A Simple Graphene NH3 Gas Sensor via Laser Direct Writing
Abstract
:1. Introduction
2. Experiments
2.1. Gas Sensor Fabrication
2.2. Characterization Techniques
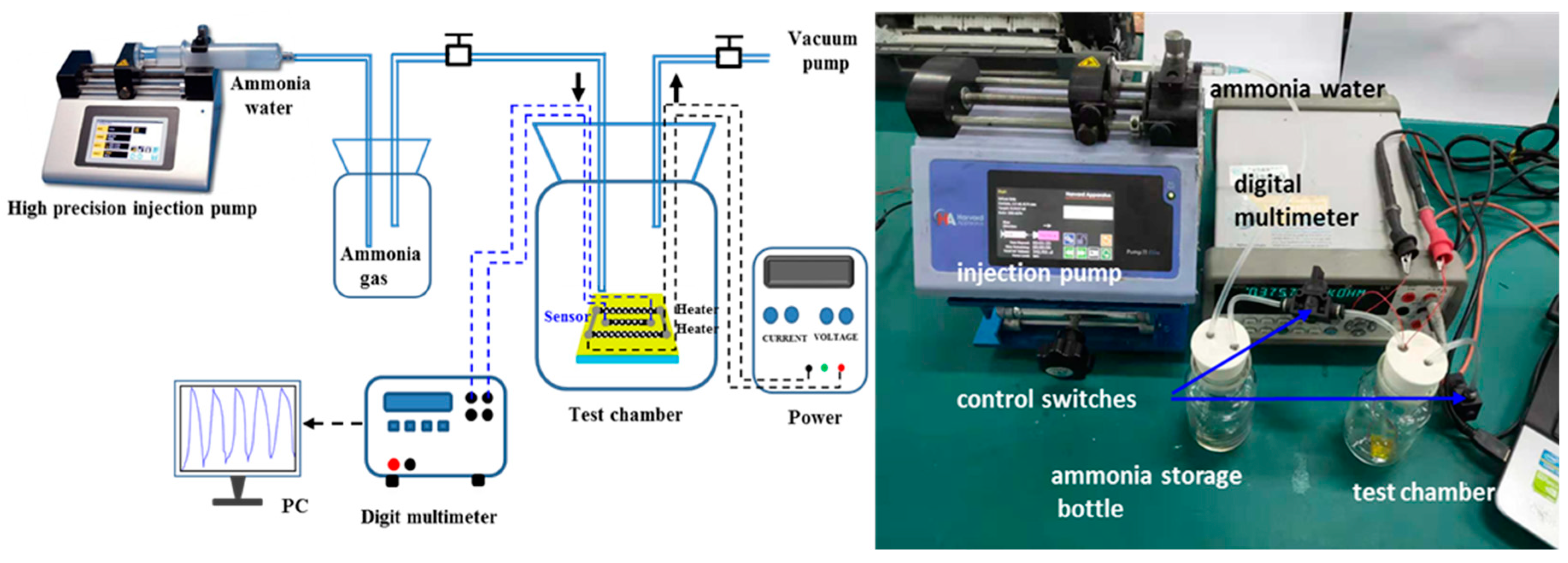
2.3. Tests of the Gas Sensor
3. Results and Discussions
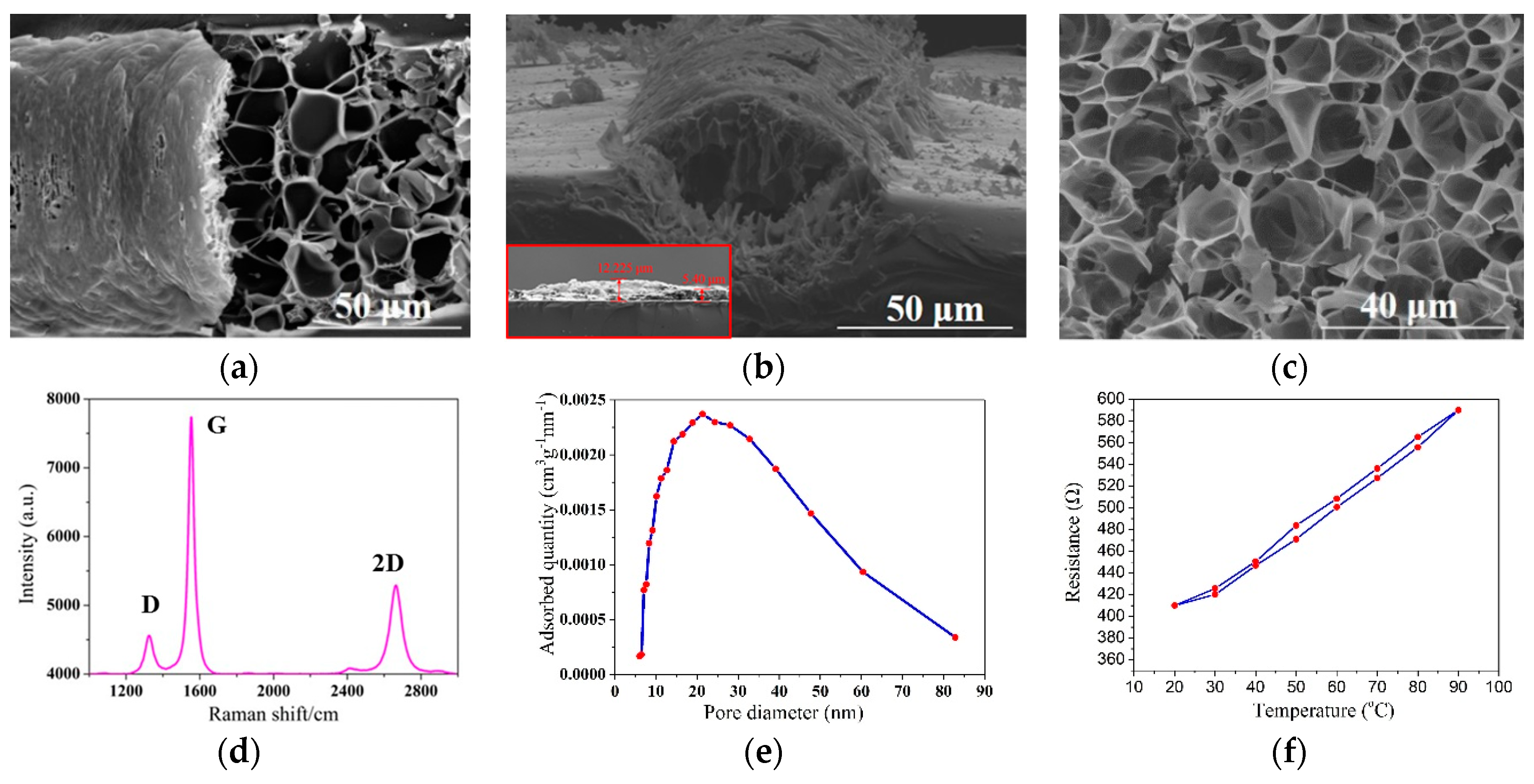
3.1. Structure Characterization
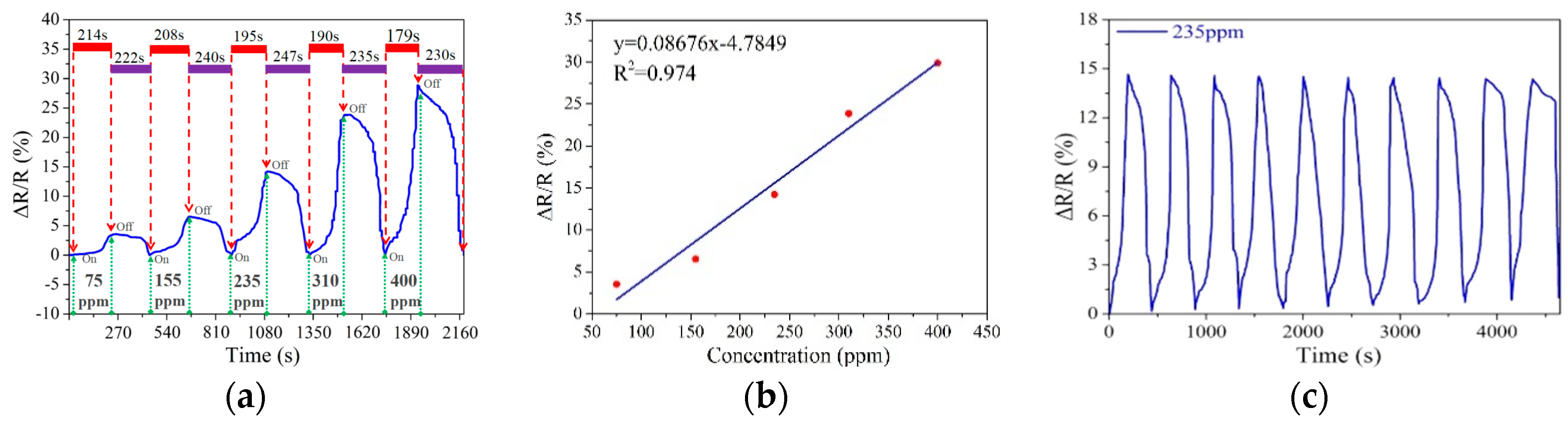
3.2. Response/Recovery Behavior and Its Sensitivity without Heating
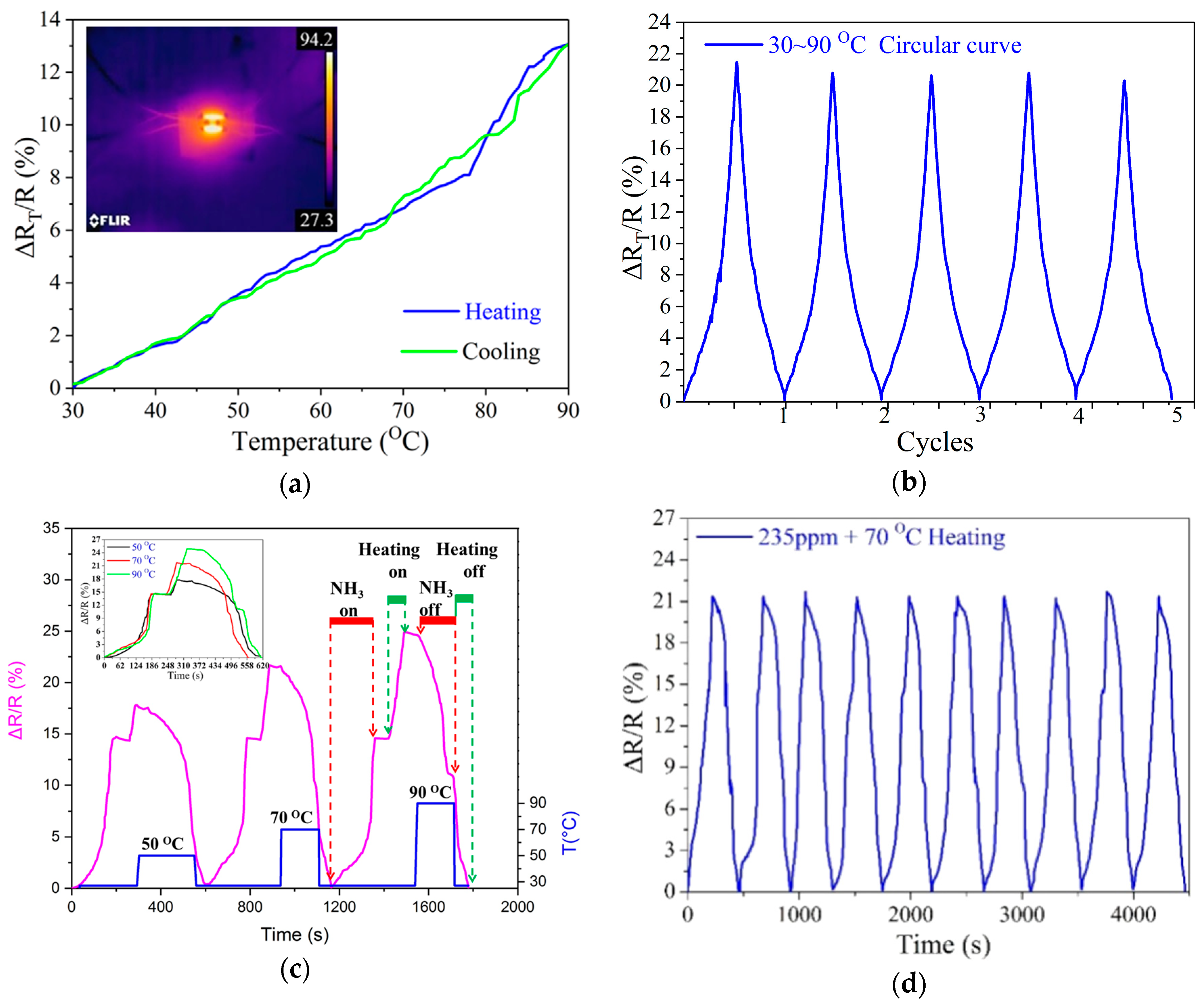
3.3. Ammonia Molecules Desorption of the Sensor with Heating
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brosha, E.L.; Mukundan, R.; Garzon, F.H. YSZ-Based Mixed Potential Sensors for the Detection of Explosives. Electrochem. Solid-State Lett. 2008, 11, J92–J95. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; Berg, A.V.D. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Yoo, K.P.; Kwon, K.H.; Min, N.K.; Lee, M.J.; Lee, C.J. Effects of O2 plasma treatment on NH3 sensing characteristics of multiwall carbon nanotube/polyaniline composite films. Sens. Actuators B Chem. 2009, 143, 333–340. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef] [PubMed]
- Carquigny, S.; Sanchez, J.B.; Berger, F.; Lakard, B.; Lallemand, F. Ammonia gas sensor based on electrosynthesized polypyrrole films. Talanta 2009, 78, 199–206. [Google Scholar] [CrossRef]
- Joshi, A.; Gangal, S.A.; Gupta, S.K. Ammonia sensing properties of polypyrrole thin films at room temperature. Sens. Actuators B Chem. 2011, 156, 938–942. [Google Scholar] [CrossRef]
- Kumar, L.; Rawal, I.; Kaur, A.; Annapoorni, S. Flexible room temperature ammonia sensor based on polyaniline. Sens. Actuators B Chem. 2017, 240, 408–416. [Google Scholar] [CrossRef]
- Akbari, E.; Buntat, Z.; Ahmad, M.H.; Enzevaee, A.; Yousof, R.; Iqbal, S.M.; Ahmadi, M.T.; Sidik, M.A.; Karimi, H. Analytical calculation of sensing parameters on carbon nanotube based gas sensors. Sensors 2014, 14, 5502–5515. [Google Scholar] [CrossRef]
- Guo, P.; Pan, H. Selectivity of Ti-doped In2O3 ceramics as an ammonia sensor. Sens. Actuators B Chem. 2006, 114, 762–767. [Google Scholar] [CrossRef]
- Hieu, N.V.; Le, D.T.T.; Khoang, N.D.; Quy, N.V.; Hoa, N.D.; Tam, P.D.; Le, A.T.; Trung, T. A comparative study on the NH3 gas-sensing properties of ZnO, SnO2, and WO3 nanowires. Int. J. Nanotechnol. 2011, 8, 174–187. [Google Scholar] [CrossRef]
- Ivanov, P.; Hubalek, J.; Malysz, K.; Prášek, J.; Vilanova, X.; Llobet, E.; Correig, X. A route toward more selective and less humidity sensitive screen-printed SnO and WO gas sensitive layers. Sens. Actuators B Chem. 2004, 100, 221–227. [Google Scholar] [CrossRef]
- Shimizu, Y.; Okamoto, T.; Takao, Y.; Egashira, M. Desorption behavior of ammonia from TiO2-based specimens—Ammonia sensing mechanism of double-layer sensors with TiO2-based catalyst layers. J. Mol. Catal. A Chem. 2000, 155, 183–191. [Google Scholar] [CrossRef]
- Hu, N.; Yang, Z.; Wang, Y.; Zhang, L.; Wang, Y.; Huang, X.; Wei, H.; Wei, L.; Zhang, Y. Ultrafast and sensitive room temperature NH3 gas sensors based on chemically reduced graphene oxide. Nanotechnology 2014, 25, 025502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhang, Q.; Xie, G.; Su, Y.; Zhao, K.; Du, H.; Jiang, Y. Gas sensors based on polyaniline/zinc oxide hybrid film for ammonia detection at room temperature. Chem. Phys. Lett. 2016, 665, 147–152. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, P.; Wang, D.; Luo, Y.; Li, M.; Lee, H.; Gerhardt, R.A. A novel paper-based flexible ammonia gas sensor via silver and SWNT-PABS inkjet printing. Sens. Actuators B Chem. 2014, 197, 308–313. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly Sensitive, Room Temperature Gas Sensor based on Polyaniline-Multiwalled Carbon Nanotubes (PANI/MWCNTs) Nanocomposite for Trace-Level Ammonia Detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Khuspe, G.D.; Navale, S.T.; Chougule, M.A.; Patil, V.B. Ammonia gas sensing properties of CSA doped PANi-SnO2 nanohybrid thin films. Synth. Met. 2013, 185–186, 1–8. [Google Scholar] [CrossRef]
- Singh, A.; Salmi, Z.; Joshi, N.; Jha, P.; Kumar, A.; Lecoq, H.; Lau, S.; Chehimi, M.M.; Aswal, D.K.; Gupta, S.K. Photo-induced synthesis of polypyrrole-silver nanocomposite films on N-(3-trimethoxysilylpropyl)pyrrole-modified biaxially oriented polyethylene terephthalate flexible substrates. RSC Adv. 2013, 3, 5506–5523. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, A.; Kumar, A.; Samanta, S.; Joshi, N.; Balouria, V.; Debnath, A.K.; Prasad, R.; Salmi, Z.; Chehimi, M.M. Bending stress induced improved chemiresistive gas sensing characteristics of flexible cobalt-phthalocyanine thin films. Appl. Phys. Lett. 2013, 102, 132107. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Mikhaylov, S.; Ogurtsov, N.; Noskov, Y.; Redon, N.; Coddeville, P.; Wojkiewicz, J.L.; Pud, A. Ammonia/amine electronic gas sensors based on hybrid polyaniline-TiO2 nanocomposites. The effects of titania and the surface active doping acid. RSC Adv. 2015, 5, 20218–20226. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, X.; Xu, E.; Tong, C.; Wu, J. Optical ammonia gas sensor based on a porous silicon rugate filter coated with polymer-supported dye. Anal. Chim. Acta 2011, 685, 58–64. [Google Scholar] [CrossRef]
- Gomes, T.C.; Constantino, C.J.L.; Lopes, E.M.; Job, A.E.; Alves, N. Thermal inkjet printing of polyaniline on paper. Thin Solid Films 2012, 520, 7200–7204. [Google Scholar] [CrossRef]
- Zhang, T.; Mubeen, S.; Bekyarova, E.; Yoo, B.Y.; Haddon, R.C.; Myung, N.; Deshusses, M.A. Poly(m-aminobenzene sulfonic acid) functionalized single-walled carbon nanotubes based gas sensor. Nanotechnology 2007, 18, 165504–165506. [Google Scholar] [CrossRef]
- Gong, J.; Li, Y.; Hu, Z.; Zhou, Z.; Deng, Y. Ultrasensitive NH3 Gas Sensor from Polyaniline Nanograin Enchased TiO2 Fibers. J. Phys. Chem. C 2010, 114, 9970–9974. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon Nanotube Sensors for Gas and Organic Vapor Detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Gautam, M.; Jayatissa, A.H. Adsorption kinetics of ammonia sensing by graphene films decorated with platinum nanoparticles. J. Appl. Phys. 2012, 111, 094317. [Google Scholar] [CrossRef]
- Yang, F.; Taggart, D.K.; Penner, R.M. Joule heating a palladium nanowire sensor for accelerated response and recovery to hydrogen gas. Small 2010, 6, 1422–1429. [Google Scholar] [CrossRef]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-Dimensional Nanostructured Materials for Gas Sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar] [CrossRef]
- Yavari, F.; Chen, Z.; Thomas, A.V.; Ren, W.; Cheng, H.M.; Koratkar, N. High Sensitivity Gas Detection Using a Macroscopic Three-Dimensional Graphene Foam Network. Sci. Rep. 2011, 1, 166. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Zhang, X.; Huang, B.; Zhao, Y.; Cheng, C.; Chen, H. High-Performance Wireless Ammonia Gas Sensors Based on Reduced Graphene Oxide and Nano-Silver Ink Hybrid Material Loaded on a Patch Antenna. Sensors 2017, 17, 2070. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.; Kurra, N.; Xia, C.; Alshareef, H.N. Highly Efficient Laser Scribed Graphene Electrodes for On-Chip Electrochemical Sensing Applications. Adv. Electron. Mater. 2016, 2, 1600185. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.Q.; Tian, H.; Liu, Y.; Ju, Z.Y.; Pang, Y.; Chen, Y.Q.; Wang, D.Y.; Tian, X.G.; Yan, J.C.; Deng, N.Q. An intelligent artificial throat with sound-sensing ability based on laser induced graphene. Nat. Commun. 2017, 8, 14579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Deng, L.; Mei, X.; Teh, K.S.; Cai, W.; Tan, Q.; Yang, Z.; Wang, L.; Zhao, L.; Luo, G. Direct-write Graphene Resistors on Aromatic Polyimide for Transparent Heating Glass. Sens. Actuators A Phys. 2017, 267, 327–333. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, S.; Lin, L. A versatile gas sensor with selectivity using a single graphene transistor. In Proceedings of the 2015 Transducers—18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 961–964. [Google Scholar]
- Mani, G.K.; Rayappan, J.B.B. A highly selective room temperature ammonia sensor using spray deposited zinc oxide thin film. Sens. Actuators B Chem. 2013, 183, 459–466. [Google Scholar] [CrossRef]
- Teerapanich, P.; Myint, M.T.Z.; Joseph, C.M.; Hornyak, G.L.; Dutta, J. Development and Improvement of Carbon Nanotube-Based Ammonia Gas Sensors Using Ink-Jet Printed Interdigitated Electrodes. IEEE Trans. Nanotechnol. 2013, 12, 255–262. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially Resolved Raman Spectroscopy of Single- and Few-Layer Graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ding, P.; Hu, R.; Zhang, J.; Ma, X.; Luo, Z.; Li, G. A Dibutyl Phthalate Sensor Based on a Nanofiber Polyaniline Coated Quartz Crystal Monitor. Sensors 2013, 13, 3765–3775. [Google Scholar] [CrossRef] [Green Version]
- Gautam, M.; Jayatissa, A.H. Ammonia gas sensing behavior of graphene surface decorated with gold nanoparticles. Solid-State Electron. 2012, 78, 159–165. [Google Scholar] [CrossRef]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Peng, Q.; Wu, S.; Wang, G.; Deng, L.; Tai, H.; Wang, L.; Yang, Y.; Dong, L.; Zhao, Y.; et al. A Simple Graphene NH3 Gas Sensor via Laser Direct Writing. Sensors 2018, 18, 4405. https://doi.org/10.3390/s18124405
Wu D, Peng Q, Wu S, Wang G, Deng L, Tai H, Wang L, Yang Y, Dong L, Zhao Y, et al. A Simple Graphene NH3 Gas Sensor via Laser Direct Writing. Sensors. 2018; 18(12):4405. https://doi.org/10.3390/s18124405
Chicago/Turabian StyleWu, Dezhi, Qianqian Peng, Shan Wu, Guangshun Wang, Lei Deng, Huiling Tai, Lingyun Wang, Yajie Yang, Linxi Dong, Yang Zhao, and et al. 2018. "A Simple Graphene NH3 Gas Sensor via Laser Direct Writing" Sensors 18, no. 12: 4405. https://doi.org/10.3390/s18124405




