Application of Gold-Nanoparticle Colorimetric Sensing to Rapid Food Safety Screening
Abstract
:1. Introduction
2. Synthesis of Gold Nanoparticles
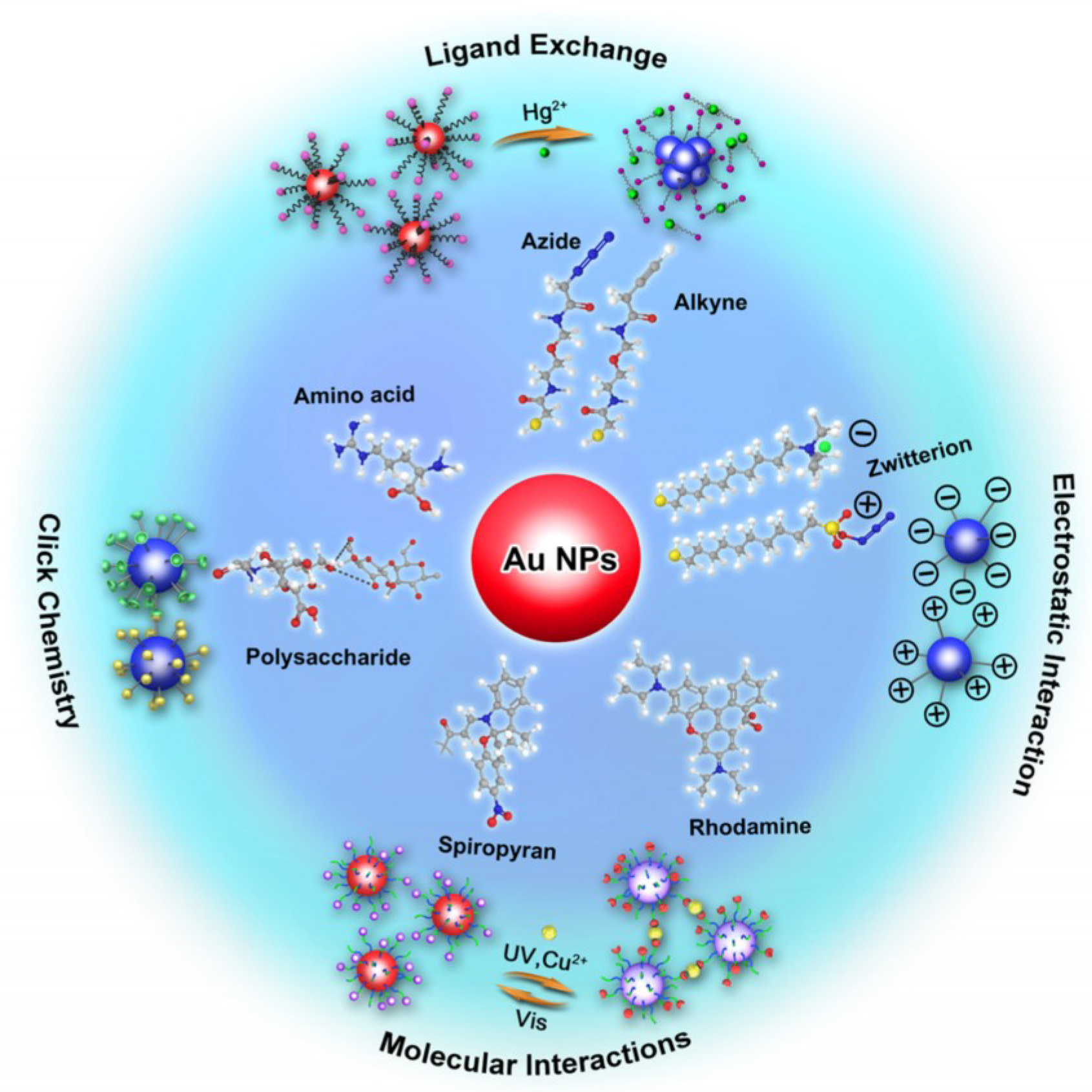
3. Functional Modification of Gold Nanoparticles
4. Optical Properties of Gold Nanoparticles
5. Principle of Gold Nanoparticles-Based Colorimetric Sensors and Application in Food Quality and Safety Testing
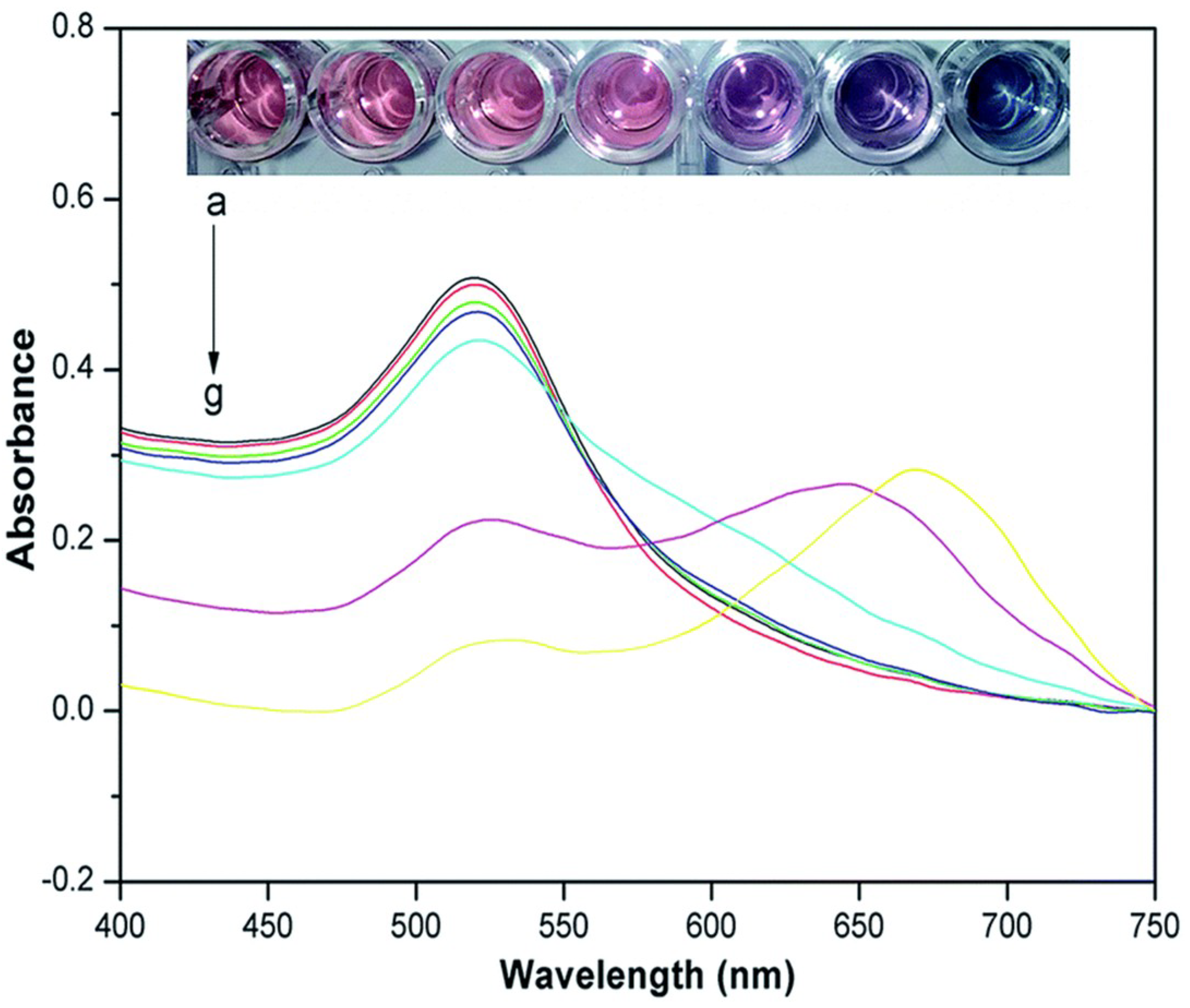
5.1. Cross-Linking Methods and Application
5.2. De-Protection Method and Application
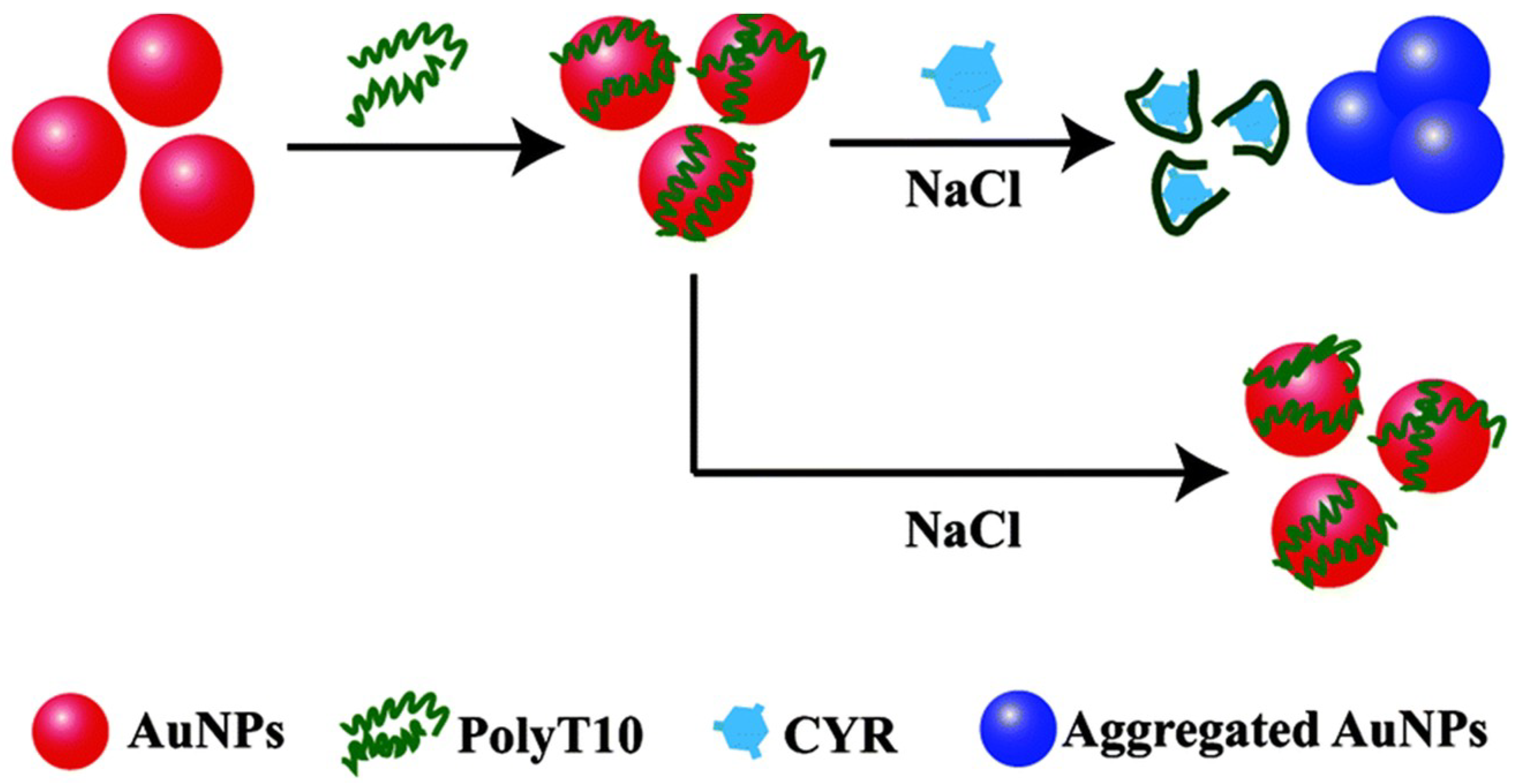
5.3. Anti-Aggregation Method and Application
6. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Neves Dias, A.; Simão, V.; Merib, J.; Carasek, E. Use of green coating (cork) in solid-phase microextraction for the determination of organochlorine pesticides in water by gas chromatography-electron capture detection. Talanta 2015, 134, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, X.; Li, T.; She, Y.; Wang, S.; Wang, J.; Zhang, M.; Jin, F.; Jin, M.; Shao, H.; et al. Preparation of a magnetic molecularly imprinted polymer using g-C3N4–Fe3O4 for atrazine adsorption. Mater. Lett. 2015, 160, 472–475. [Google Scholar] [CrossRef]
- La Torre, G.L.; Saitta, M.; Vilasi, F.; Pellicanò, T.; Dugo, G. Direct determination of phenolic compounds in Sicilian wines by liquid chromatography with PDA and MS detection. Food Chem. 2006, 94, 640–650. [Google Scholar] [CrossRef]
- Omar, M.M.A.; Elbashir, A.A.; Schmitz, O.J. Capillary electrophoresis method with UV-detection for analysis of free amino acids concentrations in food. Food Chem. 2017, 214, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Guan, J.; Zhang, D.; Li, B.; Liu, H.; He, Z. Supercritical fluid chromatography coupled with tandem mass spectrometry: A high-efficiency detection technique to quantify Taxane drugs in whole-blood samples. J. Sep. Sci. 2017, 40, 3914–3921. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qu, B.; Liu, H.; Ding, J.; Ren, N. Analysis of organochlorine pesticides in surface water of the Songhua River using magnetoliposomes as adsorbents coupled with GC-MS/MS detection. Sci. Total Environ. 2018, 618, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Malachová, A.; Stránská, M.; Václavíková, M.; Elliott, C.T.; Black, C.; Meneely, J.; Hajšlová, J.; Ezekiel, C.N.; Schuhmacher, R.; Krska, R. Advanced LC–MS-based methods to study the co-occurrence and metabolization of multiple mycotoxins in cereals and cereal-based food. Anal. Bioanal. Chem. 2018, 410, 801–825. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, L.; Huang, X.; Zheng, S.; Xu, D.; Xu, X.; Zhang, Y.; Lin, H. Determination of triazole pesticides in aqueous solution based on magnetic graphene oxide functionalized MOF-199 as solid phase extraction sorbents. Microporous Mesoporous Mater. 2018, 270, 258–264. [Google Scholar] [CrossRef]
- Liu, J.; Meng, L.; Fei, Z.; Dyson, P.J.; Jing, X.; Liu, X. MnO2 nanosheets as an artificial enzyme to mimic oxidase for rapid and sensitive detection of glutathione. Biosens. Bioelectron. 2017, 90, 69–74. [Google Scholar] [CrossRef]
- Liu, A.; Xiong, Q.; Shen, L.; Li, W.; Zeng, Z.; Li, C.; Liu, S.; Liu, Y.; Han, G. A sandwich-type ELISA for the detection of Listeria monocytogenes using the well-oriented single chain Fv antibody fragment. Food Control 2017, 79, 156–161. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.J. Gold Nanostar Enhanced Surface Plasmon Resonance Detection of an Antibiotic at Attomolar Concentrations via an Aptamer-Antibody Sandwich Assay. Anal. Chem. 2017, 89, 6624–6630. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhao, G.; Dou, W. An electrochemical immunoassay for Escherichia coli O157:H7 using double functionalized Au@Pt/SiO2 nanocomposites and immune magnetic nanoparticles. Talanta 2018, 182, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Qaddare, S.H.; Salimi, A. Amplified fluorescent sensing of DNA using luminescent carbon dots and AuNPs/GO as a sensing platform: A novel coupling of FRET and DNA hybridization for homogeneous HIV-1 gene detection at femtomolar level. Biosens. Bioelectron. 2017, 89, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, X.; Peng, Y.; Hong, X.; Liu, Y.; Jiang, S.; Ning, B.; Gao, Z. Ultrasensitive sensing of diethylstilbestrol based on AuNPs/MWCNTs-CS composites coupling with sol-gel molecularly imprinted polymer as a recognition element of an electrochemical sensor. Sens. Actuat. B-Chem. 2017, 238, 420–426. [Google Scholar] [CrossRef]
- Mascini, M.; Gaggiotti, S.; Della Pelle, F.; Wang, J.; Pingarrón, J.M.; Compagnone, D. Hairpin DNA-AuNPs as molecular binding elements for the detection of volatile organic compounds. Biosens. Bioelectron. 2018, 123, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, P.A.; Sandhyarani, N. Electrochemical DNA sensors based on the use of gold nanoparticles: A review on recent developments. Microchim. Acta 2017, 184, 981–1000. [Google Scholar] [CrossRef]
- Wang, P.; Lin, Z.; Su, X.; Tang, Z. Application of Au based nanomaterials in analytical science. Nano Today 2017, 12, 64–97. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; You, W.; Cheng, D.; Ni, W. Gold nanorod@iron oxide core–shell heterostructures: Synthesis, characterization, and photocatalytic performance. Nanoscale 2017, 9, 3925–3933. [Google Scholar] [CrossRef]
- Shi, J.; Chan, C.; Pang, Y.; Ye, W.; Tian, F.; Lyu, J.; Zhang, Y.; Yang, M. A fluorescence resonance energy transfer (FRET) biosensor based on graphene quantum dots (GQDs) and gold nanoparticles (AuNPs) for the detection of mecA gene sequence of Staphylococcus aureus. Biosens. Bioelectron. 2015, 67, 595–600. [Google Scholar] [CrossRef]
- Chen, M.; Shen, X.; Liu, P.; Wei, Y.; Meng, Y.; Zheng, G.; Diao, G. β-Cyclodextrin polymer as a linker to fabricate ternary nanocomposites AuNPs/pATP-β-CDP/rGO and their electrochemical application. Carbohyd. Polym. 2015, 119, 26–34. [Google Scholar] [CrossRef]
- Fusco, G.; Gallo, F.; Tortolini, C.; Bollella, P.; Ietto, F.; De Mico, A.; D’Annibale, A.; Antiochia, R.; Favero, G.; Mazzei, F. AuNPs-functionalized PANABA-MWCNTs nanocomposite-based impedimetric immunosensor for 2,4-dichlorophenoxy acetic acid detection. Biosens. Bioelectron. 2017, 93, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Deiminiat, B.; Rounaghi, G.H.; Arbab-Zavar, M.H.; Razavipanah, I. A novel electrochemical aptasensor based on f-MWCNTs/AuNPs nanocomposite for label-free detection of bisphenol A. Sens. Actuators B-Chem. 2017, 242, 158–166. [Google Scholar] [CrossRef]
- Lee, O.S.; Prytkova, T.R.; Schatz, G.C. Using DNA to Link Gold Nanoparticles, Polymers, and Molecules: A Theoretical Perspective. J. Phys. Chem. Lett. 2010, 1, 1781–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, P.K.; Periyasamy, G.; Manna, A.K.; Pati, S.K. Computational studies on structural and optical properties of single-stranded DNA encapsulated silver/gold clusters. J. Mater. Chem. 2012, 22, 6774–6781. [Google Scholar] [CrossRef]
- Baetsen-Young, A.M.; Vasher, M.; Matta, L.L.; Colgan, P.; Alocilja, E.C.; Day, B. Direct colorimetric detection of unamplified pathogen DNA by dextrin-capped gold nanoparticles. Biosens. Bioelectron. 2018, 101, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- De Luca, G.; Bonaccorsi, P.; Trovato, V.; Mancuso, A.; Papalia, T.; Pistone, A.; Casaletto, M.P.; Mezzi, A.; Brunetti, B.; Minuti, L.; et al. Tripodal tris-disulfides as capping agents for a controlled mixed functionalization of gold nanoparticles. New J. Chem. 2018, 42, 16436–16440. [Google Scholar] [CrossRef]
- Wu, S.; Li, D.; Wang, J.; Zhao, Y.; Dong, S.; Wang, X. Gold nanoparticles dissolution based colorimetric method for highly sensitive detection of organophosphate pesticides. Sens. Actuators B-Chem. 2017, 238, 427–433. [Google Scholar] [CrossRef]
- Memon, S.S.; Nafady, A.; Solangi, A.R.; Al-Enizi, A.M.; Sirajuddin; Shah, M.R.; Sherazi, S.T.H.; Memon, S.; Arain, M.; Abro, M.I.; et al. Sensitive and selective aggregation based colorimetric sensing of Fe3+ via interaction with acetyl salicylic acid derived gold nanoparticles. Sens. Actuators B-Chem. 2018, 259, 1006–1012. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, K.; Zhao, G. Gold nanoparticles: From synthesis, properties to their potential application as colorimetric sensors in food safety screening. Trends Food Sci. Tech. 2018, 78, 83–94. [Google Scholar] [CrossRef]
- Matteo, S.; Alessandra, A.; Saverio, M. Optical nanoprobes based on gold nanoparticles for sugar sensing. Nanotechnology 2009, 20, 135501. [Google Scholar]
- Vilela, D.; González, M.C.; Escarpa, A. (Bio)-synthesis of Au NPs from soy isoflavone extracts as a novel assessment tool of their antioxidant capacity. RSC Adv. 2014, 4, 3075–3081. [Google Scholar] [CrossRef]
- Della Pelle, F.; González, M.C.; Sergi, M.; Del Carlo, M.; Compagnone, D.; Escarpa, A. Gold Nanoparticles-based Extraction-Free Colorimetric Assay in Organic Media: An Optical Index for Determination of Total Polyphenols in Fat-Rich Samples. Anal. Chem. 2015, 87, 6905–6911. [Google Scholar] [CrossRef] [PubMed]
- Zohora, N.; Kumar, D.; Yazdani, M.; Rotello, V.M.; Ramanathan, R.; Bansal, V. Rapid colorimetric detection of mercury using biosynthesized gold nanoparticles. Colloid Surf. A 2017, 532, 451–457. [Google Scholar] [CrossRef]
- Jazayeri, M.H.; Aghaie, T.; Avan, A.; Vatankhah, A.; Ghaffari, M.R.S. Colorimetric detection based on gold nano particles (GNPs): An easy, fast, inexpensive, low-cost and short time method in detection of analytes (protein, DNA, and ion). Sens. Bio-Sens. Res. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.Y.; Takeda, Y.; Kondow, T. Full Physical Preparation of Size-Selected Gold Nanoparticles in Solution: Laser Ablation and Laser-Induced Size Control. J. Phys. Chem. B 2002, 106, 7575–7577. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Chen, N.; Liu, H.; Zhang, Y.; Zhou, Z.; Fan, W.; Yu, G.; Shen, Z.; Wu, A. A colorimetric sensor based on citrate-stabilized AuNPs for rapid pesticide residue detection of terbuthylazine and dimethoate. Sens. Actuators B-Chem. 2018, 255, 3093–3101. [Google Scholar] [CrossRef]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Liu, Q.; Jiao, Y.; Jia, R.; Chen, Z. Colorimetric aggregation based cadmium(II) assay by using triangular silver nanoplates functionalized with 1-amino-2-naphthol-4-sulfonate. Microchim. Acta 2017, 185, 6. [Google Scholar] [CrossRef] [PubMed]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically Controlled Seeded Growth Synthesis of Citrate-Stabilized Gold Nanoparticles of up to 200 nm: Size Focusing versus Ostwald Ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Akshath, U.S.; Bhatt, P.; Thakur, M.S.; Raghavarao, K.S.M.S. Gold Nanoparticles Based Enzyme Biosensor for the Detection of Chloramphenicol. Procedia Technol. 2017, 27, 282–286. [Google Scholar] [CrossRef]
- Jana, N.R.; Peng, X. Single-Phase and Gram-Scale Routes toward Nearly Monodisperse Au and Other Noble Metal Nanocrystals. J. Am. Chem. Soc. 2003, 125, 14280–14281. [Google Scholar] [CrossRef] [PubMed]
- Gentili, D.; Ori, G.; Comes Franchini, M. Double phase transfer of gold nanorods for surface functionalization and entrapment into PEG-based nanocarriers. Chem. Commun. 2009, 5874–5876. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Tripp, S.L.; Wei, A. Self-Organization of Large Gold Nanoparticle Arrays. J. Am. Chem. Soc. 2001, 123, 7955–7956. [Google Scholar] [CrossRef]
- Cheng, Y.; Samia, A.C.; Meyers, J.D.; Panagopoulos, I.; Fei, B.; Burda, C. Highly Efficient Drug Delivery with Gold Nanoparticle Vectors for in Vivo Photodynamic Therapy of Cancer. J. Am. Chem. Soc. 2008, 130, 10643–10647. [Google Scholar] [CrossRef] [Green Version]
- Kumari, M.; Mishra, A.; Pandey, S.; Singh, S.P.; Chaudhry, V.; Mudiam, M.K.R.; Shukla, S.; Kakkar, P.; Nautiyal, C.S. Physico-Chemical Condition Optimization during Biosynthesis lead to development of Improved and Catalytically Efficient Gold Nano Particles. Sci. Rep. 2016, 6, 27575. [Google Scholar] [CrossRef] [Green Version]
- Sau, T.K.; Murphy, C.J. Room Temperature, High-Yield Synthesis of Multiple Shapes of Gold Nanoparticles in Aqueous Solution. J. Am. Chem. Soc. 2004, 126, 8648–8649. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Fang, C.; Dharmarajan, R.; Megharaj, M.; Naidu, R. Gold nanoparticle-based optical sensors for selected anionic contaminants. TRAC-Trends Anal. Chem. 2017, 86, 143–154. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface Modification of Gold Nanoparticles with Small Molecules for Biochemical Analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, J.; Li, M.; Gao, P.; Zhou, Y.; Zhang, G.; Shuang, S.; Dong, C. Colorimetric sensor for cysteine in human urine based on novel gold nanoparticles. Talanta 2016, 161, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Bhamore, J.; Rawat, K.A.; Basu, H.; Singhal, R.K.; Kailasa, S.K. Influence of molecular assembly and NaCl concentration on gold nanoparticles for colorimetric detection of cysteine and glutathione. Sens. Actuators B-Chem. 2015, 212, 526–535. [Google Scholar] [CrossRef]
- Liu, G.; Wang, S.; Yang, X.; Li, T.; She, Y.; Wang, J.; Zou, P.; Jin, F.; Jin, M.; Shao, H. Colorimetric sensing of atrazine in rice samples using cysteamine functionalized gold nanoparticles after solid phase extraction. Anal. Methods 2016, 8, 52–56. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.; Zhang, X.; Zhao, L.; Li, S. Enantiomeric separation of adrenaline, noradrenaline, and isoprenaline by capillary electrophoresis using streptomycin-modified gold nanoparticles. Microchim. Acta 2018, 185, 227. [Google Scholar] [CrossRef] [PubMed]
- Shahrivari, S.; Faridbod, F.; Ganjali, M.R. Highly selective and sensitive colorimetric determination of Cr3+ ion by 4-amino-5-methyl-4H-1,2,4-triazole-3-thiol functionalized Au nanoparticles. Spectrochim. Acta A 2018, 191, 189–194. [Google Scholar] [CrossRef]
- Tolessa, T.; Tan, Z.-Q.; Liu, J.-F. Hydride generation coupled with thioglycolic acid coated gold nanoparticles as simple and sensitive headspace colorimetric assay for visual detection of Sb(III). Anal. Chim. Acta 2018, 1004, 67–73. [Google Scholar] [CrossRef]
- Gukowsky, J.C.; Tan, C.; Han, Z.; He, L. Cysteamine-Modified Gold Nanoparticles as a Colorimetric Sensor for the Rapid Detection of Gentamicin. J. Food Sci. 2018, 83, 1631–1638. [Google Scholar] [CrossRef]
- Raj, V.; Vijayan, A.N.; Joseph, K. Cysteine capped gold nanoparticles for naked eye detection of E. coli bacteria in UTI patients. Sens. Bio-Sens. Res. 2015, 5, 33–36. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Yang, X. Highly specific colorimetric recognition and sensing of sulfide with glutathione-modified gold nanoparticle probe based on an anion-for-molecule ligand exchange reaction. Analyst 2012, 137, 1556–1558. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Lu, S.; Chen, C.; Sun, J.; Yang, X. Colorimetric sandwich immunosensor for Aβ(1-42) based on dual antibody-modified gold nanoparticles. Sens. Actuators B-Chem. 2017, 243, 792–799. [Google Scholar] [CrossRef]
- Zhou, M.; Lin, T.; Gan, X. Colorimetric aggregation assay for silver(I) based on the use of aptamer modified gold nanoparticles and C-Ag(I)-C interaction. Microchim. Acta 2017, 184, 4671–4677. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, M.; Liu, Y.; Xie, X.; Bao, W.; Song, A.; Hao, J. Chitosan gel incorporated peptide-modified AuNPs for sustained drug delivery with smart pH responsiveness. J. Mater. Chem. B 2017, 5, 1174–1181. [Google Scholar] [CrossRef]
- Xu, L.; Dong, S.; Hao, J.; Cui, J.; Hoffmann, H. Surfactant-Modified Ultrafine Gold Nanoparticles with Magnetic Responsiveness for Reversible Convergence and Release of Biomacromolecules. Langmuir 2017, 33, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Emrani, A.S.; Danesh, N.M.; Ramezani, M.; Taghdisi, S.M.; Abnous, K. A novel fluorescent aptasensor based on hairpin structure of complementary strand of aptamer and nanoparticles as a signal amplification approach for ultrasensitive detection of cocaine. Biosens. Bioelectron. 2016, 79, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Abnous, K.; Danesh, N.M.; Ramezani, M.; Emrani, A.S.; Taghdisi, S.M. A novel colorimetric sandwich aptasensor based on an indirect competitive enzyme-free method for ultrasensitive detection of chloramphenicol. Biosens. Bioelectron. 2016, 78, 80–86. [Google Scholar] [CrossRef]
- Zanoli, L.M.; D’Agata, R.; Spoto, G. Functionalized gold nanoparticles for ultrasensitive DNA detection. Anal. Bioanal. Chem. 2012, 402, 1759–1771. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; McKelvie, I.D.; Cattrall, R.W.; Kolev, S.D. Colorimetric detection based on localised surface plasmon resonance of gold nanoparticles: Merits, inherent shortcomings and future prospects. Talanta 2016, 152, 410–422. [Google Scholar] [CrossRef]
- Gopinath, S.C.B.; Lakshmipriya, T.; Awazu, K. Colorimetric detection of controlled assembly and disassembly of aptamers on unmodified gold nanoparticles. Biosens. Bioelectron. 2014, 51, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Zhou, B.; Huang, N.; Jiang, M.; Zhang, J.; Liu, L. Visual and fluorescent assays for selective detection of beta-amyloid oligomers based on the inner filter effect of gold nanoparticles on the fluorescence of CdTe quantum dots. Biosens. Bioelectron. 2016, 85, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chang, H.; Li, J.-J.; Li, X.; Zhao, J.-W. Dual-mode melamine detection based on gold nanoparticles aggregation-induced fluorescence “turn-on” and “turn-off” of CdTe quantum dots. Sens. Actuators B-Chem. 2017, 239, 906–915. [Google Scholar] [CrossRef]
- Chen, G.-H.; Chen, W.-Y.; Yen, Y.-C.; Wang, C.-W.; Chang, H.-T.; Chen, C.-F. Detection of Mercury(II) Ions Using Colorimetric Gold Nanoparticles on Paper-Based Analytical Devices. Anal. Chem. 2014, 86, 6843–6849. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Minhaz, A.; Khan, N.A.; Kalantari, K.; Afifi, A.B.M.; Shah, M.R. Synthesis of gold nanoparticles stabilized by a pyrazinium thioacetate ligand: A new colorimetric nanosensor for detection of heavy metal Pd(II). Sens. Actuators B-Chem. 2018, 257, 875–881. [Google Scholar] [CrossRef]
- Dong, H.; Zou, F.; Hu, X.; Zhu, H.; Koh, K.; Chen, H. Analyte induced AuNPs aggregation enhanced surface plasmon resonance for sensitive detection of paraquat. Biosens. Bioelectron. 2018, 117, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Vilela, D.; González, M.C.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay. A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef]
- Zhu, J.; Chang, H.; Li, J.-J.; Li, X.; Zhao, J.-W. Using silicon-coated gold nanoparticles to enhance the fluorescence of CdTe quantum dot and improve the sensing ability of mercury (II). Spectrochim. Acta A 2018, 188, 170–178. [Google Scholar] [CrossRef]
- Sun, C.; Yuan, F.; Li, H.; Wu, X. A specific fluorescent nanoprobe for dopamine based on the synergistic action of citrate and gold nanoparticles on Tb(III) luminescence. Microchim. Acta 2018, 185, 317. [Google Scholar] [CrossRef]
- Kavosi, B.; Navaee, A.; Salimi, A. Amplified fluorescence resonance energy transfer sensing of prostate specific antigen based on aggregation of CdTe QDs/antibody and aptamer decorated of AuNPs-PAMAM dendrimer. J. Lumin. 2018, 204, 368–374. [Google Scholar] [CrossRef]
- Rao, H.; Ge, H.; Wang, X.; Zhang, Z.; Liu, X.; Yang, Y.; Liu, Y.; Liu, W.; Zou, P.; Wang, Y. Colorimetric and fluorometric detection of protamine by using a dual-mode probe consisting of carbon quantum dots and gold nanoparticles. Microchim. Acta 2017, 184, 3017–3025. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, J.; Jia, Z. Efficient Fluorescence Resonance Energy Transfer between Quantum Dots and Gold Nanoparticles Based on Porous Silicon Photonic Crystal for DNA Detection. Sensors 2017, 17, 1078. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, H.; Li, Y.; Su, X. Visual and fluorescent detection of acetamiprid based on the inner filter effect of gold nanoparticles on ratiometric fluorescence quantum dots. Anal. Chim. Acta 2014, 852, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-W.; Zou, X.-M.; Song, S.-H.; Chen, G.-H. Quantum Dots Applied to Methodology on Detection of Pesticide and Veterinary Drug Residues. J. Agric. Food Chem. 2018, 66, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, F.; Li, J.; Zhu, J.-J.; Lu, Y. Fluorescent nanoprobes for sensing and imaging of metal ions: Recent advances and future perspectives. Nano Today 2016, 11, 309–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, X.; Zhong, Y.; Chen, R.; Wang, F.; Liu, Y.; Luo, D. A “turn-on” fluorescence sensor for Pb2+ detection based on graphene quantum dots and gold nanoparticles. Sens. Actuators B-Chem. 2018, 255, 1577–1581. [Google Scholar] [CrossRef]
- Stanisavljevic, M.; Krizkova, S.; Vaculovicova, M.; Kizek, R.; Adam, V. Quantum dots-fluorescence resonance energy transfer-based nanosensors and their application. Biosens. Bioelectron. 2015, 74, 562–574. [Google Scholar] [CrossRef]
- Yuan, Z.; Hu, C.-C.; Chang, H.-T.; Lu, C. Gold nanoparticles as sensitive optical probes. Analyst 2016, 141, 1611–1626. [Google Scholar] [CrossRef] [Green Version]
- Lo, S.-H.; Wu, M.-C.; Venkatesan, P.; Wu, S.-P. Colorimetric detection of chromium(III) using O-phospho-l-serine dithiocarbamic acid functionalized gold nanoparticles. Sens. Actuators B-Chem. 2015, 220, 772–778. [Google Scholar] [CrossRef]
- Yue, G.; Su, S.; Li, N.; Shuai, M.; Lai, X.; Astruc, D.; Zhao, P. Gold nanoparticles as sensors in the colorimetric and fluorescence detection of chemical warfare agents. Coordin. Chem. Rev. 2016, 311, 75–84. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Pradhan, N. Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: A review. Sens. Actuators B-Chem. 2017, 238, 888–902. [Google Scholar] [CrossRef]
- Sharma, R.; Ragavan, K.V.; Thakur, M.S.; Raghavarao, K.S.M.S. Recent advances in nanoparticle based aptasensors for food contaminants. Biosens. Bioelectron. 2015, 74, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Morales-Narváez, E.; Golmohammadi, H.; Naghdi, T.; Yousefi, H.; Kostiv, U.; Horák, D.; Pourreza, N.; Merkoçi, A. Nanopaper as an Optical Sensing Platform. ACS Nano 2015, 9, 7296–7305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Zhang, J.; Wang, Y.; Chen, J.; Li, Y.; Duan, Y. An aptamer based method for small molecules detection through monitoring salt-induced AuNPs aggregation and surface plasmon resonance (SPR) detection. Sens. Actuators B-Chem. 2016, 236, 474–479. [Google Scholar] [CrossRef]
- Tsogas, G.Z.; Kappi, F.A.; Vlessidis, A.G.; Giokas, D.L. Recent Advances in Nanomaterial Probes for Optical Biothiol Sensing: A Review. Anal. Lett. 2018, 51, 443–468. [Google Scholar] [CrossRef]
- Häkkinen, H. The gold–sulfur interface at the nanoscale. Nat. Chem. 2012, 4, 443. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Z.; Jiang, X. Gold nanoparticles for the colorimetric and fluorescent detection of ions and small organic molecules. Nanoscale 2011, 3, 1421–1433. [Google Scholar] [CrossRef]
- Ma, X.; Guo, Z.; Mao, Z.; Tang, Y.; Miao, P. Colorimetric theophylline aggregation assay using an RNA aptamer and non-crosslinking gold nanoparticles. Microchim. Acta 2017, 185, 33. [Google Scholar] [CrossRef]
- Liu, G.; Yang, X.; Li, T.; Yu, H.; Du, X.; She, Y.; Wang, J.; Wang, S.; Jin, F.; Jin, M.; et al. Spectrophotometric and visual detection of the herbicide atrazine by exploiting hydrogen bond-induced aggregation of melamine-modified gold nanoparticles. Microchim. Acta 2015, 182, 1983–1989. [Google Scholar] [CrossRef]
- Giannoulis, K.M.; Giokas, D.L.; Tsogas, G.Z.; Vlessidis, A.G. Ligand-free gold nanoparticles as colorimetric probes for the non-destructive determination of total dithiocarbamate pesticides after solid phase extraction. Talanta 2014, 119, 276–283. [Google Scholar] [CrossRef]
- Liu, G.; Huang, X.; Zheng, S.; Li, L.; Xu, D.; Xu, X.; Zhang, Y.; Lin, H. Novel triadimenol detection assay based on fluorescence resonance energy transfer between gold nanoparticles and cadmium telluride quantum dots. Dyes Pigment. 2018, 149, 229–235. [Google Scholar] [CrossRef]
- Gao, N.; Huang, P.; Wu, F. Colorimetric detection of melamine in milk based on Triton X-100 modified gold nanoparticles and its paper-based application. Spectrochim. Acta A 2018, 192, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Shellaiah, M.; Steffi, P.; Sun, K.W.; Ko, F.-H. Development of extremely stable dual functionalized gold nanoparticles for effective colorimetric detection of clenbuterol and ractopamine in human urine samples. Anal. Chim. Acta 2018, 1023, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, P.; Su, X.; Zhao, H.; He, Y. Colorimetric detection of ractopamine and salbutamol using gold nanoparticles functionalized with melamine as a probe. Talanta 2013, 112, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Ratnarathorn, N.; Chailapakul, O.; Dungchai, W. Highly sensitive colorimetric detection of lead using maleic acid functionalized gold nanoparticles. Talanta 2015, 132, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, X.; Cui, X.; Zhang, Y.; Zhao, H.; Li, X.; He, Y. A highly sensitive colorimetric probe for Cd2+, Hg2+ and ascorbic acid determination based on trithiocyanuric acid-AuNPs. Talanta 2018, 188, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Hu, X.; Song, S.; Fan, C. Unmodified gold nanoparticles as a colorimetric probe for potassium DNA aptamers. Chem. Commun. 2006, 3780–3782. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cao, F.; Zheng, W.; Tian, Y.; Xianyu, Y.; Xu, P.; Zhang, W.; Wang, Z.; Deng, K.; Jiang, X. Detection of the nanomolar level of total Cr[(iii) and (vi)] by functionalized gold nanoparticles and a smartphone with the assistance of theoretical calculation models. Nanoscale 2015, 7, 2042–2049. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Z.; Cui, Z.; Li, H. Ionic liquid functionalized gold nanoparticles: Synthesis, rapid colorimetric detection of imidacloprid. Sens. Actuators B-Chem. 2014, 191, 313–319. [Google Scholar] [CrossRef]
- Liu, J.; Bai, W.; Zhu, C.; Yan, M.; Yang, S.; Chen, A. Sensitive colorimetric detection of cyromazine in cucumber samples by using label-free gold nanoparticles and polythymine. Analyst 2015, 140, 3064–3069. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Marty, J.-L.; Yang, X. Aptamer-based colorimetric biosensing of Ochratoxin A using unmodified gold nanoparticles indicator. Biosens. Bioelectron. 2011, 26, 2724–2727. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.-P.; Wang, Z.-X.; Zhang, L.; Qiu, J.-D. Label-Free Colorimetric Detection of Arsenite Utilizing G-/T-Rich Oligonucleotides and Unmodified Au Nanoparticles. Chem.-A Eur. J. 2013, 19, 5029–5033. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhan, S.; Wang, F.; He, L.; Zhi, W.; Zhou, P. Cationic polymers and aptamers mediated aggregation of gold nanoparticles for the colorimetric detection of arsenic(iii) in aqueous solution. Chem. Commun. 2012, 48, 4459–4461. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Jiang, X.; Zhang, W.; Chen, G.; Zhao, Y.; Tunio, T.M.; Liu, J.; Lv, Z.; Li, C.; Yang, S. High sensitive rapid visual detection of sulfadimethoxine by label-freeaptasensor. Biosens. Bioelectron. 2013, 42, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Song, K.-M.; Jeong, E.; Jeon, W.; Cho, M.; Ban, C. Aptasensor for ampicillin using gold nanoparticle based dual fluorescence–colorimetric methods. Anal. Bioanal. Chem. 2012, 402, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.H.; Kim, I.A.; Lee, S.J.; Jurng, J.; Gu, M.B. A novel colorimetric aptasensor using gold nanoparticle for a highly sensitive and specific detection of oxytetracycline. Biosens. Bioelectron. 2010, 26, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Wang, J.; Zhang, J.; Li, C.; Tian, Y.; Wang, J. Selection and identification of streptomycin-specific single-stranded DNA aptamers and the application in the detection of streptomycin in honey. Talanta 2013, 108, 109–116. [Google Scholar] [CrossRef]
- Sun, J.; Ge, J.; Liu, W.; Fan, Z.; Zhang, H.; Wang, P. Highly sensitive and selective colorimetric visualization of streptomycin in raw milk using Au nanoparticles supramolecular assembly. Chem. Commun. 2011, 47, 9888–9890. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, R.; Li, L.; Huang, X.; Li, T.; Lu, M.; Xu, D.; Wang, J. Anti-Agglomeration Behavior and Sensing Assay of Chlorsulfuron Based on Acetamiprid-Gold Nanoparticles. Nanomaterials 2018, 8, 499. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, R.; Huang, X.; Li, L.; Liu, N.; Wang, J.; Xu, D. Visual and Colorimetric Sensing of Metsulfuron-Methyl by Exploiting Hydrogen Bond-Induced Anti-Aggregation of Gold Nanoparticles in the Presence of Melamine. Sensors 2018, 18, 1595. [Google Scholar] [CrossRef]
- Jin, W.; Huang, P.; Wei, G.; Cao, Y.; Wu, F. Visualization and quantification of Hg2+ based on anti-aggregation of label-free gold nanoparticles in the presence of 2-mercaptobenzothiazole. Sens. Actuators B-Chem. 2016, 233, 223–229. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Kang, J.; Dong, C.; Chen, N.; Li, X.; Guo, Z.; Wu, A. Detection of herbicide glyphosates based on an anti-aggregation mechanism by using unmodified gold nanoparticles in the presence of Pb2+. Anal. Methods 2017, 9, 2890–2896. [Google Scholar] [CrossRef]
- Huang, P.-C.; Gao, N.; Li, J.-F.; Wu, F.-Y. Colorimetric detection of methionine based on anti-aggregation of gold nanoparticles in the presence of melamine. Sens. Actuators B-Chem. 2018, 255, 2779–2784. [Google Scholar] [CrossRef]
- He, P.; Shen, L.; Liu, R.; Luo, Z.; Li, Z. Direct Detection of β-Agonists by Use of Gold Nanoparticle-Based Colorimetric Assays. Anal. Chem. 2011, 83, 6988–6995. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.-Y.; Zhang, L.-X.; Chen, D.-D.; Lin, K.; Fan, H.-C.; Wang, Y.; Xia, C.-G. Colorimetric detection of melamine based on methanobactin-mediated synthesis of gold nanoparticles. Food Chem. 2015, 174, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Rawat, K.A.; Kailasa, S.K. 4-Amino nicotinic acid mediated synthesis of gold nanoparticles for visual detection of arginine, histidine, methionine and tryptophan. Sens. Actuators B-Chem. 2016, 222, 780–789. [Google Scholar] [CrossRef]



| Type of Food Contaminants | Analytes | Colorimetric Strategy based on AuNPs | LOD | References |
|---|---|---|---|---|
| Heavy metals | Pd2+ | Cross-linking | 4230 nM | [75] |
| Pb2+ | Cross-linking | 1.54 nM | [105] | |
| Cd2+ | Cross-linking | 3.5 nM | [106] | |
| Hg2+ | Cross-linking | 2.8 nM | [106] | |
| Cr3+ | Cross-linking | 10 nM | [107] | |
| As3+ | De-protection | 10 nM | [112] | |
| Hg2+ | Anti-aggregation | 6.0 nM | [121] | |
| Pb2+ | Anti-aggregation | 2.38 nM | [122] | |
| Pesticides residues | Atrazine | Cross-linking | 165 nM | [99] |
| Dithiocarbamate | Cross-linking | 1.05 nM | [100] | |
| Triadimenol | Cross-linking | 182 nM | [101] | |
| Imidacloprid | De-protection | 500 nM | [109] | |
| Cyromazine | De-protection | 12 nM | [110] | |
| Chlorsulfuron | Anti-aggregation | 70 nM | [119] | |
| Metsulfuron methyl | Anti-aggregation | 131 nM | [120] | |
| Veterinary drugs | Clenbuterol; Ractopamine | Cross-linking | 0.23 nM; 0.43 nM | [103] |
| Ractopamine; Salbutamol | Cross-linking | 100 nM | [104] | |
| Sulfadimethoxine | De-protection | 179 nM | [114] | |
| Ampicillin | De-protection | 4.9 nM | [115] | |
| Oxytetracycline | De-protection | 25 nM | [116] | |
| Melamine | Cross-linking | 5.1 nM | [102] | |
| Melamine | Anti-aggregation | 238 nM | [125] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Lu, M.; Huang, X.; Li, T.; Xu, D. Application of Gold-Nanoparticle Colorimetric Sensing to Rapid Food Safety Screening. Sensors 2018, 18, 4166. https://doi.org/10.3390/s18124166
Liu G, Lu M, Huang X, Li T, Xu D. Application of Gold-Nanoparticle Colorimetric Sensing to Rapid Food Safety Screening. Sensors. 2018; 18(12):4166. https://doi.org/10.3390/s18124166
Chicago/Turabian StyleLiu, Guangyang, Meng Lu, Xiaodong Huang, Tengfei Li, and Donghui Xu. 2018. "Application of Gold-Nanoparticle Colorimetric Sensing to Rapid Food Safety Screening" Sensors 18, no. 12: 4166. https://doi.org/10.3390/s18124166




