Temperature-Corrected Fluidic Glucose Sensor Based on Microwave Resonator
Abstract
:1. Introduction
2. Materials and Methods
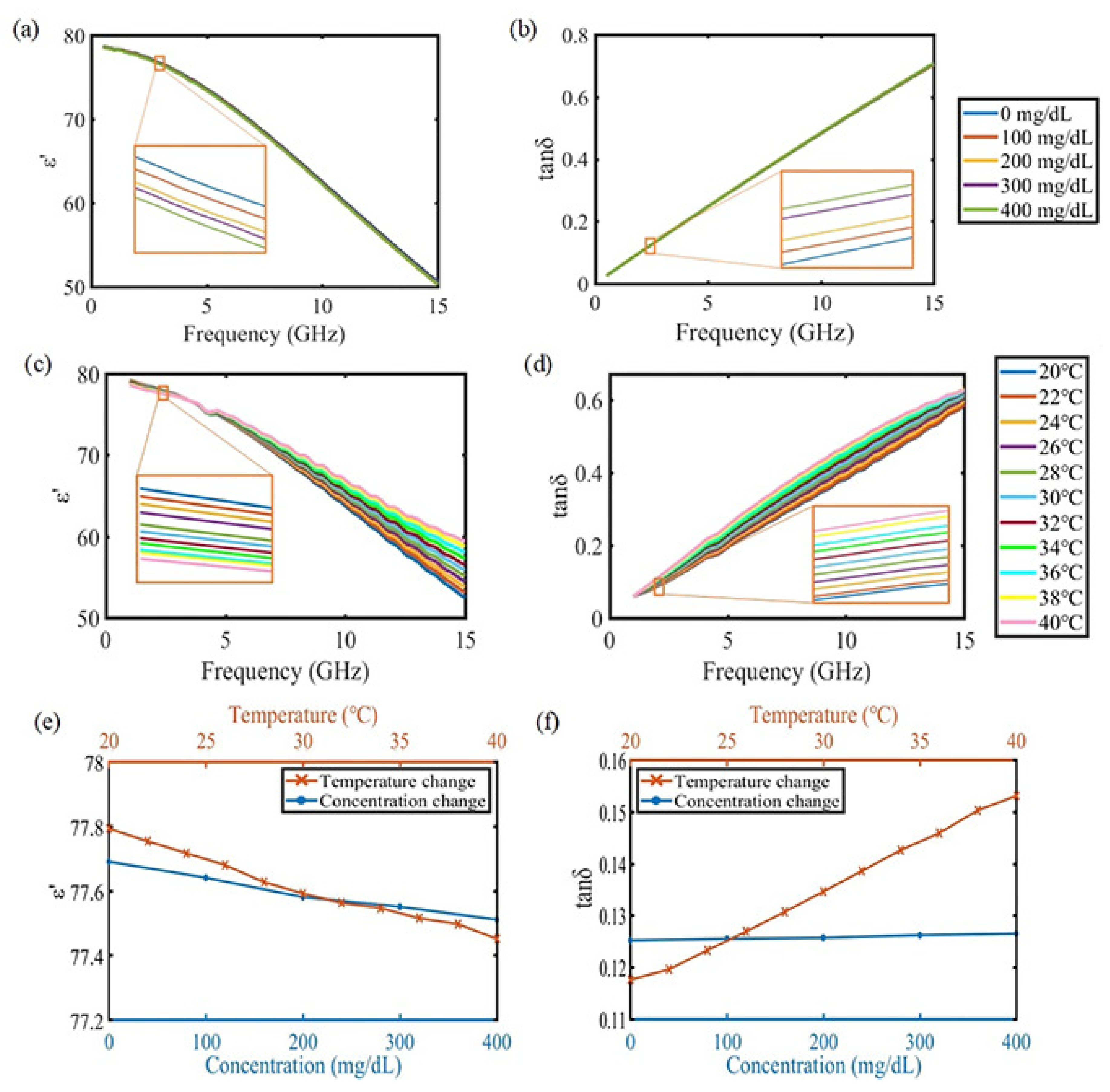
2.1. Material Property Specification
2.2. Description of the Proposed Sensor
2.3. Sensing Mechanism
3. Results and Discussion
3.1. Experimental Result under Stable Temperature Condition
3.2. Experimental Result under Varying Temperature Conditions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaw, J.; Sicree, R.; Zimmet, P. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.; Guariguata, L.; Weil, C.; Shaw, J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.H.; Litwin, S.E. Hyperglycemia and Adverse Outcomes in Acute Coronary Syndromes: Is Serum Glucose the Provocateur or Innocent Bystander? Diabetes 2014, 63, 2209–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayan, K.V.; Gujral, U.P. Evidence Tips the Scale Toward Screening for Hyperglycemia. Diabetes Care 2015, 38, 1399–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umpierrez, G.E.; Pasquel, F.J. Management of Inpatient Hyperglycemia and Diabetes in Older Adults. Diabetes Care 2017, 40, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, S.W.; Park, S.; Lee, Y.h.; Balkau, B.; Yi, J.J. Fasting Glucose and All-Cause Mortality by Age in Diabetes: A Prospective Cohort Study. Diabetes Care 2018, 41, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Long, N.; Moussy, Y.; Moussy, F. A long-term flexible minimally-invasive implantable glucose biosensor based on an epoxy-enhanced polyurethane membrane. Biosens. Bioelectron. 2006, 21, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Koinkar, P.; Fuchiwaki, Y.; Yasuzawa, M. A fine pointed glucose oxidase immobilized electrode for low-invasive amperometric glucose monitoring. Biosens. Bioelectron. 2016, 86, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Kuroki, Y.; Nitta, H.; Chouhan, P.; Toma, K.; Ichi Sawada, S.; Takeuchi, S.; Sekita, T.; Akiyoshi, K.; Minakuchi, S.; et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens. Bioelectron. 2016, 84, 106–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leboulanger, B.; Guy, R.H.; Delgado-Charro, M.B. Reverse iontophoresis for non-invasive transdermal monitoring. Physiol. Meas. 2004, 25, R35. [Google Scholar] [CrossRef] [PubMed]
- Ermolina, I.; Polevaya, Y.; Feldman, Y. Analysis of dielectric spectra of eukaryotic cells by computer modeling. Eur. Biophys. J. 2000, 29, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, Y.; Yang, Y.; Spencer, S. Current problems and potential techniques in in vivo glucose monitoring. J. Fluoresc. 2004, 14, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.H.; Jha, A.K.; Akhtar, M.J. Design and application of the CSRR-based planar sensor for noninvasive measurement of complex permittivity. IEEE Sens. J. 2015, 15, 7181–7189. [Google Scholar] [CrossRef]
- Bailly, G.; Harrabi, A.; Rossignol, J.; Stuerga, D.; Pribetich, P. Microwave gas sensing with a microstrip interDigital capacitor: Detection of NH3 with TiO2 nanoparticles. Sens. Actuators B Chem. 2016, 236, 554–564. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, G.; Tan, Q.; Wei, T.; Wu, D.; Shen, S.; Dong, H.; Zhang, W. Dielectrically-Loaded Cylindrical Resonator-Based Wireless Passive High-Temperature Sensor. Sensors 2016, 16, 2037. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Ashraf, F.B.; Alam, T.; Misran, N.; Mat, K.B. A Compact Ultrawideband Antenna Based on Hexagonal Split-Ring Resonator for pH Sensor Application. Sensors 2016, 18, 2959. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lee, H.J.; Kim, S.G.; Kim, B.H.; Yun, G.H.; Yook, J.G. A label-free biosensing platform using a PLL circuit and Biotin-Streptavidin Binding system. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.H.; Choi, S.; Jang, I.S.; Choi, J.S.; Jung, H.I. Asymmetric split-ring resonator-based biosensor for detection of label-free stress biomarkers. Appl. Phys. Lett. 2013, 103, 053702. [Google Scholar] [CrossRef]
- Kim, S.G.; Yun, G.H.; Yook, J.G. Compact vital signal sensor using oscillation frequency deviation. IEEE Trans. Microw. Theory Tech. 2012, 60, 393–400. [Google Scholar] [CrossRef]
- Rahman, M.; NaghshvarianJahromi, M.; Mirjavadi, S.S.; Hamouda, A.M. Resonator Based Switching Technique between Ultra Wide Band (UWB) and Single/Dual Continuously Tunable-Notch Behaviors in UWB Radar for Wireless Vital Signs Monitoring. Sensors 2018, 18, 3330. [Google Scholar] [CrossRef] [PubMed]
- An, Y.J.; Yun, G.H.; Kim, S.W.; Yook, J.G. Wrist pulse detection system based on changes in the near-field reflection coefficient of a resonator. IEEE Microw. Wirel. Compon. Lett. 2014, 24, 719–721. [Google Scholar] [CrossRef]
- Aguilar, S.M.; Al-Joumayly, M.A.; Burfeindt, M.J.; Behdad, N.; Hagness, S.C. Multiband miniaturized patch antennas for a compact, shielded microwave breast imaging array. IEEE Trans. Antennas Propag. 2014, 62, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Kang, T.G.; Kim, B.H.; Lee, H.J.; Choi, H.H.; Yook, J.G. Real-time Humidity Sensor Based on Microwave Resonator Coupled with PEDOT: PSS Conducting Polymer Film. Sci. Rep. 2018, 8, 439. [Google Scholar] [CrossRef] [PubMed]
- Qiang, T.; Wang, C.; Kim, N.Y. Quantitative detection of glucose level based on radiofrequency patch biosensor combined with volume-fixed structures. Biosens. Bioelectron. 2017, 98, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.h.; Shin, K.s.; Kang, S.; Lee, S.H.; Kang, J.Y.; Kim, S.; Jun, S.C. Fundamental monomeric biomaterial diagnostics by radio frequency signal analysis. Biosens. Bioelectron. 2016, 82, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Dhakal, R.; Adhikari, K.; Kim, E.; Wang, C. A reusable robust radio frequency biosensor using microwave resonator by integrated passive device technology for quantitative detection of glucose level. Biosens. Bioelectron. 2015, 67, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yoon, H.S.; Patil, U.; Anoop, R.; Lee, J.; Lim, J.; Lee, W.; Jun, S.C. Radio frequency based label-free detection of glucose. Biosens. Bioelectron. 2014, 54, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Liebe, H.J.; Hufford, G.A.; Manabe, T. A model for the complex permittivity of water at frequencies below 1 THz. Int. J. Infrared Millim. Waves 1991, 12, 659–675. [Google Scholar] [CrossRef]
- Haller, M.J.; Shuster, J.J.; Schatz, D.; Melker, R.J. Adverse Impact of Temperature and Humidity on Blood Glucose Monitoring Reliability: A Pilot Study. Diabetes Technol. Ther. 2007, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Kim, K.; Lee, J.H.; Babajanyan, A.; Friedman, B.; Lee, K. In vitro monitoring of goat-blood glycemia with a microwave biosensor. Curr. Appl. Phys. 2014, 14, 563–569. [Google Scholar] [CrossRef]
- Lee, C.; Yang, C. Complementary split-ring resonators for measuring dielectric constants and loss tangents. IEEE Microw. Wirel. Compon. Lett. 2014, 24, 563–565. [Google Scholar] [CrossRef]
- Boybay, M.S.; Ramahi, O.M. Material Characterization Using Complementary Split-Ring Resonators. IEEE Trans. Instrum. Meas. 2012, 61, 3039–3046. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Withayachumnankul, W.; Al-Sarawi, S.F.; Abbott, D. Dual-mode behavior of the complementary electric-LC resonators loaded on transmission line: Analysis and applications. J. Appl. Phys. 2014, 116, 083705. [Google Scholar] [CrossRef]
- Baena, J.D.; Bonache, J.; Martin, F.; Sillero, R.M.; Falcone, F.; Lopetegi, T.; Laso, M.A.; Garcia-Garcia, J.; Gil, I.; Portillo, M.F.; et al. Equivalent-circuit models for split-ring resonators and complementary split-ring resonators coupled to planar transmission lines. IEEE Trans. Microw. Theory Tech. 2005, 53, 1451–1461. [Google Scholar] [CrossRef]

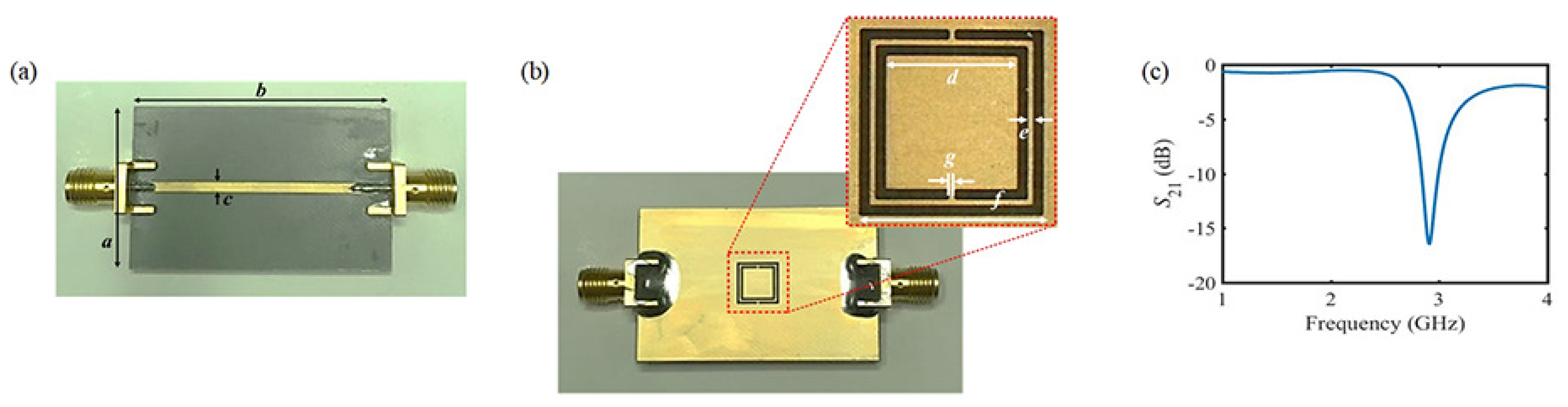





| Parameter | a | b | c | d | e | f | g |
|---|---|---|---|---|---|---|---|
| mm | 26 | 40 | 2.4 | 5.02 | 0.22 | 7 | 0.22 |
| Concentration (mg/dL) | (×10 dB) | ||
|---|---|---|---|
| 25% | Mean | 75% | |
| 0 | 1.2 | 2.3 | 3.3 |
| 100 | 8.0 | 9.1 | 10.3 |
| 200 | 14.8 | 15.7 | 16.7 |
| 300 | 20.0 | 21.2 | 22.3 |
| 400 | 26.4 | 28.2 | 29.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, C.; Park, J.-K.; Lee, H.-J.; Yun, G.-H.; Yook, J.-G. Temperature-Corrected Fluidic Glucose Sensor Based on Microwave Resonator. Sensors 2018, 18, 3850. https://doi.org/10.3390/s18113850
Jang C, Park J-K, Lee H-J, Yun G-H, Yook J-G. Temperature-Corrected Fluidic Glucose Sensor Based on Microwave Resonator. Sensors. 2018; 18(11):3850. https://doi.org/10.3390/s18113850
Chicago/Turabian StyleJang, Chorom, Jin-Kwan Park, Hee-Jo Lee, Gi-Ho Yun, and Jong-Gwan Yook. 2018. "Temperature-Corrected Fluidic Glucose Sensor Based on Microwave Resonator" Sensors 18, no. 11: 3850. https://doi.org/10.3390/s18113850






