Design of an Aluminum/Polymer Plasmonic 2D Crystal for Label-Free Optical Biosensing
Abstract
:1. Introduction
2. Sensor Structure and Modeling
3. Design Parameters and Performance Functions
4. Results
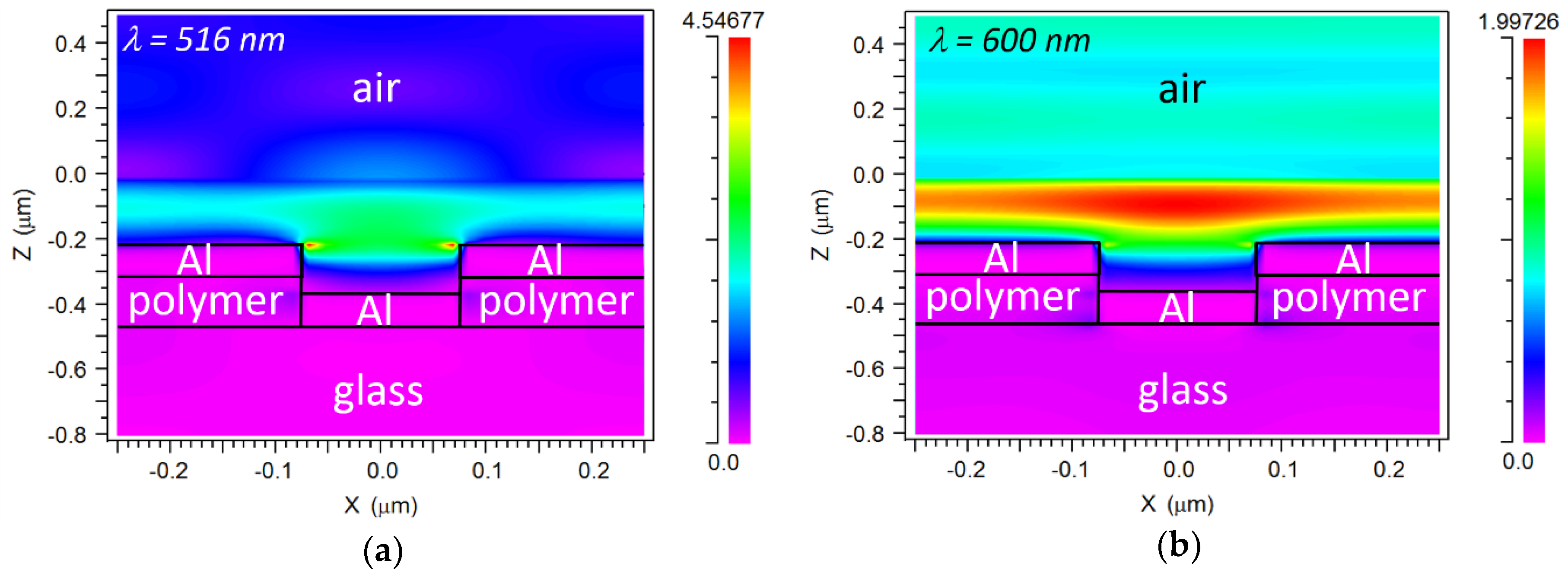
4.1. Sensor Response and Analysis of Resonances
4.2. Dimensions Optimization
4.3. Effect of Al Oxidation
5. Discussion
- (a)
- selection of design parameters,
- (b)
- selection of performance functions,
- (c)
- analysis of resonances, and
- (d)
- design optimization,
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lindquist, N.C.; Nagpal, P.; McPeak, K.M.; Norris, D.J.; Oh, S.-H. Engineering metallic nanostructures for plasmonics and nanophotonics. Rep. Prog. Phys. 2012, 75, 036501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, J.F.; Zhao, S.S. Plasmonic Sensors for Analysis of Proteins and an Oncologic Drug in Human Serum. In Nanobiosensors and Nanobioanalyses; Vestergaard, M., Kerman, K., Hsing, I.M., Tamiya, E., Eds.; Springer: Tokyo, Japan. [CrossRef]
- Gomez-Cruz, J.; Nair, S.; Manjarrez-Hernández, A.; Gavilanes-Parra, S.; Ascanio, G.; Escobedo, C. Cost-effective flow-through nanohole array-based biosensing platform for the label-free detection of uropathogenic E. coli in real time. Biosens. Bioelectron. 2018, 106, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Raschke, G.; Kowarik, S.; Franzl; Sönnichsen, T.; Klar, T.A.; Feldmann, J. Biomolecular recognition based on single gold nanoparticle light scattering. Nano Lett. 2003, 3, 935–938. [Google Scholar] [CrossRef]
- Sakono, M.; Zako, T.; Maeda, M. Naked-eye detection of amyloid aggregates using gold nanoparticles modified with amyloid beta antibody. Anal. Sci. 2012, 28, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Ultrasensitive chemical analysis by Raman spectroscopy. Chem. Rev. 1999, 99, 2957–2976. [Google Scholar] [CrossRef] [PubMed]
- Brolo, A.G.; Gordon, R.; Leathem, B.; Kavanagh, K.L. Surface plasmon sensor based on the enhanced light transmission through arrays of nanoholes in gold films. Langmuir 2004, 20, 4813–4815. [Google Scholar] [CrossRef] [PubMed]
- De Leebeeck, A.; Kumar, L.K.; de Lange, V.; Sinton, D.; Gordon, R.; Brolo, A.G. On-Chip Surface-Based Detection with Nanohole Arrays. Anal. Chem. 2007, 79, 4094–4100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Yonzon, C.R.; Haes, A.J.; van Duyne, R.P. Localized surface plasmon resonance biosensors. Nanomedicine 2006, 1, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhou, J.; Liu, T.; Tao, Y.; Jiang, R.; Liu, M.; Xiao, G.; Zhu, J.; Zhou, Z.-K.; Wang, X.; et al. Plasmonic gold mushroom arrays with refractive index sensing figures of merit approaching the theoretical limit. Nature Commun. 2013, 4, 2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpe, J.C.; Mitchell, J.S.; Lin, L.; Sedoglavich, N.; Blaikie, R.J. Gold nanohole array substrates as immunobiosensors. Anal. Chem. 2008, 80, 2244–2249. [Google Scholar] [CrossRef] [PubMed]
- Artar, A.; Yanik, A.A.; Altug, H. Fabry–Pérot nanocavities in multilayered plasmonic crystals for enhanced biosensing. Appl. Phys. Lett. 2009, 95, 051105. [Google Scholar] [CrossRef]
- Yang, J.-C.; Ji, J.; Hogle, J.M.; Larson, D.N. Multiplexed plasmonic sensing based on small-dimension nanohole arrays and intensity interrogation. Biosens. Bioelectron. 2009, 15, 2334–2338. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, C. On-chip nanohole array based sensing: A review. Lab Chip 2013, 13, 2445–2463. [Google Scholar] [CrossRef] [PubMed]
- Pilát, Z.; Kizovský, M.; Ježek, J.; Krátký, S.; Sobota, J.; Šiler, M.; Samek, O.; Buryška, T.; Vaňáček, P.; Damborský, J.; et al. Detection of Chloroalkanes by Surface-Enhanced Raman Spectroscopy in Microfluidic Chips. Sensors 2018, 18, 3212. [Google Scholar] [CrossRef] [PubMed]
- Sinton, D.; Gordon, R.; Brolo, A.G. Nanohole arrays in metal films as optofluidic elements: Progress and potential. Microfluid. Nanofluid. 2008, 4, 107–116. [Google Scholar] [CrossRef]
- Yang, J.-C.; Ji, J.; Hogle, J.M.; Larson, D.N. Metallic Nanohole Arrays on Fluoropolymer Substrates as Small Label-Free Real-Time Bioprobes. Nano Lett. 2008, 8, 2718–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuo, Y.; Hohertz, D.; Landrock, C.; Omrane, B.; Kavanagh, K.L.; Kaminska, B. Large-Area Low-Cost Flexible Plastic Nanohole Arrays for Integrated Bio-Chemical Sensing. IEEE Sens. J. 2013, 13, 3982–3990. [Google Scholar] [CrossRef]
- Polyanskiy, M.N. Refractive Index Database. Available online: https://refractiveindex.info/ (accessed on 1 October 2018).
- Martin, J.; Kociak, M.; Mahfoud, Z.; Proust, J.; Gerard, D.; Plain, J. High-resolution imaging and spectroscopy of multipolar plasmonic resonances in aluminum nanoantennas. Nano Lett. 2014, 14, 5517–5523. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, A.; Manjavacas, A.; Cao, Y.; McClain, M.J.; de Abajo, J.F.; Nordlander, P.; Halas, N.J. Pronounced linewidth narrowing of an aluminum nanoparticle metallic film. Nano Lett. 2015, 15, 6946–6951. [Google Scholar] [CrossRef] [PubMed]
- Canalejas-Tejero, V.; Herranz, S.; Bellingham, A.; Moreno-Bondi, M.C.; Barrios, C.A. Passivated Aluminum Nanohole Arrays for Label-Free Biosensing Applications. ACS Appl. Mater. Interfaces 2014, 6, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.A.; Canalejas-Tejero, V.; Herranz, S.; Moreno-Bondi, M.C.; Avella-Oliver, M.; Puchades, R.; Maquieira, A. Aluminum nanohole arrays fabricated on polycarbonate for compact disc-based label-free optical biosensing. Plasmonics 2014, 9, 645–649. [Google Scholar] [CrossRef]
- Li, W.; Qiu, Y.; Zhang, L.; Jiang, L.; Zhou, Z.; Chen, H.; Zhou, J. Aluminum nanopyramid array with tunable ultraviolet–visible–infrared wavelength plasmon resonances for rapid detection of carbohydrate antigen 199. Biosens. Bioelectron. 2016, 79, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, J.; Whitney, A.V.; Elam, J.W.; Van Duyne, R.P. Ultrastable substrates for surface-enhanced Raman spectroscopy: Al2O3 overlayers fabricated by atomic layer deposition yield improved anthrax biomarker detection. J. Am. Chem. Soc. 2006, 128, 10304–10309. [Google Scholar] [CrossRef] [PubMed]
- Block, I.; Ganesh, N.; Lu, M.; Cunningham, B.T. A sensitivity model for predicting photonic crystal biosensor performance. IEEE Sens. J. 2008, 8, 274–280. [Google Scholar] [CrossRef]
- Ju, J.; Han, Y.-A.; Kim, S.-M. Design Optimization of Structural Parameters for Highly Sensitive Photonic Crystal Label-Free Biosensors. Sensors 2013, 13, 3232–3241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, S.; García-Vidal, F.; Martín-Moreno, L. Influence of material propertieson extraordinary optical transmission through hole arrays. Phys. Rev. B Condens. Matter 2008, 77, 075401. [Google Scholar] [CrossRef]
- Otte, M.A.; Sepúlveda, B.; Ni, W.; Pérez Juste, J.; Liz-Marzán, L.M.; Lechuga, L.M. Identification of the optimal spectral region for plasmonic and nanoplasmonic sensing. ACS Nano 2010, 4, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K. Efficient coupling of a sub-5-nm-gap plasmonic crystal cavity with an integrated waveguide. Opt. Express 2015, 23, 2040–2047. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, M.S.; Wu, M.C. Efficient Coupling of an Antenna-Enhanced nanoLED into an Integrated InP Waveguide. Nano Lett. 2015, 15, 3329–3333. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D. Design and Analysis of Experiments, 5th ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Barrios, C.A.; Bañuls, M.J.; González-Pedro, V.; Gylfason, K.B.; Sánchez, B.; Griol, A.; Maquieira, A.; Sohlström, H.; Holgado, M.; Casquel, R. Label-free optical biosensing with slot-waveguides. Opt. Lett. 2008, 33, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Sanza, F.J.; Holgado, M.; Ortega, F.J.; Casquel, R.; López-Romero, D.; Bañuls, M.J.; Laguna, M.F.; Barrios, C.A.; Puchades, R.; Maquieira, A. Bio-Photonic Sensing Cells over transparent substrates for anti-gestrinone antibodies biosensing. Biosens. Bioelectron. 2011, 26, 4842–4847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banica, F.-G. Chemical Sensors and Biosensors Fundamentals and Applications; John Wiley & Sons: West Sussex, UK, 2012; p. 486. [Google Scholar]
- Lee, K.-L.; Hsu, H.-Y.; You, M.-L.; Chang, C.-C.; Pan, M.-Y.; Shi, X.; Ueno, K.; Misawa, H.; Wei, P.-K. Highly Sensitive Aluminum-Based Biosensors using Tailorable Fano Resonances in Capped Nanostructures. Sci. Rep. 2017, 7, 44104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Lin, J. Field enhancement of a metal grating with nanocavities and its sensing applications. J. Opt. 2017, 19, 055004. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tramarin, L.; Angulo Barrios, C. Design of an Aluminum/Polymer Plasmonic 2D Crystal for Label-Free Optical Biosensing. Sensors 2018, 18, 3335. https://doi.org/10.3390/s18103335
Tramarin L, Angulo Barrios C. Design of an Aluminum/Polymer Plasmonic 2D Crystal for Label-Free Optical Biosensing. Sensors. 2018; 18(10):3335. https://doi.org/10.3390/s18103335
Chicago/Turabian StyleTramarin, Luca, and Carlos Angulo Barrios. 2018. "Design of an Aluminum/Polymer Plasmonic 2D Crystal for Label-Free Optical Biosensing" Sensors 18, no. 10: 3335. https://doi.org/10.3390/s18103335




