 5, 666–675. [Google Scholar] [CrossRef]
- Jiang, X.L.; Xiang, Z.; Zhang, Z.J. Predictive Model for Equivalent Ice Thickness Load on Overhead Transmission Lines Based on Measured Insulator String Deviations. IEEE Trans. Power Deliv. 2014, 4, 1659–1665. [Google Scholar] [CrossRef]
- Owusu, K.P.; Kuhn, D.C.; Bibeau, E.L. Capacitive probe for ice detection and accretion rate measurement: Proof of concept. Renew. Energy 2013, 50, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Bhattu, S.K.; Mughal, U.N.; Virk, M.S. Experimental study of relative permittivity of atmospheric ice. Int. J. Energy Environ. 2013, 3, 369–376. [Google Scholar]
- Huang, X.B.; Zhang, F.; Li, H.S. An Online Technology for Measuring Icing Shape on Conductor Based on Vision and Force Sensors. IEEE Trans. Instrum. Meas. 2017, 12, 3180–3189. [Google Scholar] [CrossRef]
- Hao, Y.P.; Wei, J.; Jiang, X.L. Icing Condition Assessment of In-Service Glass Insulators Based on Graphical Shed Spacing and Graphical Shed Overhang. Energies 2018, 2, 318. [Google Scholar] [CrossRef]
- Luo, J.B.; Hao, Y.P.; Ye, Q. Development of optical fiber sensors based on Brillouin scattering and FBG for on-line monitoring in overhead transmission line. J. Lightw. Technol. 2013, 10, 1559–1565. [Google Scholar] [CrossRef]
- Wydra, M.; Kisala, P.; Harasim, D.; Kacejko, P. Overhead Transmission Line Sag Estimation Using a Simple Optomechanical System with Chirped Fiber Bragg Gratings. Part 1: Preliminary Measurements. Sensors 2018, 18, 309. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Mao, N.; Li, Y. The Reusable Load Cell with Protection Applied for Online Monitoring of Overhead Transmission Lines Based on Fiber Bragg Grating. Sensors 2016, 16, 922. [Google Scholar] [CrossRef] [PubMed]
- Takei, I. Dielectric relaxation of ice samples grown from vaporphase or liquid-phase water. In Proceedings of the 11th International Conference on the Physics and Chemistry of Ice, Bremerhaven, Germany, 23–28 July 2006; p. 577, ISBN 978-0-85404-350-7. [Google Scholar]
- Mughal, U.N.; Virk, M.S.; Mustafa, M.Y. Dielectric based sensing of atmospheric ice. In AIP Conference Proceedings; AIP: College Park, MD, USA, 2013; Volume 1570, pp. 212–220. [Google Scholar] [CrossRef]
- Niang, M.; Bernier, M.; Stacheder, M. Influence of snow temperature interpolation algorithm and dielectric mixing-model coefficient on density and liquid water content determination in a cold seasonal snow pack. Subsurf. Sens. Technol. Appl. 2006, 1, 1–22. [Google Scholar] [CrossRef]
- Andersson, O. Dielectric relaxation of the amorphous ices. J. Phys. 2008, 24, 244115. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Huang, X.; Tian, Y.; Ji, C.; Cao, W.; Zhao, L. Experimental Study on the Icing Dielectric Constant for the Capacitive Icing Sensor. Sensors 2018, 18, 3325. https://doi.org/10.3390/s18103325
Zhu Y, Huang X, Tian Y, Ji C, Cao W, Zhao L. Experimental Study on the Icing Dielectric Constant for the Capacitive Icing Sensor. Sensors. 2018; 18(10):3325. https://doi.org/10.3390/s18103325
Chicago/Turabian StyleZhu, Yongcan, Xinbo Huang, Yi Tian, Chao Ji, Wen Cao, and Long Zhao. 2018. "Experimental Study on the Icing Dielectric Constant for the Capacitive Icing Sensor" Sensors 18, no. 10: 3325. https://doi.org/10.3390/s18103325
APA StyleZhu, Y., Huang, X., Tian, Y., Ji, C., Cao, W., & Zhao, L. (2018). Experimental Study on the Icing Dielectric Constant for the Capacitive Icing Sensor. Sensors, 18(10), 3325. https://doi.org/10.3390/s18103325




