Carbon Monoxide Sensing Technologies for Next-Generation Cyber-Physical Systems
Abstract
:1. Introduction
2. Necessity of Carbon Monoxide Detection
3. Cyber-Physical System Framework for CO Monitoring
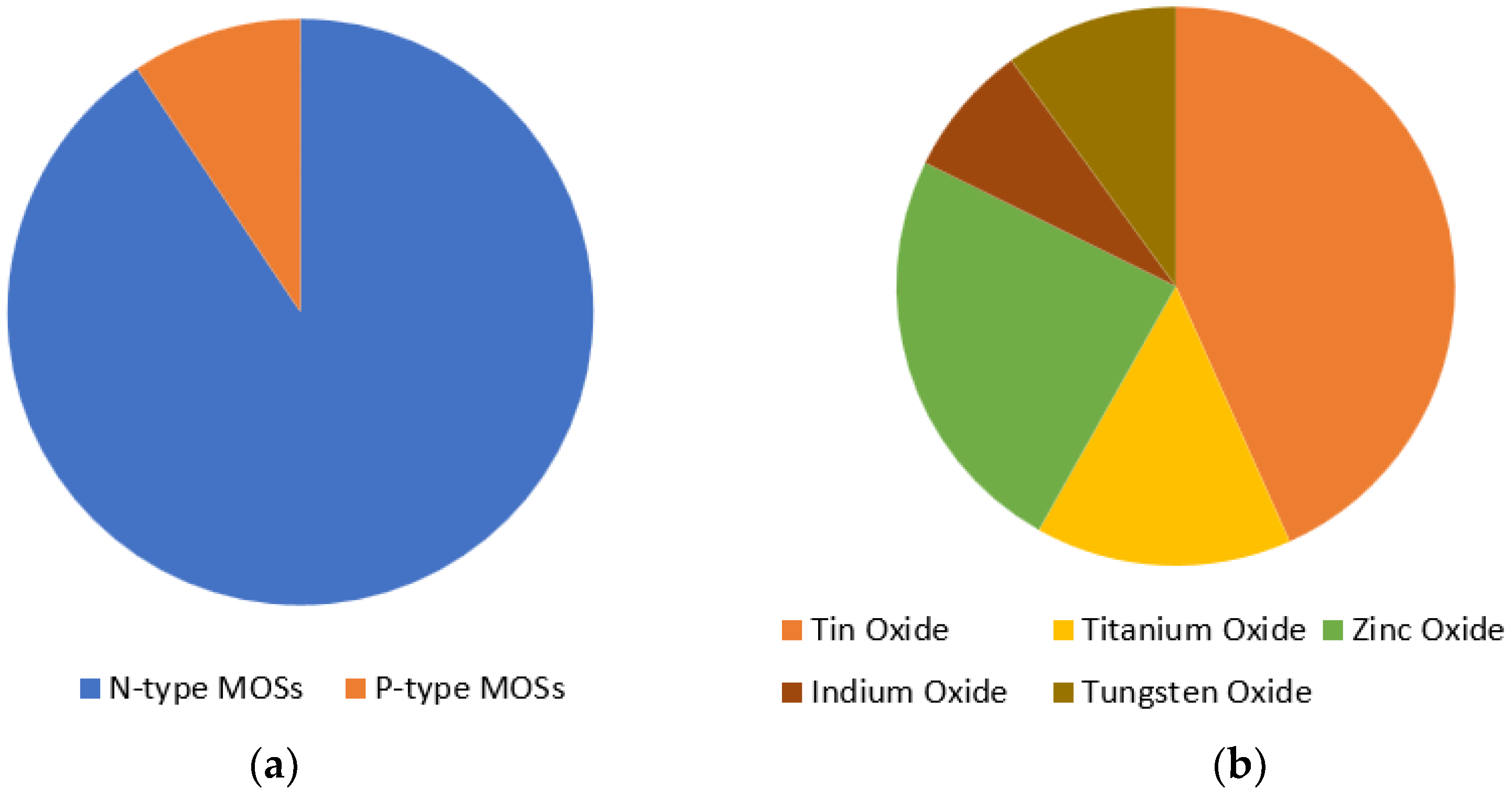
4. Metal Oxide Semiconductors Based CO Sensing
5. N-Type Metal Oxides for CO Sensing
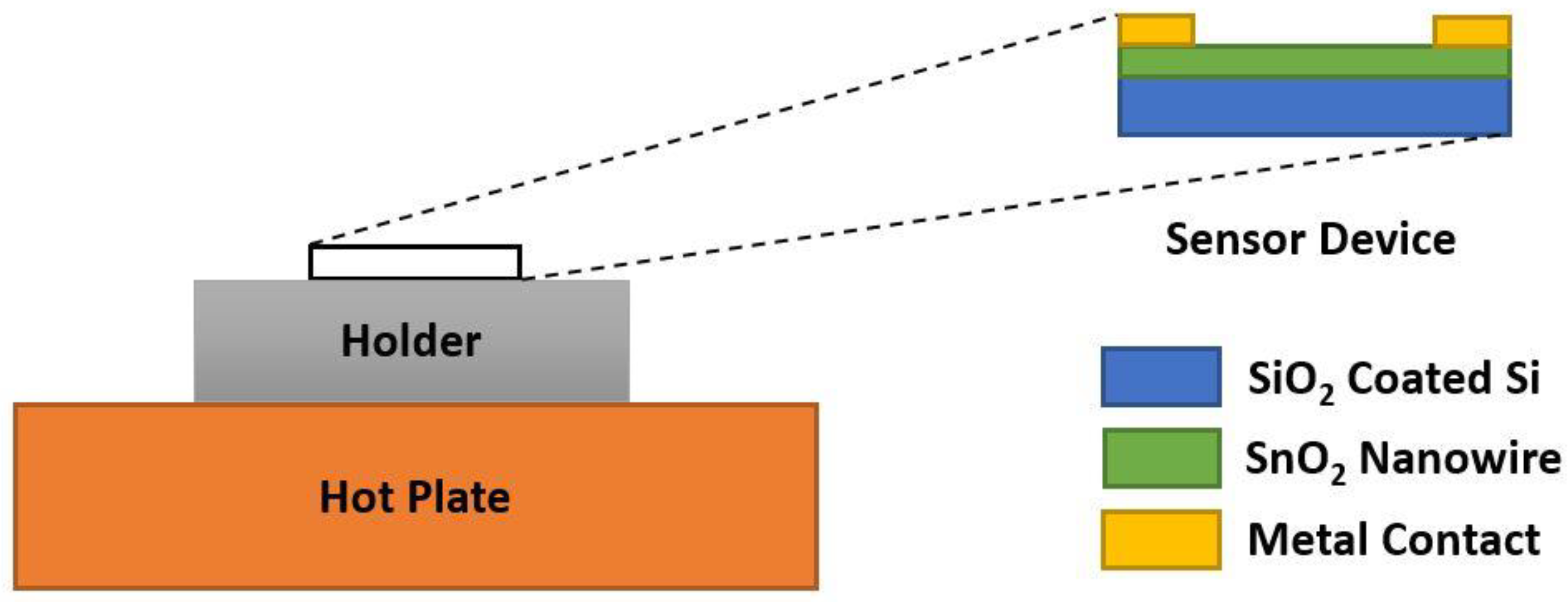
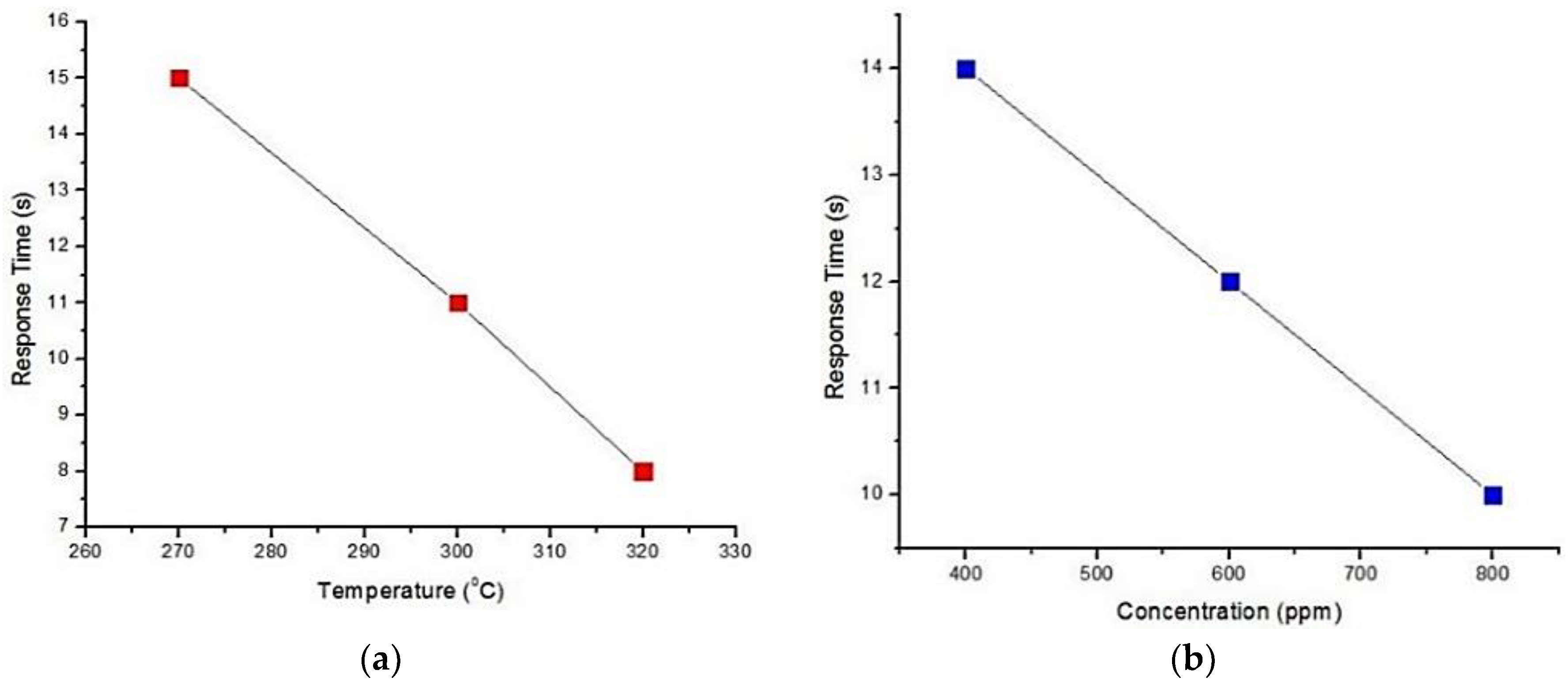
5.1. Tin Oxide (SnO2)
5.1.1. Copper Doped Tin Oxide
5.1.2. Palladium and Platinum Doped Tin Oxide
5.1.3. Gold Doped Tin Oxide
5.1.4. Multiwalled Carbon Nanotube Doped Tin Oxide
5.1.5. Vanadium Doped Tin Oxide
5.1.6. Calcium Doped Tin Oxide
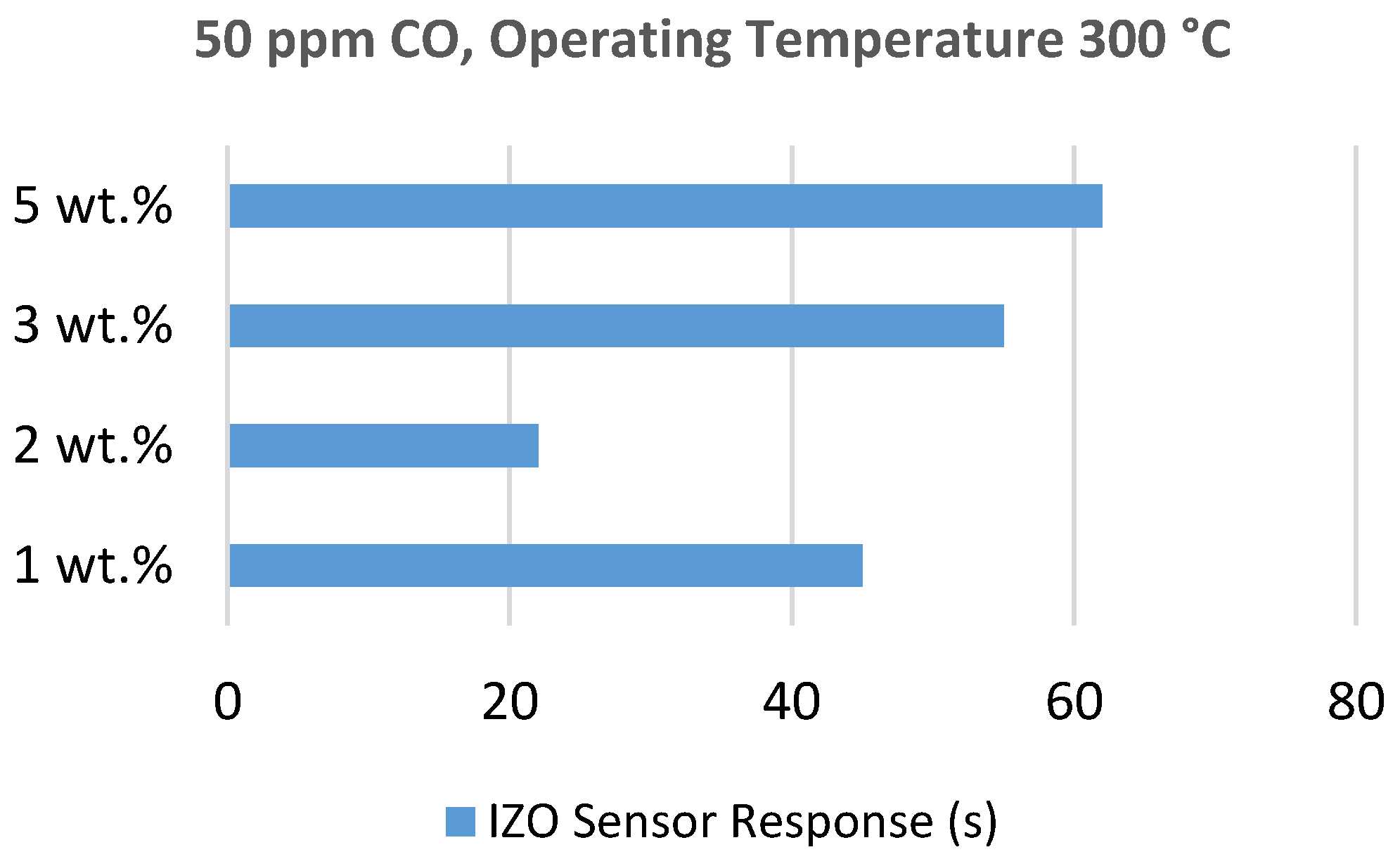
5.1.7. Zinc Oxide Doped Tin Oxide
5.1.8. Copper Oxide Doped Tin Oxide
5.1.9. Tin Oxide with Graphene and Silver Nanoparticles
5.1.10. Summary of the SnO2 Based CO Sensors
5.2. Titanium Oxide (TiO2)
Multiwalled Carbon Nanotube Doped TiO2 thin film
5.3. Zinc Oxide (ZnO)
5.3.1. Aluminum Doped Zinc Oxide
5.3.2. Copper, Palladium, Indium and Gallium Doped Zinc Oxide
5.3.3. Cerium Oxide Doped Zinc Oxide
5.4. Indium Oxide (In2O3)
Gold Doped Indium Oxide
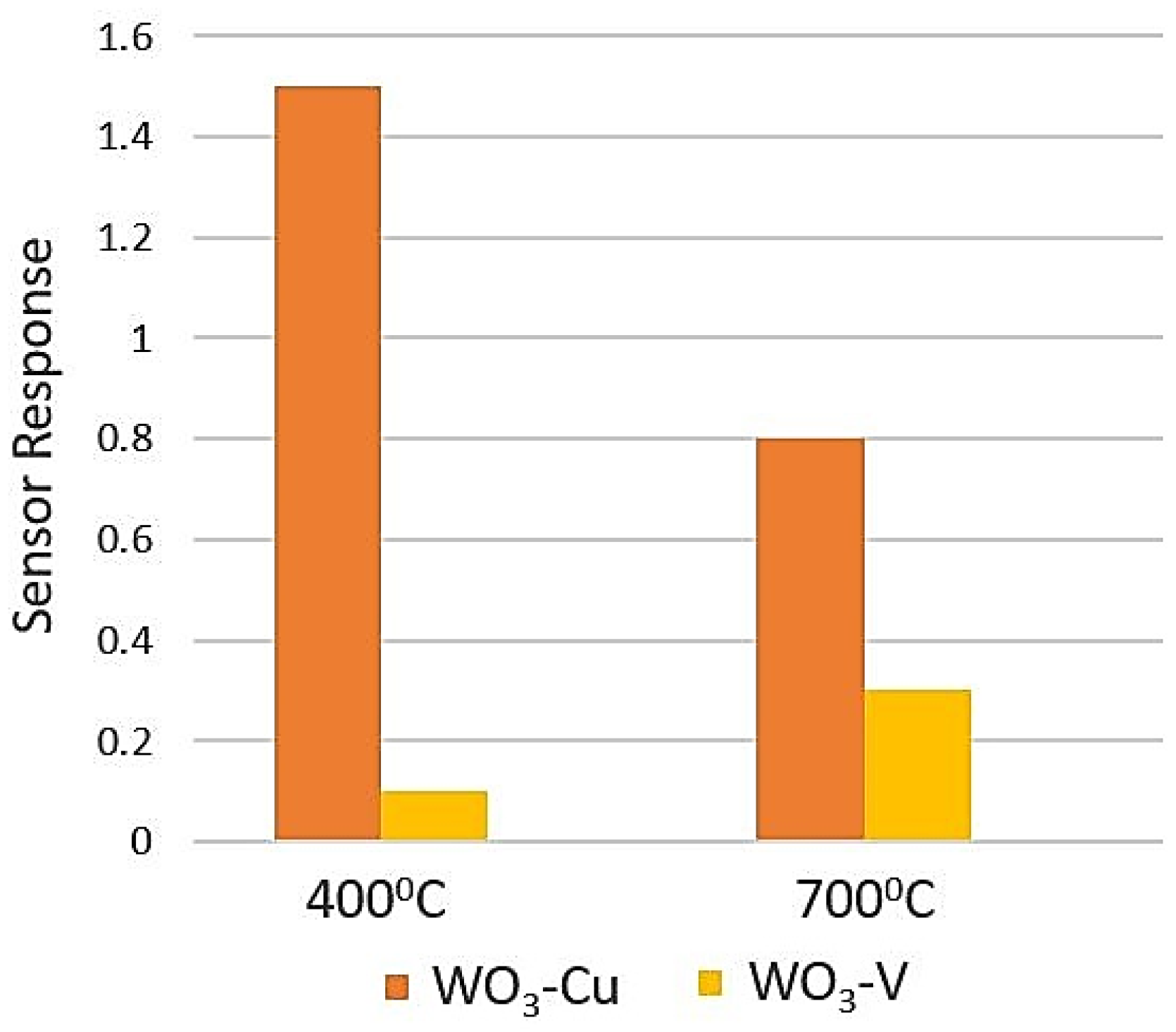
5.5. Tungsten Oxide (WO3)
6. P-Type Metal Oxides for CO Sensing
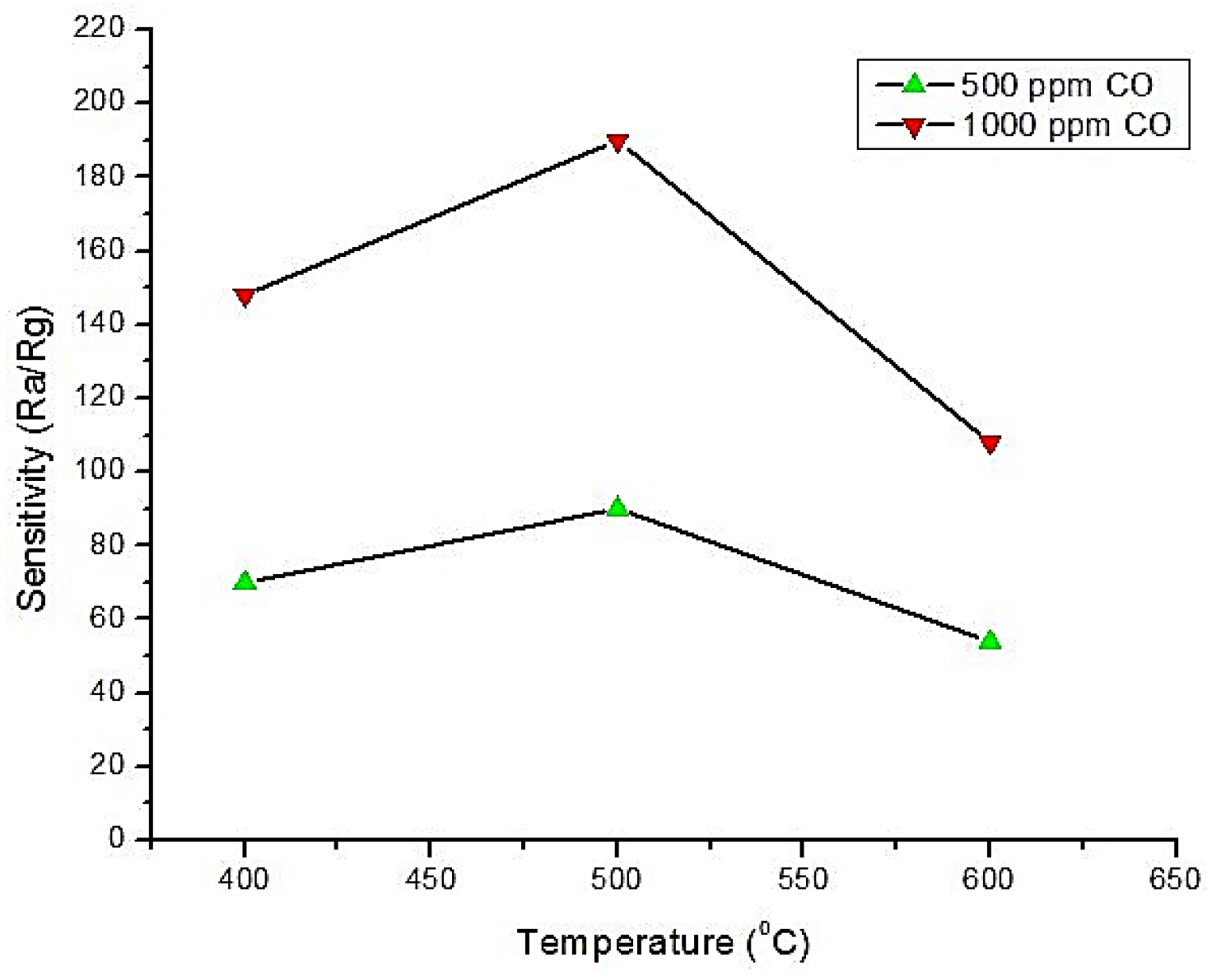
6.1. Cobalt Oxide (CoO and Co3O4)
6.1.1. Gold-Palladium-Platinum Doped Cobalt Oxide
6.1.2. Tin Oxide Doped Cobalt Oxide
6.2. Nickel Oxide (NiO)
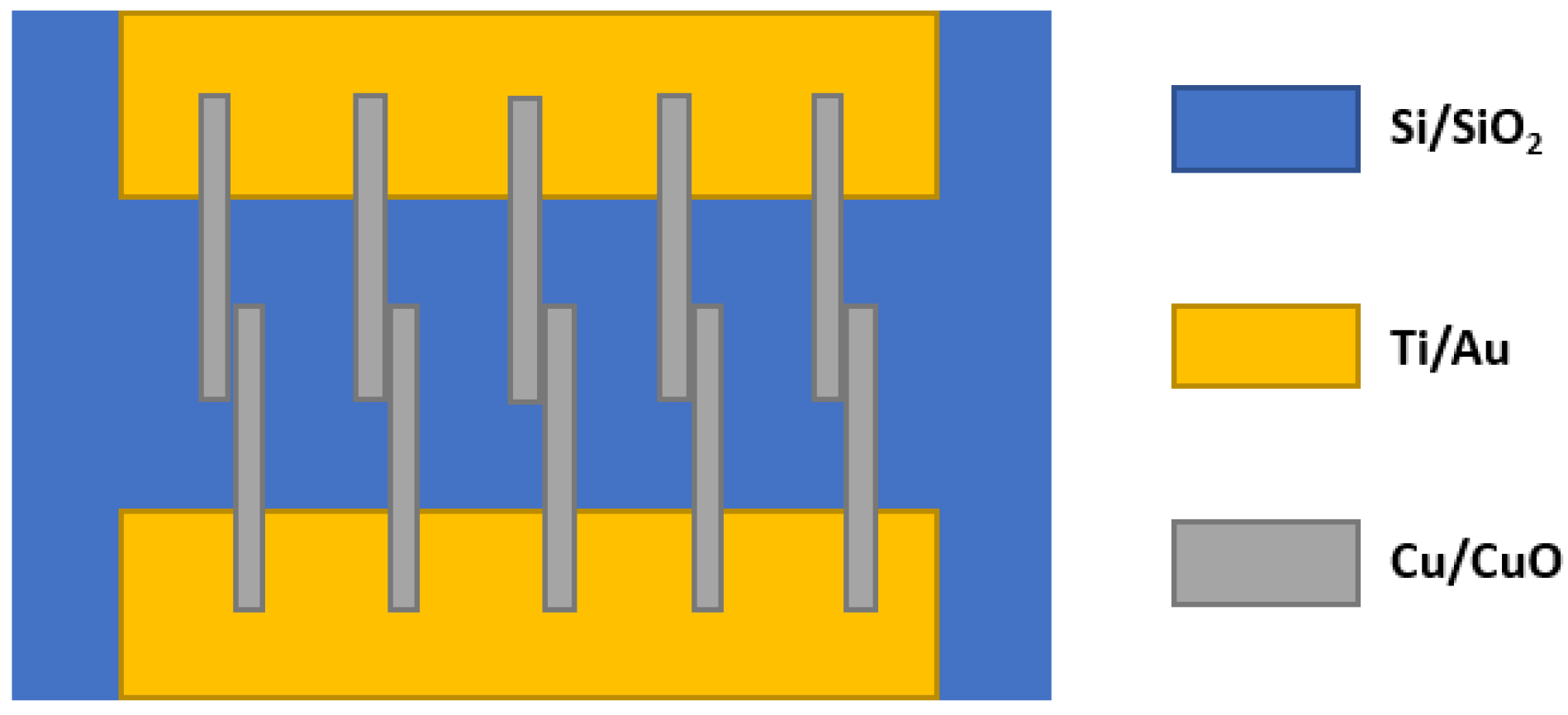
6.3. Copper Oxide (CuO)
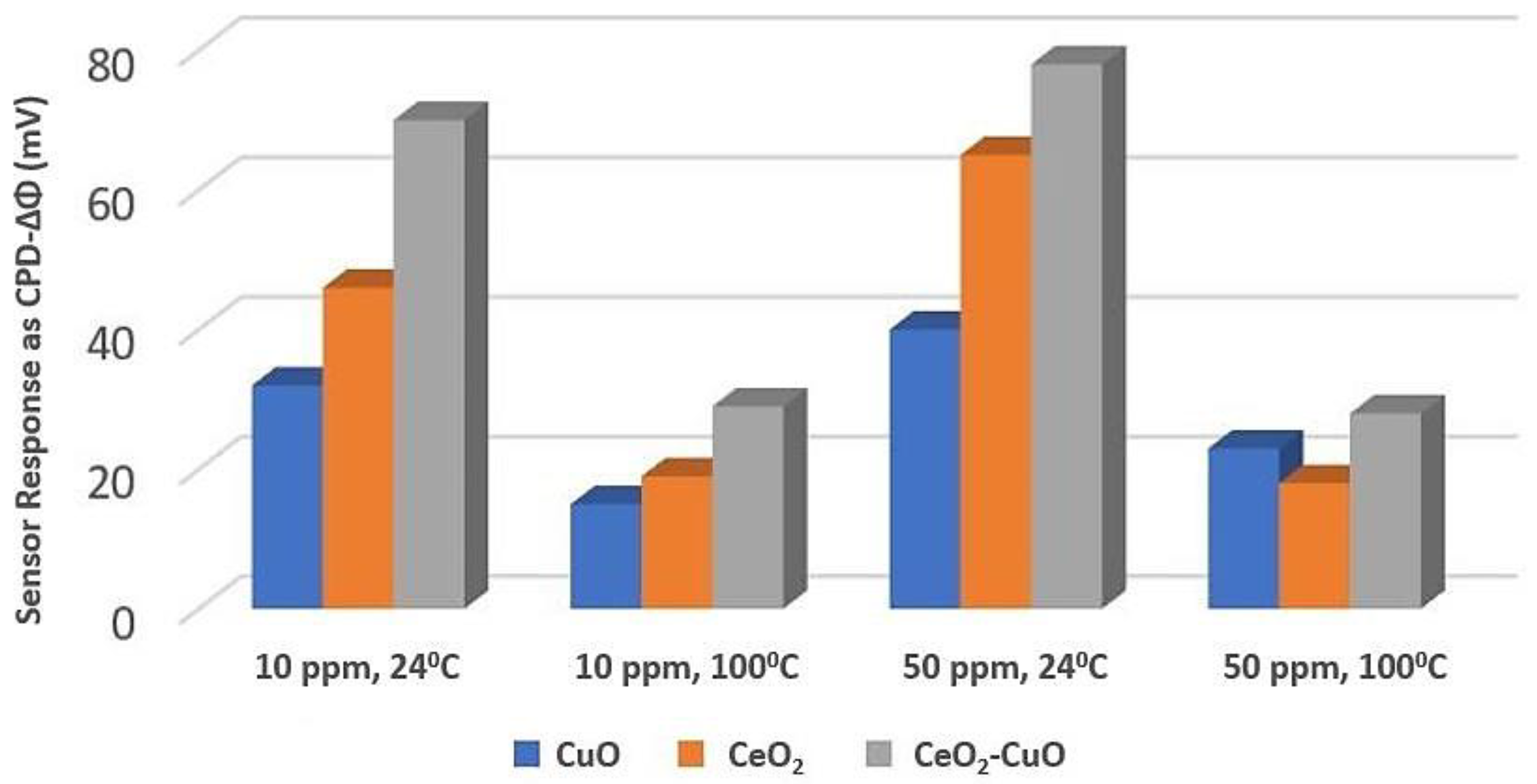
Copper Oxide (CuO) with Cerium Oxide (CeO2)
7. Summary of MOS Based (except SnO2) CO Sensors
8. Optical Based CO Detection System
8.1. Quantum Cascade Laser Based Sensing
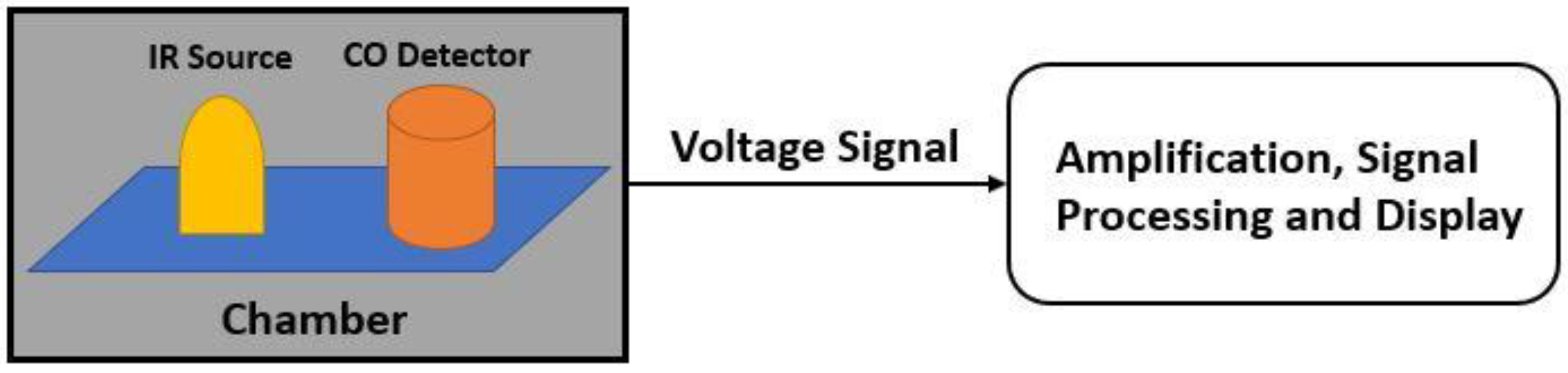
8.2. Non-Dispersive Infrared (NDIR) Based Sensing
9. Conclusions and Summary
Author Contributions
Funding
Conflicts of Interest
References
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A new detector for gaseous components using semi-conductive thin films. Anal. Chem. 1962, 34, 1502. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Moseley, P.T. Solid state gas sensors. Meas. Sci. Technol. 1997, 8, 223–237. [Google Scholar] [CrossRef]
- Shimizu, Y.; Egashira, M. Basic aspects and challenges of semiconductor gas sensors. MRS Bull. 1999, 24, 18–24. [Google Scholar] [CrossRef]
- Tomchenko, A.; Harmer, G.P.; Marquis, B.T.; Allen, J.W. Semiconducting metal oxide sensor array for the selective detection of combustion gases. Sens. Actuator B Chem. 2003, 93, 126–134. [Google Scholar] [CrossRef]
- Kung, M.C.; Kung, H.H. IR studies of NH3, Pyridine, CO, and NO adsorbed on transition metal oxides. Catal. Rev.-Sci. Eng. 1985, 27, 425–460. [Google Scholar] [CrossRef]
- Kamegawa, T.; Takeuchi, R.; Matsuoka, M.; Anpo, M. Photocatalytic oxidation of CO with various oxidants by Mo oxide species highly dispersed on SiO2 at 293 K. Catal. Today 2006, 111, 248–253. [Google Scholar] [CrossRef]
- Elmi, I.; Zampolli, S.; Cozzani, E.; Mancarella, F.; Cardinali, G.C. Development of ultra-low-power consumption MOX sensors with ppb-level VOC detection capabilities for emerging applications. Sens. Actuat. B-Chem. 2008, 135, 342–351. [Google Scholar] [CrossRef]
- Williams, D.E. Semiconducting oxides as gas-sensitive resistors. Sens. Actuator B-Chem. 1999, 57, 1–16. [Google Scholar] [CrossRef]
- Cordos, E.; Ferenczi, L.; Cadar, S.; Costiug, S.; Pitl, G.; Aciu, A.; Ghita, A.; Chintoanu, M. Methane and carbon monoxide gas detection system based on semiconductor sensor. In Proceedings of the 2006 IEEE International Conference on Automation, Quality and Testing, Robotics, Bucharest, Romania, 25–28 May 2006; pp. 208–211. [Google Scholar] [CrossRef]
- Bakrania, S.D.; Wooldridge, M.S. The effects of the location of Au additives on combustion-generated SnO2 nanopowders for co gas sensing. Sensors 2010, 10, 7002–7017. [Google Scholar] [CrossRef] [PubMed]
- Sofian, M.K.; Oussama, M.E.; Imad, A.A.; Marsha, C.K. Semiconducting metal oxide based sensors for selective gas pollutant detection. Sensors 2009, 9, 8158–8196. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wan, Q. Gas sensors based on semiconducting metal oxide one-dimensional nanostructures. Sensors 2009, 9, 9903–9924. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Jang, H.W. One-dimensional oxide nanostructures as gas-sensing materials: Review and issues. Sensors 2010, 10, 4083–4099. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.; Moussa, W. Studying the effect of deposition conditions on the performance and reliability of MEMS gas sensors. Sensors 2007, 7, 319–340. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Varon, J.; Marik, P.E.; Fromm, R.E., Jr.; Gueler, A. Carbon monoxide poisoning: A review for clinicians. J. Emerg. Med. 1999, 17, 87–93. [Google Scholar] [CrossRef]
- Raub, J.A.; Mathieu-Nolf, M.; Hampson, N.B.; Thom, S.R. Carbon monoxide poisoning—A public health perspective. Toxicology 2000, 145, 1–14. [Google Scholar] [CrossRef]
- Respiratory Health–Air Pollution, Boston University School of Public Health. Available online: http://sphweb.bumc.bu.edu/otlt/MPH-Modules/PH/RespiratoryHealth/RespiratoryHealth7.html (accessed on 28 September 2018).
- Harper, A.; Croft-Baker, J. Carbon monoxide poisoning: Undetected by both patients and their doctors. Age Ageing 2004, 33, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M. Carbon monoxide poisoning. J. Emerg. Nurs. 2008, 34, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Sanislav, T.; Miclea, L. Cyber-physical systems-concept, challenges and research areas. J. Control Eng. Appl. Inf. 2012, 14, 28–33. [Google Scholar]
- Tan, Y.; Goddard, S.; Perez, L.C. A prototype architecture for cyber-physical systems. ACM Sigbed Rev. 2008, 5, 26. [Google Scholar] [CrossRef]
- Cardenas, A.A.; Amin, S.; Sastry, S. Secure control: Towards survivable cyber-physical systems. In Proceedings of the 28th IEEE International Conference on Distributed Computing Systems Workshops, Beijing, China, 17–20 June 2008; pp. 495–500. [Google Scholar] [CrossRef]
- Lee, E.A. Cyber physical systems: Design challenges. In Proceedings of the 11th IEEE International Symposium on Object Oriented Real-Time Distributed Computing, Orlando, FL, USA, 5–7 May 2008; pp. 363–369. [Google Scholar] [CrossRef]
- Rajkumar, R.R.; Lee, I.; Sha, L.; Stankovic, J. Cyber-physical systems: The next computing revolution. In Proceedings of the ACM 47th Design Automation Conference, Anaheim, CA, USA, 13–18 June 2010; pp. 731–736. [Google Scholar] [CrossRef]
- Wan, K.; Man, K.L.; Hughes, D. Specification, Analyzing Challenges and Approaches for Cyber-Physical Systems (CPS). Eng. Lett. 2010, 18, 3. [Google Scholar]
- Wan, J.; Suo, H.; Yan, H.; Liu, J. A general test platform for cyber-physical systems: Unmanned vehicle with wireless sensor network navigation. Proced. Eng. 2011, 24, 123–127. [Google Scholar] [CrossRef]
- Shi, J.; Wan, J.; Yan, H.; Suo, H. A survey of cyber-physical systems. In Proceedings of the IEEE International Conference on Wireless Communications and Signal Processing (WCSP), Nanjing, China, 9–11 November 2011; pp. 1–6. [Google Scholar]
- Conti, M.; Das, S.K.; Bisdikian, C.; Kumar, M.; Ni, L.M.; Passarella, A.; Roussos, G.; Tröster, G.; Tsudik, G.; Zambonelli, F. Looking ahead in pervasive computing: Challenges and opportunities in the era of cyber–physical convergence. Pervasive Mob. Comput. 2012, 8, 2–21. [Google Scholar] [CrossRef]
- Lin, C.Y.; Zeadally, S.; Chen, T.S.; Chang, C.Y. Enabling cyber physical systems with wireless sensor networking technologies. Int. J. Distrib. Sens. Netw. 2012, 8, 489794. [Google Scholar] [CrossRef]
- Schirner, G.; Erdogmus, D.; Chowdhury, K.; Padir, T. The future of human-in-the-loop cyber-physical systems. Computer 2013, 46, 36–45. [Google Scholar] [CrossRef]
- Hu, X.; Chu, T.H.; Chan, H.C.; Leung, V.C. Vita: A crowdsensing-oriented mobile cyber-physical system. IEEE Trans. Emerg. Top. Comput. 2013, 1, 148–165. [Google Scholar] [CrossRef]
- Hackmann, G.; Guo, W.; Yan, G.; Sun, Z.; Lu, C.; Dyke, S. Cyber-physical codesign of distributed structural health monitoring with wireless sensor networks. IEEE Trans. Parallel Distrib. Syst. 2014, 25, 63–72. [Google Scholar] [CrossRef]
- Khaitan, S.K.; McCalley, J.D. Design techniques and applications of cyber physical systems: A survey. IEEE Syst. J. 2015, 9, 350–365. [Google Scholar] [CrossRef]
- Ko, J.; Lu, C.; Srivastava, M.B.; Stankovic, J.A.; Terzis, A.; Welsh, M. Wireless sensor networks for healthcare. Proc. IEEE 2010, 98, 1947–1960. [Google Scholar] [CrossRef]
- Xia, F. QoS challenges and opportunities in wireless sensor/actuator networks. Sensors 2008, 8, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: State of the art and approaches. Sens. Actuator B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuator B Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Labidi, A.; Gillet, E.; Delamare, R.; Maaref, M.; Aguir, K. Ethanol and ozone sensing characteristics of WO3 based sensors activated by Au and Pd. Sens. Actuators B Chem. 2006, 120, 338–345. [Google Scholar] [CrossRef]
- Khadayate, R.S.; Chaudhari, M.T.; Disawal, S.K.; Patil, P.P. Sensing behavior printed WO3 thick films for four organic vapors. Invertis J. Sci. Technol. 2009, 2, 185–189. [Google Scholar]
- Sahay, P.P.; Tewari, S.; Jha, S.; Shamsuddin, M. Sprayed ZnO thin films for ethanol sensors. J. Mater. Sci. 2005, 40, 4791–4793. [Google Scholar] [CrossRef]
- Mädler, L.; Sahm, T.; Gurlo, A.; Grunwaldt, J.D.; Barsan, N.; Weimar, U.; Pratsinis, S.E. Sensing low concentrations of CO using flame-spray-made Pt/SnO2 nanoparticles. J. Nanopart. Res. 2006, 8, 783–796. [Google Scholar] [CrossRef]
- Firooz, A.A.; Mahjoub, A.R.; Khodadadi, A.A. Highly sensitive CO and ethanol nanoflower-like SnO2 sensor among various morphologies obtained by using single and mixed ionic surfactant templates. Sens. Actuators B Chem. 2009, 141, 89–96. [Google Scholar] [CrossRef]
- Singh, N.; Ponzoni, A.; Gupta, R.K.; Lee, P.S.; Comini, E. Synthesis of In2O3–ZnO core–shell nanowires and their application in gas sensing. Sens. Actuators B Chem. 2011, 160, 1346–1351. [Google Scholar] [CrossRef]
- Seo, M.H.; Yuasa, M.; Kida, T.; Huh, J.S.; Yamazoe, N.; Shimanoe, K. Microstructure control of TiO2 nanotubular films for improved VOC sensing. Sens. Actuators B Chem. 2011, 154, 251–256. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceramics 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Kolmakov, A.; Zhang, Y.; Cheng, G.; Moskovits, M. Detection of CO and O2 using tin oxide nanowire sensors. Adv. Mater. 2003, 15, 997–1000. [Google Scholar] [CrossRef]
- Du, X.; George, S.M. Thickness dependence of sensor response for CO gas sensing by tin oxide films grown using atomic layer deposition. Sens. Actuators B Chem. 2008, 135, 152–160. [Google Scholar] [CrossRef]
- Tischner, A.; Maier, T.; Stepper, C.; Köck, A. Ultrathin SnO2 gas sensors fabricated by spray pyrolysis for the detection of humidity and carbon monoxide. Sens. Actuators B Chem. 2008, 134, 796–802. [Google Scholar] [CrossRef]
- Köck, A.; Tischner, A.; Maier, T.; Kast, M.; Edtmaier, C.; Gspan, C.; Kothleitner, G. Atmospheric pressure fabrication of SnO2-nanowires for highly sensitive CO and CH4 detection. Sens. Actuators B Chem. 2009, 138, 160–167. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, C.C.; Lo, Y.M. Fabrication of a Flexible Micro CO Sensor for Micro Reformer Applications. Sensors 2010, 10, 10701–10713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.K.; Chan, P.C.; Tang, Z.; Yan, G.; Hsing, I.M.; Sin, J.K. Sensitive, selective and stable tin dioxide thin-films for carbon monoxide and hydrogen sensing in integrated gas sensor array applications. Sens. Actuators B Chem. 2001, 72, 160–166. [Google Scholar] [CrossRef]
- Menini, P.; Parret, F.; Guerrero, M.; Soulantica, K.; Erades, L.; Maisonnat, A.; Chaudret, B. CO response of a nanostructured SnO2 gas sensor doped with palladium and platinum. Sens. Actuators B Chem. 2004, 103, 111–114. [Google Scholar] [CrossRef]
- Kim, B.; Lu, Y.; Hannon, A.; Meyyappan, M.; Li, J. Low temperature Pd/SnO2 sensor for carbon monoxide detection. Sens. Actuators B Chem. 2013, 177, 770–775. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, T.; Lian, G.; Yu, Q.; Luan, C.; Wang, Q.; Cui, D. Room temperature CO sensor fabricated from Pt-loaded SnO2 porous nanosolid. Sens. Actuators B Chem. 2013, 184, 33–39. [Google Scholar] [CrossRef]
- Yuasa, M.; Masaki, T.; Kida, T.; Shimanoe, K.; Yamazoe, N. Nano-sized PdO loaded SnO2 nanoparticles by reverse micelle method for highly sensitive CO gas sensor. Sens. Actuators B Chem. 2009, 136, 99–104. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Huang, J.; Wang, Y.; Kong, F.; Wu, S.; Zhang, S.; Huang, W. Preparation and CO gas-sensing behavior of Au-doped SnO2 sensors. Vacuum 2006, 81, 394–397. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K.; Gulina, L.; Tolstoy, V. SnO2 thin films modified by the SnO2–Au nanocomposites: Response to reducing gases. Sens. Actuators B Chem. 2009, 141, 610–616. [Google Scholar] [CrossRef]
- Manjula, P.; Arunkumar, S.; Manorama, S.V. Au/SnO2 an excellent material for room temperature carbon monoxide sensing. Sens. Actuators B Chem. 2011, 152, 168–175. [Google Scholar] [CrossRef]
- Zhao, L.; Choi, M.; Kim, H.S.; Hong, S.H. The effect of multiwalled carbon nanotube doping on the CO gas sensitivity of SnO2-based nanomaterials. Nanotechnology 2007, 18, 445501. [Google Scholar] [CrossRef]
- Leghrib, R.; Pavelko, R.; Felten, A.; Vasiliev, A.; Cané, C.; Gràcia, I.; Pireaux, J.; Llobet, E. Gas sensors based on multiwall carbon nanotubes decorated with tin oxide nanoclusters. Sens. Actuators B Chem. 2010, 145, 411–416. [Google Scholar] [CrossRef]
- Wang, C.T.; Chen, M.T. Vanadium-promoted tin oxide semiconductor carbon monoxide gas sensors. Sens. Actuators B Chem. 2010, 150, 360–366. [Google Scholar] [CrossRef]
- Ghosh, S.; Narjinary, M.; Sen, A.; Bandyopadhyay, R.; Roy, S. Fast detection of low concentration carbon monoxide using calcium-loaded tin oxide sensors. Sens. Actuators B Chem. 2014, 203, 490–496. [Google Scholar] [CrossRef]
- Nikan, E.; Khodadadi, A.A.; Mortazavi, Y. Highly enhanced response and selectivity of electrospun ZnO-doped SnO2 sensors to ethanol and CO in presence of CH4. Sens. Actuators B Chem. 2013, 184, 196–204. [Google Scholar] [CrossRef]
- Jeun, J.H.; Kim, D.H.; Hong, S.H. SnO2/CuO nano-hybrid foams synthesized by electrochemical deposition and their gas sensing properties. Mater. Lett. 2013, 105, 58–61. [Google Scholar] [CrossRef]
- Bai, S.; Guo, W.; Sun, J.; Li, J.; Tian, Y.; Chen, A.; Luo, R.; Li, D. Synthesis of SnO2–CuO heterojunction using electrospinning and application in detecting of CO. Sens. Actuators B Chem. 2016, 226, 96–103. [Google Scholar] [CrossRef]
- Kim, H.; Park, C.S.; Kang, K.M.; Hong, M.H.; Choi, Y.J.; Park, H.H. The CO gas sensing properties of direct-patternable SnO2 films containing graphene or Ag nanoparticles. New J. Chem. 2015, 39, 2256–2260. [Google Scholar] [CrossRef]
- Park, J.A.; Moon, J.; Lee, S.J.; Kim, S.H.; Zyung, T.; Chu, H.Y. Structure and CO gas sensing properties of electrospun TiO2 nanofibers. Mater. Lett. 2010, 64, 255–257. [Google Scholar] [CrossRef]
- Moon, H.G.; Shim, Y.S.; Jang, H.W.; Kim, J.S.; Choi, K.J.; Kang, C.Y.; Choi, J.W.; Park, H.H.; Yoon, S.J. Highly sensitive CO sensors based on cross-linked TiO2 hollow hemispheres. Sens. Actuators B Chem. 2010, 149, 116–121. [Google Scholar] [CrossRef]
- Guidi, V.; Carotta, M.C.; Ferroni, M.; Martinelli, G.; Paglialonga, L.; Comini, E.; Sberveglieri, G. Preparation of nanosized titania thick and thin films as gas-sensors. Sens. Actuators B Chem. 1999, 57, 197–200. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Fray, D.J.; Ghorbani, M. Comparison of single and binary oxide sol–gel gas sensors based on titania. Solid State Sci. 2008, 10, 884–893. [Google Scholar] [CrossRef]
- Seo, M.H.; Yuasa, M.; Kida, T.; Huh, J.S.; Shimanoe, K.; Yamazoe, N. Gas sensing characteristics and porosity control of nanostructured films composed of TiO2 nanotubes. Sens. Actuators B Chem. 2009, 137, 513–520. [Google Scholar] [CrossRef]
- Landau, O.; Rothschild, A.; Zussman, E. Processing-microstructure-properties correlation of ultrasensitive gas sensors produced by electrospinning. Chem. Mater. 2008, 21, 9–11. [Google Scholar] [CrossRef]
- Lee, J.S.; Ha, T.J.; Hong, M.H.; Park, H.H. The effect of porosity on the CO sensing properties of TiO2 xerogel thin films. Thin Solid Films 2013, 529, 98–102. [Google Scholar] [CrossRef]
- Rao, L.L.R.; Singha, M.K.; Subramaniam, K.M.; Jampana, N.; Asokan, S. Molybdenum microheaters for MEMS-based gas sensor applications: Fabrication, electro-thermo-mechanical and response characterization. IEEE Sensors J. 2017, 17, 22–29. [Google Scholar] [CrossRef]
- Lee, J.S.; Ha, T.J.; Hong, M.H.; Park, C.S.; Park, H.H. The effect of multiwalled carbon nanotube doping on the CO gas sensitivity of TiO2 xerogel composite film. Appl. Surf. Sci. 2013, 269, 125–128. [Google Scholar] [CrossRef]
- Kim, H.; Hong, M.H.; Jang, H.W.; Yoon, S.J.; Park, H.H. CO gas sensing properties of direct-patternable TiO2 thin films containing multi-wall carbon nanotubes. Thin Solid Films 2013, 529, 89–93. [Google Scholar] [CrossRef]
- Chang, J.F.; Kuo, H.H.; Leu, I.C.; Hon, M.H. The effects of thickness and operation temperature on ZnO: Al thin film CO gas sensor. Sens. Actuators B Chem. 2002, 84, 258–264. [Google Scholar] [CrossRef]
- Hjiri, M.; El Mir, L.; Leonardi, S.G.; Pistone, A.; Mavilia, L.; Neri, G. Al-doped ZnO for highly sensitive CO gas sensors. Sens. Actuators B Chem. 2014, 196, 413–420. [Google Scholar] [CrossRef]
- Gong, H.; Hu, J.Q.; Wang, J.H.; Ong, C.H.; Zhu, F.R. Nano-crystalline Cu-doped ZnO thin film gas sensor for CO. Sens. Actuators B Chem. 2006, 115, 247–251. [Google Scholar] [CrossRef]
- Wei, S.; Yu, Y.; Zhou, M. CO gas sensing of Pd-doped ZnO nanofibers synthesized by electrospinning method. Mater. Lett. 2010, 64, 2284–2286. [Google Scholar] [CrossRef]
- Dhahri, R.; Hjiri, M.; El Mir, L.; Alamri, H.; Bonavita, A.; Iannazzo, D.; Leonardi, S.G.; Neri, G. CO sensing characteristics of In-doped ZnO semiconductor nanoparticles. J. Sci. Adv. Mater. Devices 2017, 2, 34–40. [Google Scholar] [CrossRef]
- Kim, K.; Song, Y.W.; Chang, S.; Kim, I.H.; Kim, S.; Lee, S.Y. Fabrication and characterization of Ga-doped ZnO nanowire gas sensor for the detection of CO. Thin Solid Films 2009, 518, 1190–1193. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.F.; Durrani, S.M.A.; Bakhtiari, I.A. Carbon monoxide gas-sensing properties of CeO2–ZnO thin films. Appl. Surf. Sci. 2008, 255, 3033–3039. [Google Scholar] [CrossRef]
- Choi, K.I.; Kim, H.R.; Lee, J.H. Enhanced CO sensing characteristics of hierarchical and hollow In2O3 microspheres. Sens. Actuators B Chem. 2009, 138, 497–503. [Google Scholar] [CrossRef]
- Lim, S.K.; Hwang, S.H.; Chang, D.; Kim, S. Preparation of mesoporous In2O3 nanofibers by electrospinning and their application as a CO gas sensor. Sens. Actuators B Chem. 2010, 149, 28–33. [Google Scholar] [CrossRef]
- Fu, H.; Hou, C.; Gu, F.; Han, D.; Wang, Z. Facile preparation of rod-like Au/In2O3 nanocomposites exhibiting high response to CO at room temperature. Sens. Actuators B Chem. 2017, 243, 516–524. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Haensch, A.; Barsan, N.; Weimar, U. CO sensing mechanism with WO3 based gas sensors. Sens. Actuators B Chem. 2010, 151, 103–106. [Google Scholar] [CrossRef]
- Jimenez, I.; Vilà, A.M.; Calveras, A.C.; Morante, J.R. Gas-sensing properties of catalytically modified WO/sub 3/with copper and vanadium for NH/sub 3/detection. IEEE Sens. J. 2005, 5, 385–391. [Google Scholar] [CrossRef]
- Patil, D.; Patil, P.; Subramanian, V.; Joy, P.A.; Potdar, H.S. Highly sensitive and fast responding CO sensor based on Co3O4 nanorods. Talanta 2010, 81, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhu, L.; Li, Y.; Zhang, Z.; Ye, Z. Mesoporous Co3O4 nanoneedle arrays for high-performance gas sensor. Sens. Actuators B Chem. 2014, 203, 873–879. [Google Scholar] [CrossRef]
- Tan, W.; Tan, J.; Li, L.; Dun, M.; Huang, X. Nanosheets-assembled hollowed-out hierarchical Co3O4 microrods for fast response/recovery gas sensor. Sens. Actuators B Chem. 2017, 249, 66–75. [Google Scholar] [CrossRef]
- Vetter, S.; Haffer, S.; Wagner, T.; Tiemann, M. Nanostructured Co3O4 as a CO gas sensor: Temperature-dependent behavior. Sens. Actuators B Chem. 2015, 206, 133–138. [Google Scholar] [CrossRef]
- Nagai, D.; Nakashima, T.; Nishibori, M.; Itoh, T.; Izu, N.; Shin, W. Thermoelectric gas sensor with CO combustion catalyst for ppm level carbon monoxide detection. Sens. Actuators B Chem. 2013, 182, 789–794. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Optimization and gas sensing mechanism of n-SnO2-p-Co3O4 composite nanofibers. Sens. Actuators B Chem. 2017, 248, 500–511. [Google Scholar] [CrossRef]
- Khaleed, A.A.; Bello, A.; Dangbegnon, J.K.; Momodu, D.Y.; Madito, M.J.; Ugbo, F.U.; Akande, A.A.; Dhonge, B.P.; Barzegar, F.; Olaniyan, O.; et al. Effect of activated carbon on the enhancement of CO sensing performance of NiO. J. Alloys Compd. 2017, 694, 155–162. [Google Scholar] [CrossRef]
- Steinhauer, S.; Brunet, E.; Maier, T.; Mutinati, G.C.; Köck, A. CuO nanowire gas sensors for CO detection in humid atmosphere. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems, Barcelona, Spain, 16–20 June 2013; pp. 1095–1098. [Google Scholar] [CrossRef]
- Tanvir, N.B.; Laubender, E.; Yurchenko, O.; Urban, G. Room temperature CO sensing with metal oxide nanoparticles using work function readout. Proced. Eng. 2016, 168, 284–288. [Google Scholar] [CrossRef]
- Elia, A.; Di Franco, C.; Lugarà, P.M.; Scamarcio, G. Photoacoustic spectroscopy with quantum cascade lasers for trace gas detection. Sensors 2006, 6, 1411–1419. [Google Scholar] [CrossRef]
- Kosterev, A.A.; Bakhirkin, Y.A.; Tittel, F.K. Ultrasensitive gas detection by quartz-enhanced photoacoustic spectroscopy in the fundamental molecular absorption bands region. Appl. Phys. B 2005, 80, 133–138. [Google Scholar] [CrossRef]
- Li, L.; Cao, F.; Wang, Y.; Cong, M.; Li, L.; An, Y.; Song, Z.; Guo, S.; Liu, S.; Wang, L. Design and characteristics of quantum cascade laser-based CO detection system. Sens. Actuators B Chem. 2009, 142, 33–38. [Google Scholar] [CrossRef]
- Li, J.; Deng, H.; Sun, J.; Yu, B.; Fischer, H. Simultaneous atmospheric CO, N2O and H2O detection using a single quantum cascade laser sensor based on dual-spectroscopy techniques. Sens. Actuators B Chem. 2016, 231, 723–732. [Google Scholar] [CrossRef]
- Tan, Q.; Tang, L.; Yang, M.; Xue, C.; Zhang, W.; Liu, J.; Xiong, J. Three-gas detection system with IR optical sensor based on NDIR technology. Opt. Lasers Eng. 2015, 74, 103–108. [Google Scholar] [CrossRef]







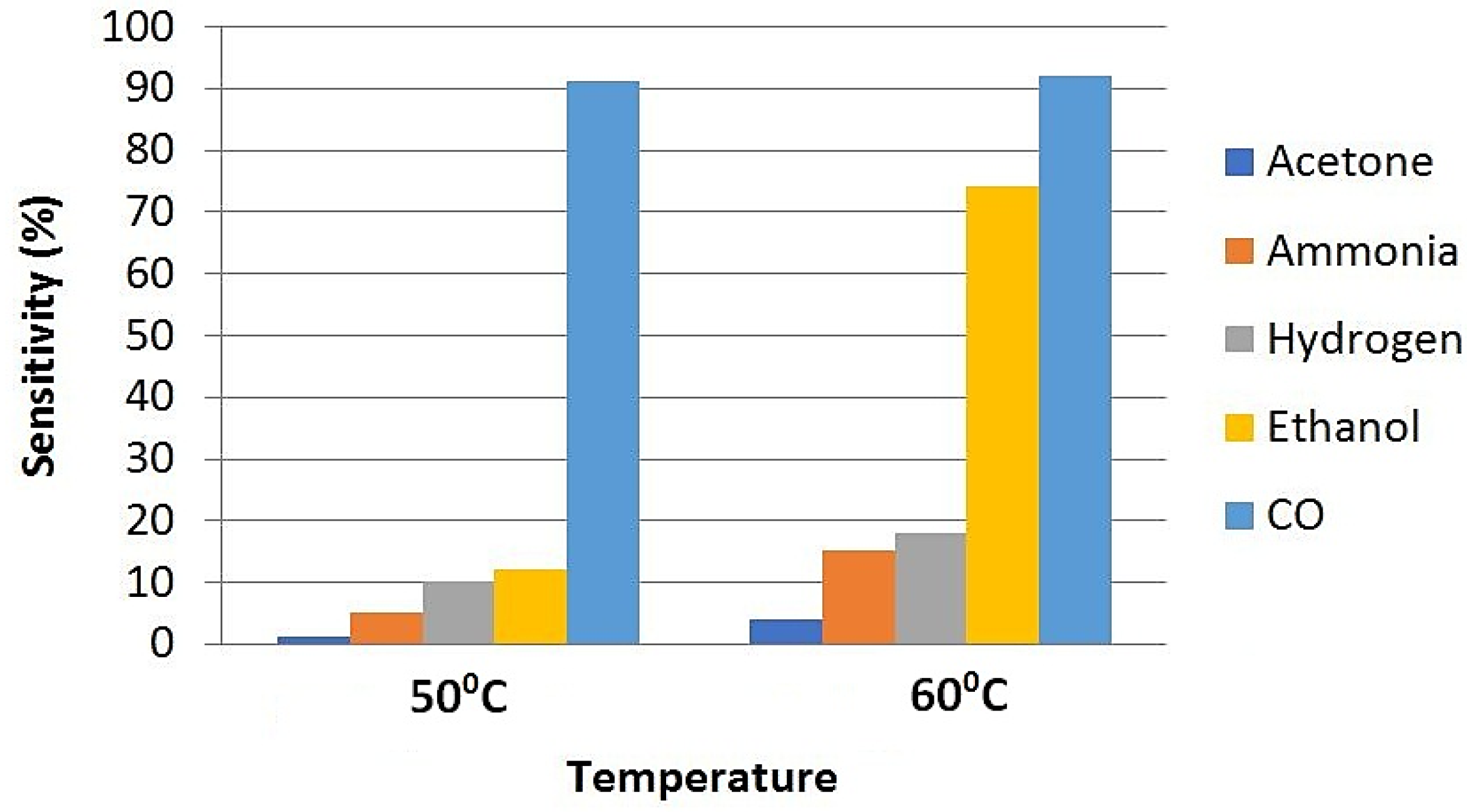
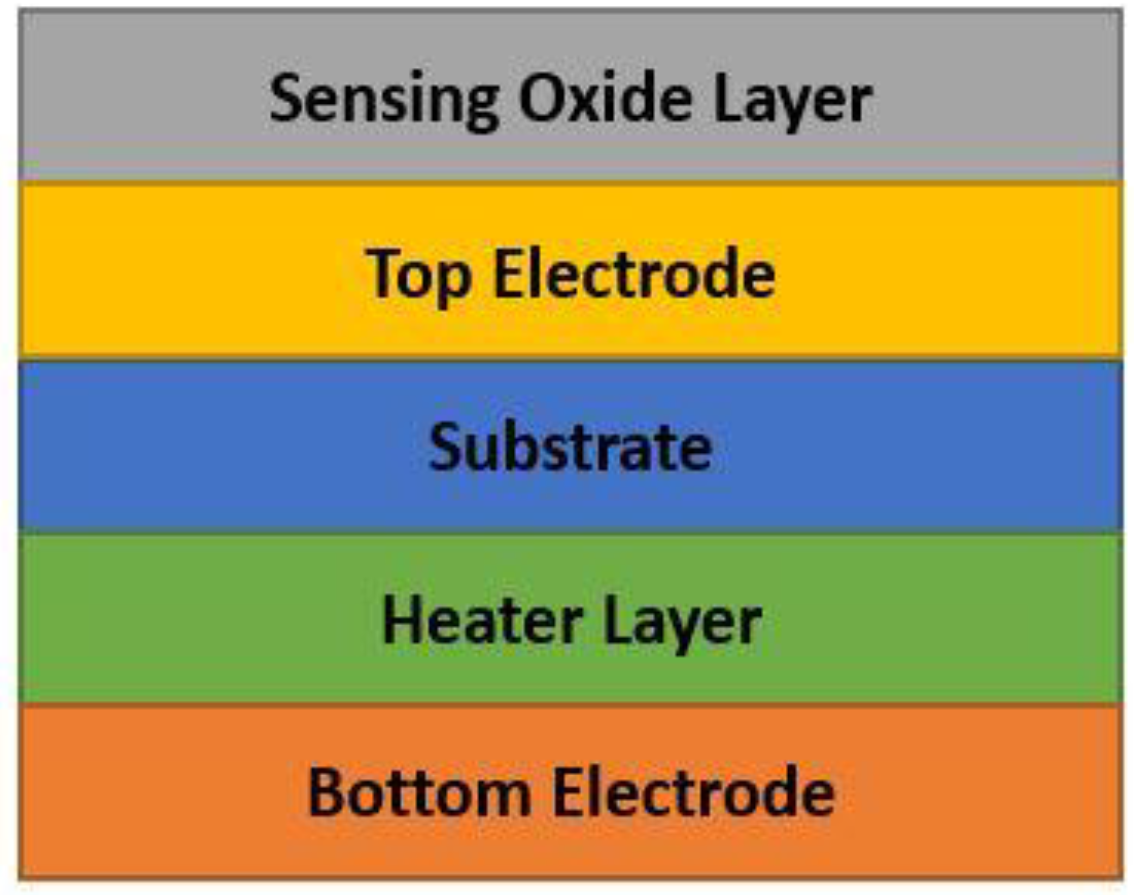
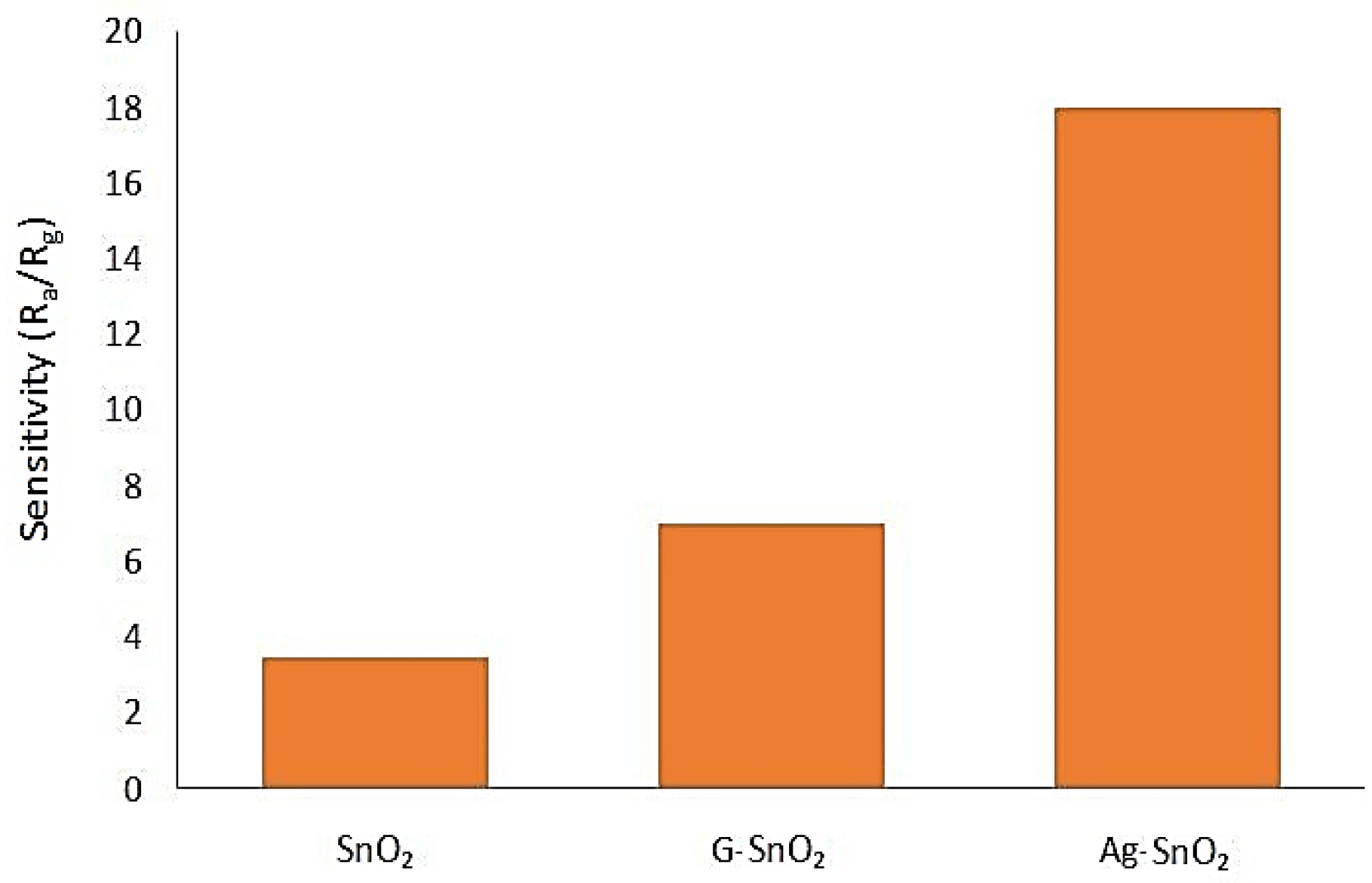
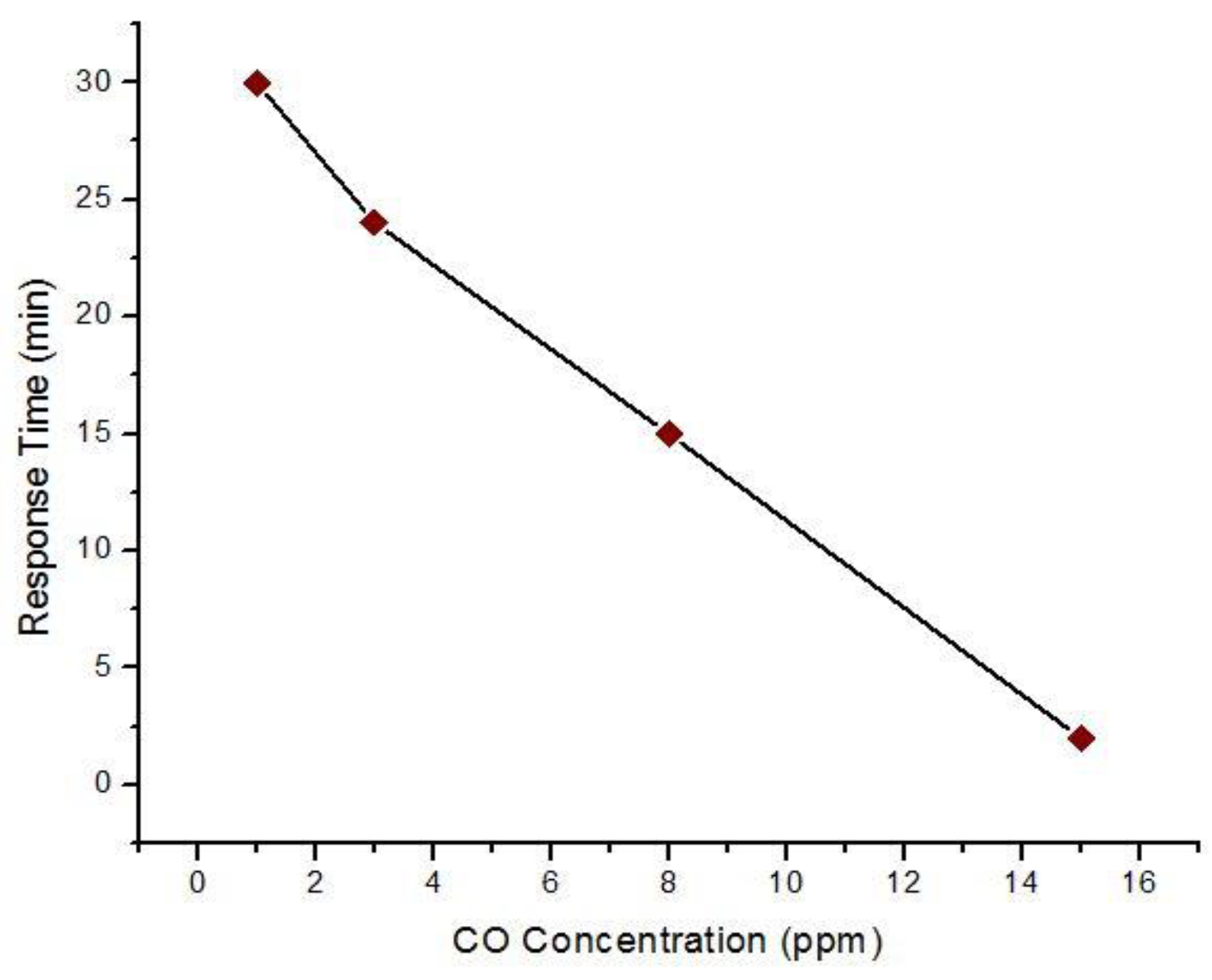





| Concentration of CO (Exposure Time) | Created Health Problems |
|---|---|
| 35 ppm (6–8 h), 100–200 ppm (2–3 h), 400 ppm (1–2 h), 800 ppm (45 min), 1600 ppm (20 min), 3200 ppm (5–10 min), 6400 ppm (1–2 min) | Headache, dizziness, nausea, loss of judgment and convulsions |
| 1600 ppm (2 h), 3200 ppm (30 min), 6400 ppm (<20 min), 12,800 ppm (<3 min) | Respiratory arrest, severe conditions (coma) and death |
| Study | Material | Performance | Optimum Temperature | Advantages | Limitations |
|---|---|---|---|---|---|
| Kock et al. [55] | SnO2 nano wire | Response time 25 s | 250 °C | Low concentration detection | Resistance suddenly increased |
| Lee et al. [56] | SnO2 thin film | Sensitivity 59% | 270 °C | MEMS based structure and good stability | High CO concentration |
| Sharma et al. [57] | Cu-SnO2 thin film | Response time 5–10 s | 320 °C | Fast response | High CO concentration and temperature |
| Menini et al. [58] | Pd-SnO2 thin film | Sensitivity ~80%, Response time ~50 min | 450 °C | Very high sensitivity | Very slow detection and high operating temperature |
| Wang et al. [60] | Pt-SnO2 porous nano solid | Sensitivity (Ra/Rg) 64.5 | Room temperature | Room temperature measurement and good selectivity | Humidity dependency |
| Manjula et al. [64] | Au-SnO2 thin film | Sensitivity ~90% | Room temperature | Room temperature measurement, good selectivity and no humidity effect | More doping caused decrease in response |
| Zhao et al. [65] | MWCNT-SnO2 nano particle | Sensitivity ~46% | 300 °C | Fast detection and good stability | High operating temperature |
| Ghosh et al. [68] | Ca-SnO2 thin film | Difference of Response and Recovery ~25 s | 350 °C | low CO concentration measurement, good selectivity and stability | High operating temperature |
| Nikan et al. [69] | ZnO-SnO2 thin film | Difference of response and recovery time ~11 min | 300 °C | Very small doping concentration | Slow recovery and high operating temperature |
| Bai et al. [71] | CuO-SnO2 nano composite | Sensitivity (Ra/Rg) ~95 | 235 °C | Low concentration detection and good selective | High annealing temperature |
| Study | Material | Performance | Optimum Temperature | Advantages | Limitations |
|---|---|---|---|---|---|
| Rao et al. [80] | TiO2 thin film | Sensitivity 84% | 500 °C | MEMS based structure, low concentration | Very high operating temperature |
| Lee et al. [81] | MWCNT-TiO2 thin film | Response time 4 s | 300 °C | Fast and low concentration detection | High operating temperature |
| Hijri et al. [84] | Al-ZnO nano particle | Sensitivity (Ra/Rg) ~6 | 300 °C | Fast and low concentration detection | Optimum doping concentration varied |
| Gong et al. [85] | Cu-ZnO thin film | Difference of response and recovery time ~100 s | 350 °C | Low concentration detection | High operating temperature |
| Wei et al. [86] | Pd-ZnO nano fiber | Response time 25–29 s, Recovery time 12–17 s | 220 °C | Low concentration detection and good selectivity | Moderate sensitivity |
| Kuhaili et al. [89] | CeO2-ZnO thin film | Response time 44 s, Recovery time 40 s | 380 °C | High resistance change | High CO concentration and high operating temperature |
| Choi et al. [90] | In3O4 micro spheres | Response time 4–8 s, Recovery time 5–10 s | 400 °C | Fast and low concentration detection | High operating temperature |
| Fu et al. [92] | Au-In2O3 nano materials | Response ~9, response and recovery time ~30/30 s | 25 °C | Room temperature measurement and very good selectivity | Influence of humidity and detection ability up to 100 ppm |
| Patil et al. [95] | Co3O4 nano rod | Response and Recovery time 3–4 and 5–6 s | 250 °C | Fast, low ppm detection and good selective | Low resistance change |
| Steinhauer et al. [102] | CuO nano wires | Sensor Response (RCO/Rair) ~1 | 325 °C | No dependency on humidity variation | High operating temperature |
| Tanvir et al. [103] | CeO2-CuO thin film | Sensor Response ~80 mV | 24 °C | Room temperature measurement | It needs more investigations as a new material |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandy, T.; Coutu, R.A., Jr.; Ababei, C. Carbon Monoxide Sensing Technologies for Next-Generation Cyber-Physical Systems. Sensors 2018, 18, 3443. https://doi.org/10.3390/s18103443
Nandy T, Coutu RA Jr., Ababei C. Carbon Monoxide Sensing Technologies for Next-Generation Cyber-Physical Systems. Sensors. 2018; 18(10):3443. https://doi.org/10.3390/s18103443
Chicago/Turabian StyleNandy, Turja, Ronald A. Coutu, Jr., and Cristinel Ababei. 2018. "Carbon Monoxide Sensing Technologies for Next-Generation Cyber-Physical Systems" Sensors 18, no. 10: 3443. https://doi.org/10.3390/s18103443





