A 40-MHz Ultrasound Transducer with an Angled Aperture for Guiding Percutaneous Revascularization of Chronic Total Occlusion: A Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Concept of the Proposed FL–IVUS Transducer
2.2. Design and Fabrication of the FL–IVUS Transducer
3. Results and Discussion
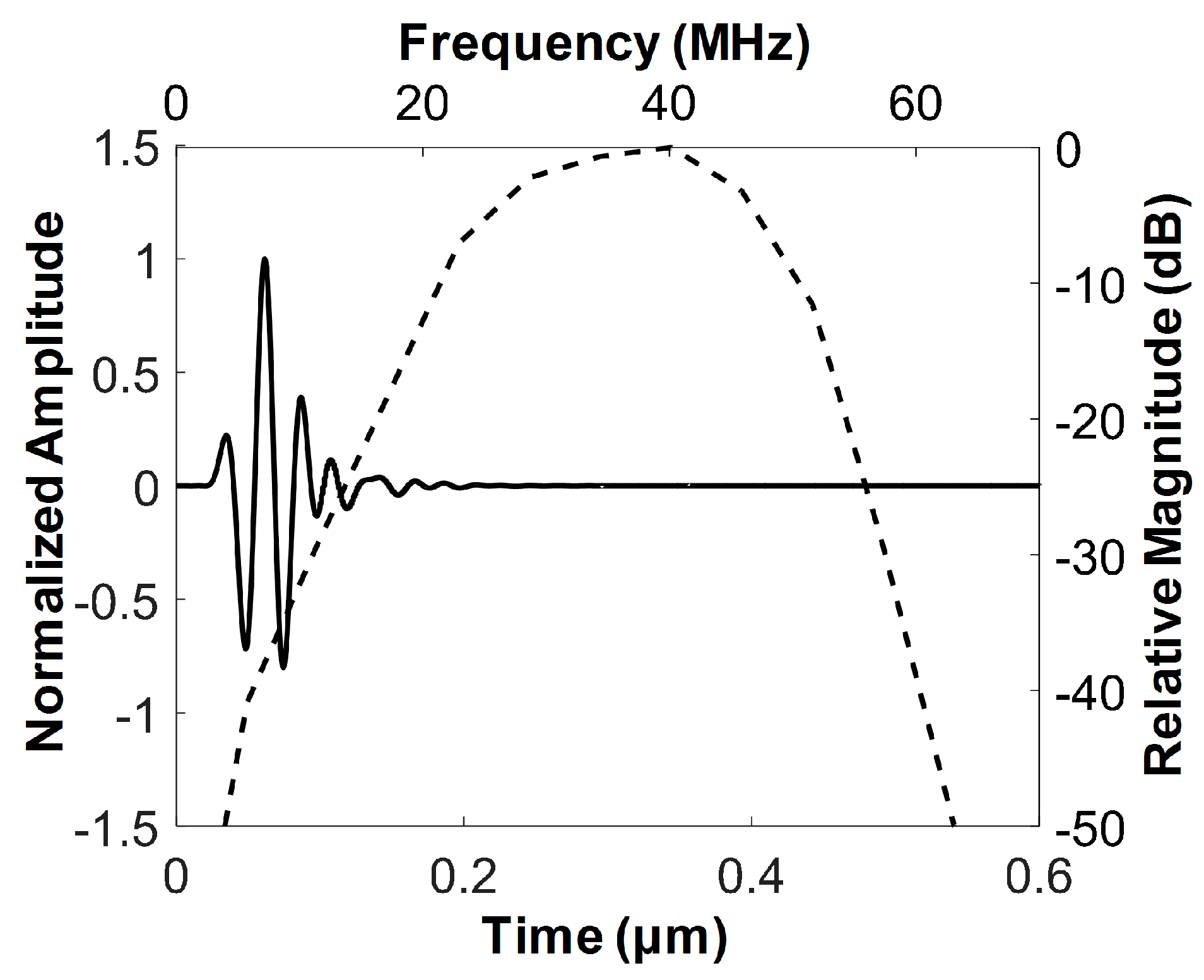
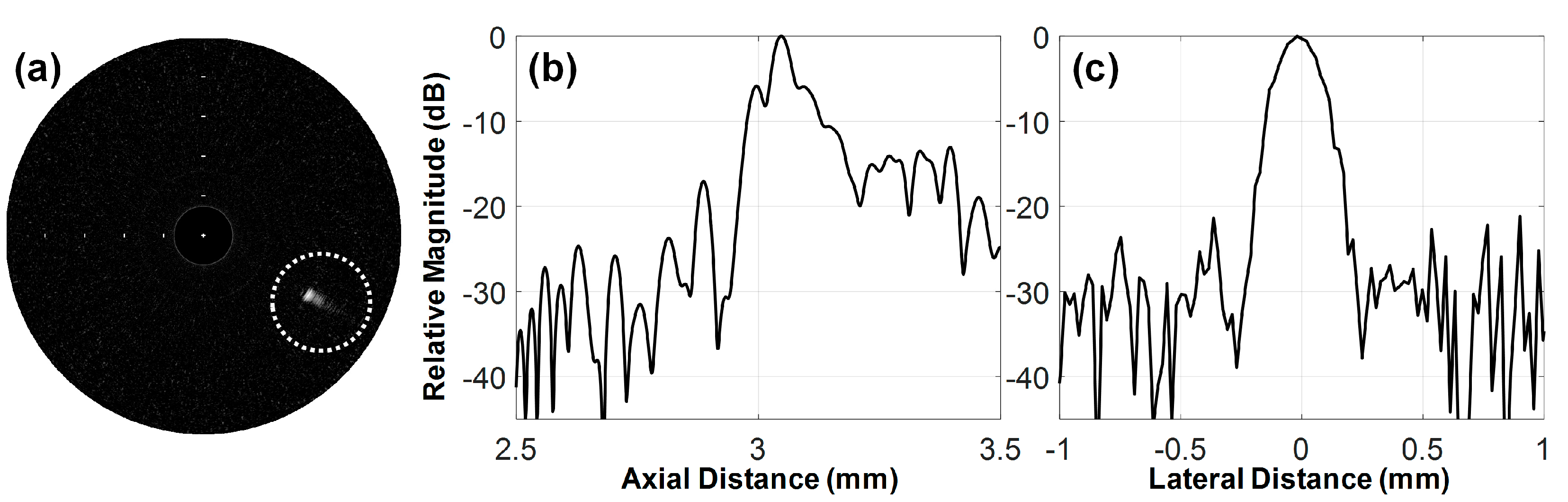
3.1. Characteristics of the Developed FL–IVUS Transducer
3.2. Feasibility Study Using Custom Blood-Vessel-Mimicking Phantoms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Matsuo, H.; Terashima, M.; Kinoshita, Y.; Kimura, M.; Tsuchikane, E.; Nasu, K.; Ehara, M.; Asakura, Y.; Katoh, O.; et al. Procedural and In-Hospital Outcomes After Percutaneous Coronary Intervention for Chronic Total Occlusions of Coronary Arteries 2002 to 2008. JACC Cardiovasc. Interv. 2009, 2, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Yock, P. Intravascular ultrasound: Novel pathophysiological insights and current clinical applications. Circulation 2001, 103, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, J.; Chang, J.H. Oblong-Shaped-Focused Transducers for Intravascular Ultrasound Imaging. IEEE Trans. Biomed. Eng. 2017, 64, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, Y.; Ma, T.; Shung, K.K.; Zhou, Q. Intravascular Ultrasound Imaging with Virtual Source Synthetic Aperture Focusing and Coherence Factor Weighting. IEEE Trans. Med. Imaging 2017, 36, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shin, E.-J.; Lee, C.; Chang, J.H. Development of Dual-Frequency Oblong-Shaped-Focused Transducers for Intravascular Ultrasound Tissue Harmonic Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1571–1582. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Moon, J.-Y.; Chang, J. A 35 MHz/105 MHz Dual-Element Focused Transducer for Intravascular Ultrasound Tissue Imaging Using the Third Harmonic. Sensors 2018, 18, 2290. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-C.; Chen, P.-Y.; Ma, T.; Zhou, Q.; Shung, K.K.; Huang, C.-C. Development of an intravascular ultrasound elastography based on a dual-element transducer. R. Soc. Open Sci. 2018, 5, 180138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanderLaan, D.; Karpiouk, A.B.; Yeager, D.; Emelianov, S. Real-Time Intravascular Ultrasound and Photoacoustic Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Morino, Y.; Abe, M.; Morimoto, T.; Kimura, T.; Hayashi, Y.; Muramatsu, T.; Ochiai, M.; Noguchi, Y.; Kato, K.; Shibata, Y.; et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes. JACC Cardiovasc. Interv. 2011, 4, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Srivatsa, S.S.; Edwards, W.D.; Boos, C.M.; Grill, D.E.; Sangiorgi, G.M.; Garratt, K.N.; Schwartz, R.S.; Holmes, D.R. Histologic correlates of angiographic chronic total coronary artery occlusions. Influence of occlusion duration on neovascular channel patterns and intimal plaque composition. J. Am. Coll. Cardiol. 1997, 29, 955–963. [Google Scholar] [CrossRef]
- Rathore, S.; Katoh, O.; Tuschikane, E.; Oida, A.; Suzuki, T.; Takase, S. A Novel Modification of the Retrograde Approach for the Recanalization of Chronic Total Occlusion of the Coronary Arteries. Intravascular Ultrasound-Guided Reverse Controlled Antegrade and Retrograde Tracking. JACC Cardiovasc. Interv. 2010, 3, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Joyal, D.; Thompson, C.A.; Grantham, J.A.; Buller, C.E.H.; Rinfret, S. The retrograde technique for recanalization of chronic total occlusions: A step-by-step approach. JACC Cardiovasc. Interv. 2012, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Chen, Y.H.; Lin, M.S.; Yeh, C.F.; Hung, C.S.; Kao, H.L.; Huang, C.C. Retrograde approach is as effective and safe as antegrade approach in contemporary percutaneous coronary intervention for chronic total occlusion: A Taiwan single-center registry study. Acta Cardiol. Sin. 2017, 33, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Suzuki, T.; Ito, T.; Katoh, O.; Ojio, S.; Sato, H.; Ehara, M.; Suzuki, T.; Kawase, Y.; Myoishi, M.; et al. Novel technique using intravascular ultrasound-guided guidewire cross in coronary intervention for uncrossable chronic total occlusions. Circ. J. 2004, 68, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.H.; Evans, J.L.; Vonesh, M.J.; Meyers, S.N.; Mills, T.A.; Kane, B.J.; Aldrich, W.N.; Jang, Y.T.; Yock, P.G.; Rold, M.D.; et al. Arterial imaging with a new forward-viewing intravascular ultrasound catheter, II: Three-dimensional reconstruction and display of data. Circulation 1994, 89, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Stephens, D.N.; O’Donnell, M. Optimizing the beam pattern of a forward-viewing ring-annular ultrasound array for intravascular imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002, 49, 1652–1664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Stephens, D.; O’Donnell, M. Initial results from a forward-viewing ring-annular ultrasound array for intravascular imaging. In Proceedings of the 2003 IEEE Symposium on Ultrasonics, Honolulu, HI, USA, 5–8 October 2003; pp. 212–215. [Google Scholar]
- Yeh, D.T.; Oralkan, Ö.; Wygant, I.O.; O’Donnell, M.; Khuri-Yakub, B.T. 3-D ultrasound imaging using forward viewing CMUT ring arrays for intravascular and intracardiac applications. Proc. IEEE Ultrason. Symp. 2005, 2, 783–786. [Google Scholar] [CrossRef]
- Tekes, C.; Karaman, M.; Degertekin, F.L. Optimizing circular ring arrays for forward-looking IVUS imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 2596–2607. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chang, J.H. Effect of Acoustic Properties of Lens Materials on Performance of Capacitive Micromachined Ultrasonic Transducers. J. Med. Biol. Eng. 2016, 36, 536–544. [Google Scholar] [CrossRef]
- Janjic, J.; Mastik, F.; Leistikow, M.D.; Bosch, J.G.; Springeling, G.; van der Steen, A.F.W.; van Soest, G. Sparse Ultrasound Image Reconstruction from a Shape-Sensing Single-Element Forward-Looking Catheter. IEEE Trans. Biomed. Eng. 2018, 65, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wu, D.; Jin, J.; Hu, C.; Xu, X.; Williams, J.; Cannata, J.M.; Lim, L.; Shung, K.K. Design and fabrication of PZN-7%PT single crystal high frequency angled needle ultrasound transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 1394–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paeng, D.-G.; Chang, J.H.; Chen, R.; Humayun, M.S.; Shung, K.K. Feasibility of rotational scan ultrasound imaging by an angled high frequency transducer for the posterior segment of the eye. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 676–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, J.H. Forward-Looking IVUS in Chronic Total Occlusions. Card. Interv. Today 2009, June/July, 21–24. [Google Scholar]
- Yoon, S.; Williams, J.; Kang, B.J.; Yoon, C.; Cabrera-Munoz, N.; Jeong, J.S.; Lee, S.G.; Shung, K.K.; Kim, H.H. Angled-focused 45MHz PMN-PT single element transducer for intravascular ultrasound imaging. Sens. Actuators A Phys. 2015, 228, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodge, J.T.; Brown, B.G.; Bolson, E.L.; Dodge, H.T. Lumen diameter of normal human coronary arteries. Influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation 1992, 86, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Chang, J.H. Development of 15 MHz 2-2 piezo-composite ultrasound linear array transducers for ophthalmic imaging. Sens. Actuators A Phys. 2014, 217, 39–48. [Google Scholar] [CrossRef]
- Jang, J.; Chang, J.H. Design and Fabrication of a Miniaturized Convex Array for Combined Ultrasound and Photoacoustic Imaging of the Prostate. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Chang, J.H. Design and fabrication of double-focused ultrasound transducers to achieve tight focusing. Sensors 2016, 16, 1248. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Sun, L.; Yen, J.T.; Shung, K.K. Low-cost, high-speed back-end processing system for high-frequency ultrasound B-mode imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 1490–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menikou, G.; Damianou, C. Acoustic and thermal characterization of agar based phantoms used for evaluating focused ultrasound exposures. J. Ther. Ultrasound 2017, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tsui, C.S.L.; Liang, H.D.; Halliwell, M.; Shere, M.; Braybrooke, J.P.; Whipp, E.; Wells, P.N.T. Coherent Ultrasonic Doppler Tomography. Ultrasound Med. Biol. 2011, 37, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-Y.; Lee, J.; Chang, J.H. Electrical impedance matching networks based on filter structures for high frequency ultrasound transducers. Sens. Actuators A Phys. 2016, 251, 225–233. [Google Scholar] [CrossRef]
- Mintz, G.S. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc. Imaging 2015, 8, 461–471. [Google Scholar] [CrossRef] [PubMed]





| Parameters | PZT-5H | 1st Matching | 2nd Matching | Backing Layer |
|---|---|---|---|---|
| Longitudinal Velocity (m/s) | 4700 | 1900 | 2350 | 1850 |
| Density (g/cm3) | 7.8 | 3.86 | 1.1 | 3.2 |
| Acoustic Impedance (MRayl) | 36.7 | 7.334 | 2.59 | 5.92 |
| Clamped dielectric constant | 1200 | - | - | - |
| Thickness (μm) | 59 | 13 | 16 | 414 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Chang, J.H. A 40-MHz Ultrasound Transducer with an Angled Aperture for Guiding Percutaneous Revascularization of Chronic Total Occlusion: A Feasibility Study. Sensors 2018, 18, 4079. https://doi.org/10.3390/s18114079
Lee J, Chang JH. A 40-MHz Ultrasound Transducer with an Angled Aperture for Guiding Percutaneous Revascularization of Chronic Total Occlusion: A Feasibility Study. Sensors. 2018; 18(11):4079. https://doi.org/10.3390/s18114079
Chicago/Turabian StyleLee, Junsu, and Jin Ho Chang. 2018. "A 40-MHz Ultrasound Transducer with an Angled Aperture for Guiding Percutaneous Revascularization of Chronic Total Occlusion: A Feasibility Study" Sensors 18, no. 11: 4079. https://doi.org/10.3390/s18114079





