Research on the Effects of Drying Temperature on Nitrogen Detection of Different Soil Types by Near Infrared Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Sample Preparation
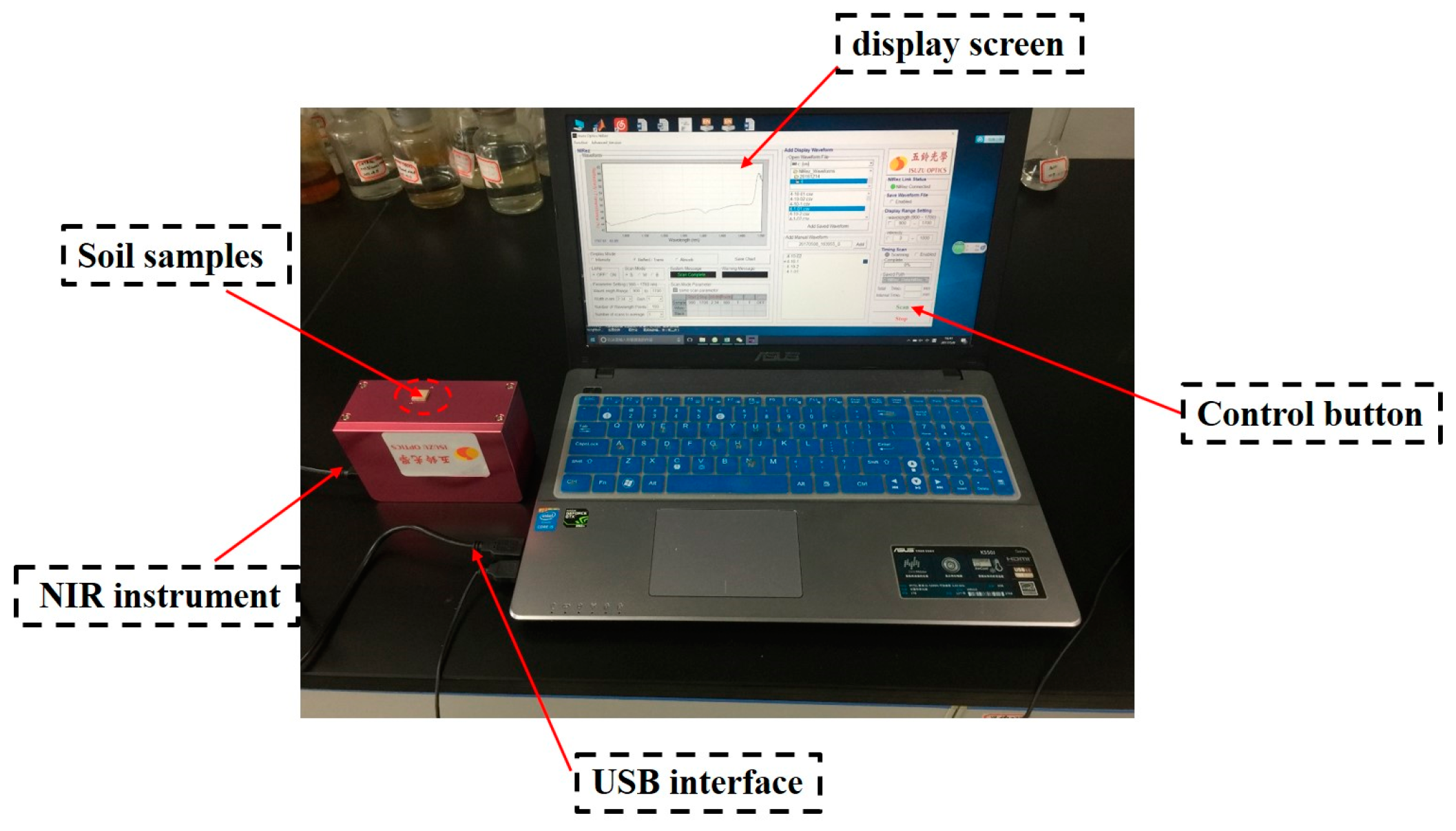
2.2. Spectrometric Determination
2.3. Data Analysis
2.4. Spectral Preprocessing Method
2.5. SPXY Method
2.6. Modeling Method
2.6.1. Partial Least Squares Method
2.6.2. Successive Projections Algorithm-Multiple Linear Regression (SPA-MLR)
2.6.3. Competitive Adaptive Weighting Method (CARS)
2.7. Model Evaluation Index
3. Results and Discussion
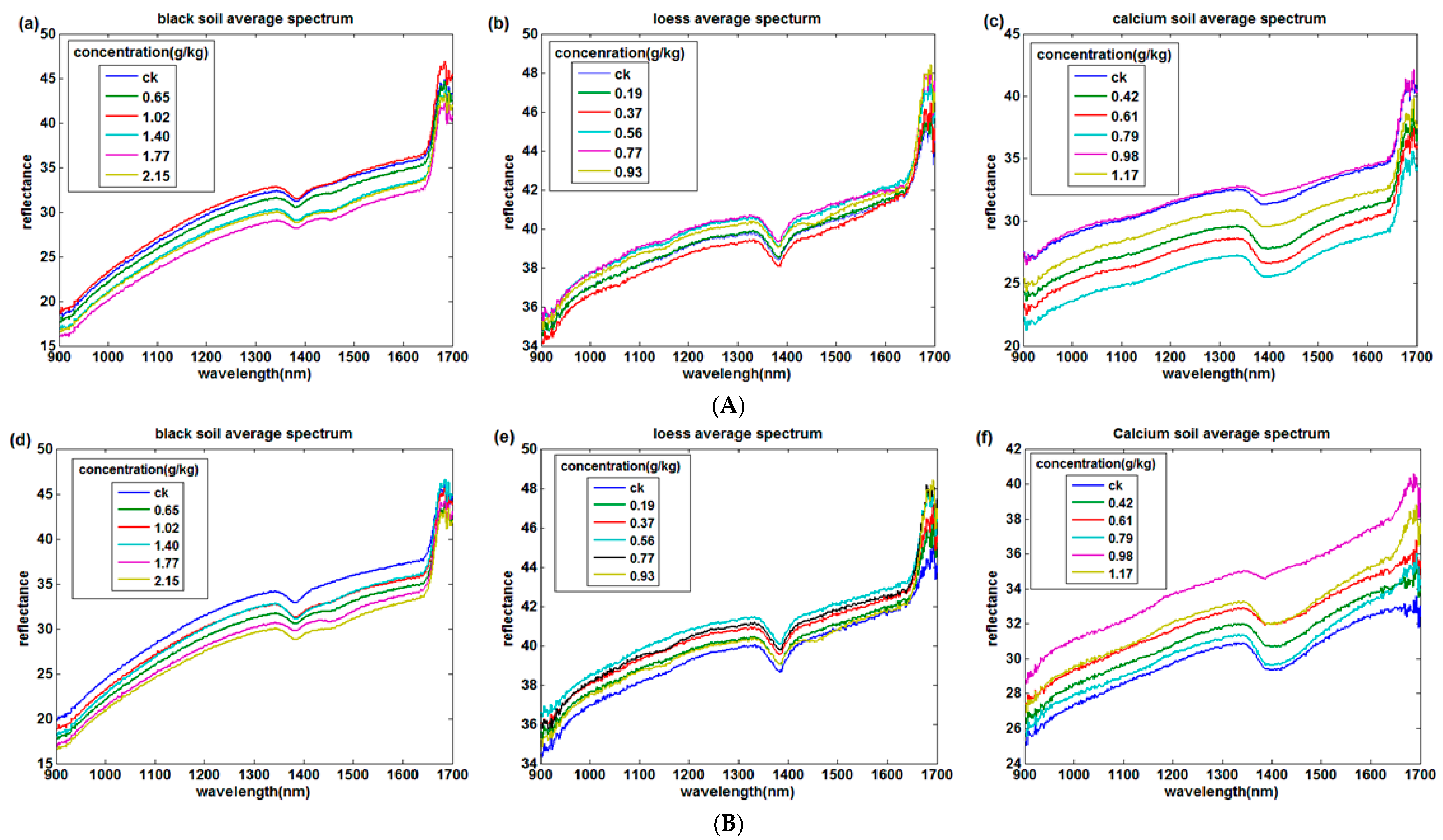
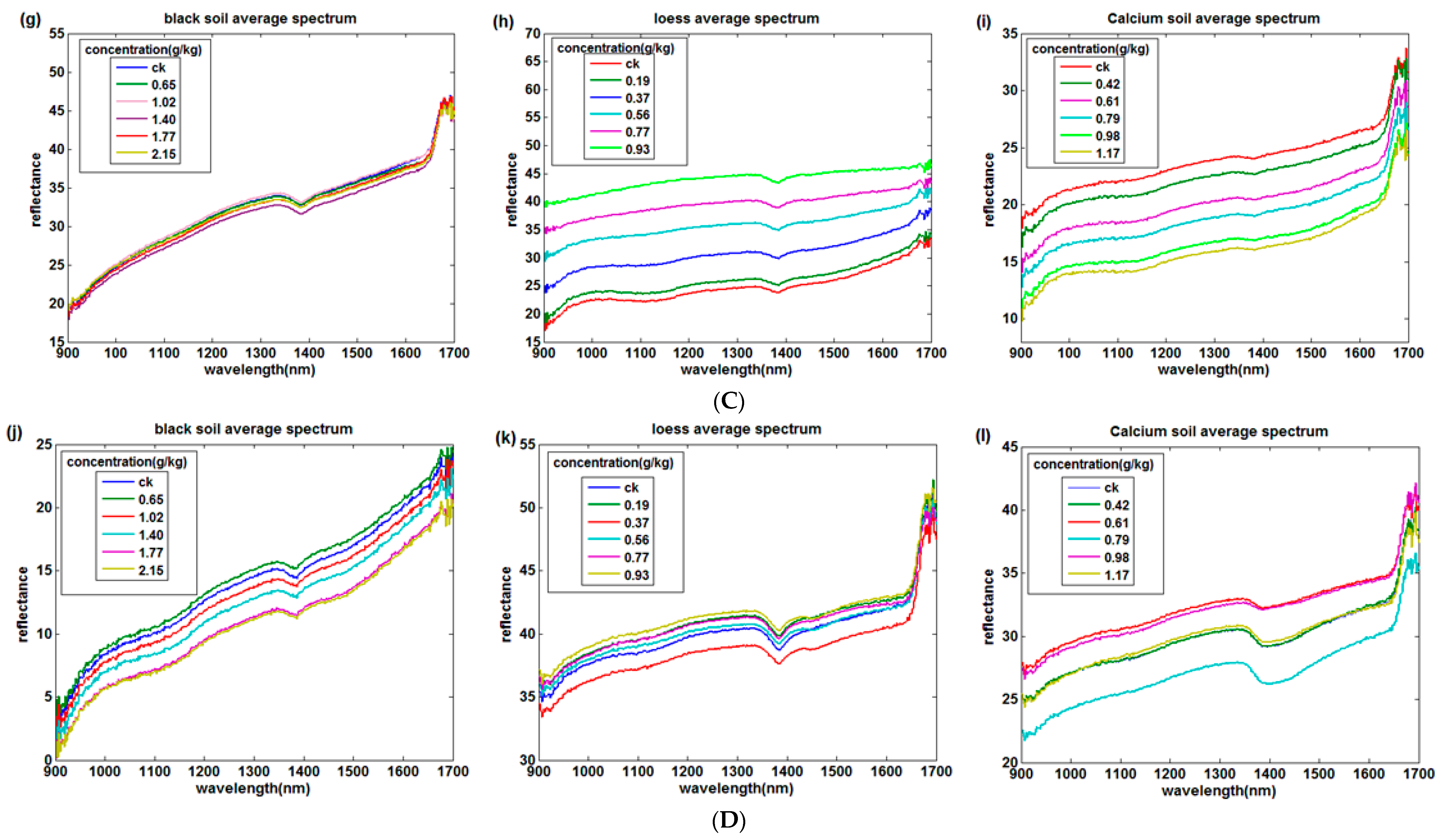
3.1. Temperature and Soil Reflectance
3.2. Data Modeling Prediction and Analysis
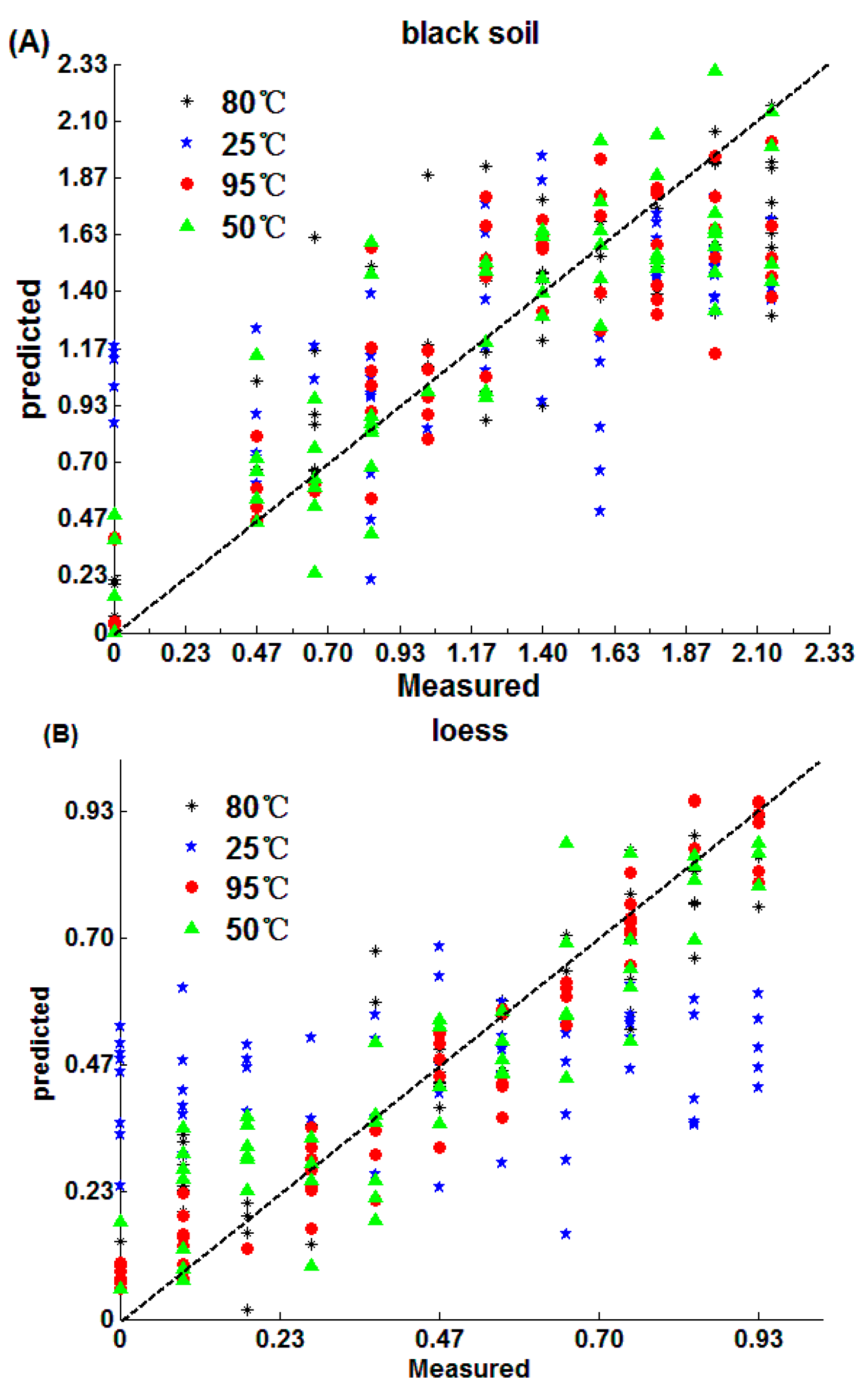
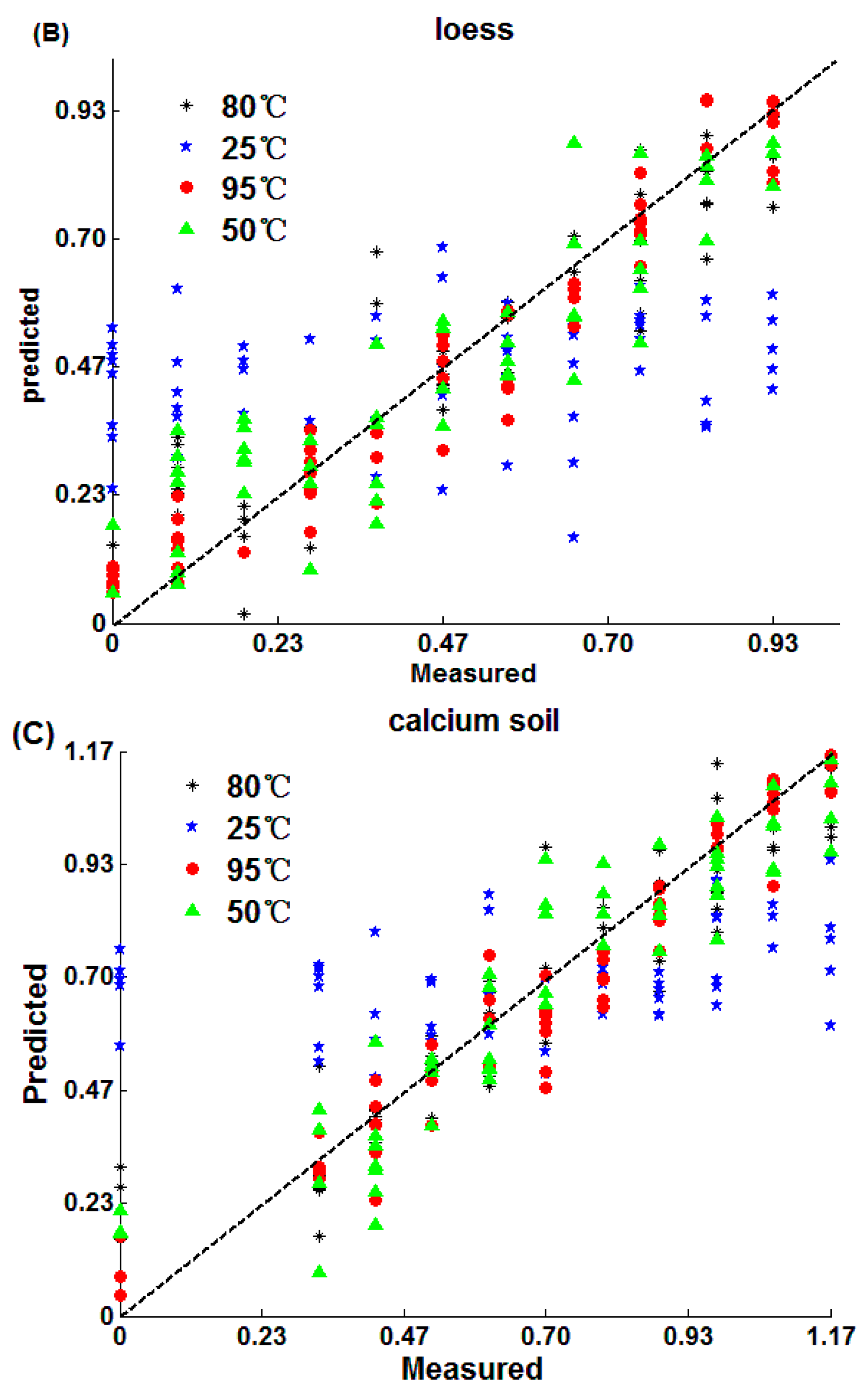
3.2.1. SPA-MLR Model
3.2.2. PLS Method Model
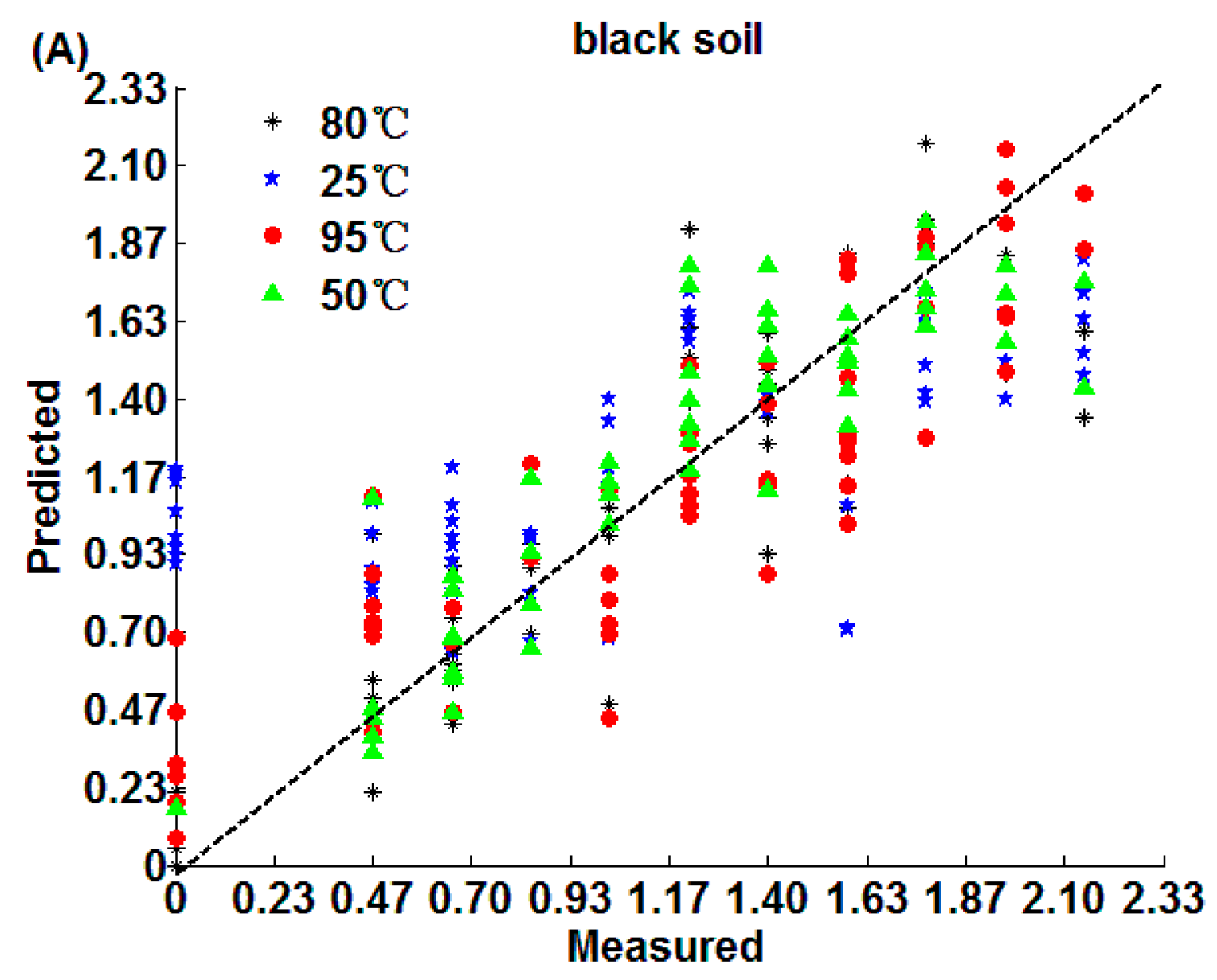
3.2.3. CARS Model Methods
3.3. Analysis and Comparison of Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Todorova, M.; Atanassova, S.; Lange, H.; Pavlov, D. Estimation of total N, total P, pH and electrical conductivity in soil by near-infrared reflectance sensors. Agric. Sci. Technol. 2011, 3, 50–54. [Google Scholar]
- Muñozhuerta, R.F.; Guevaragonzalez, R.G.; Contrerasmedina, L.M.; Torrespacheco, I.; Pradoolivarez, J.; Ocampovelazquez, R.V. A Review of Methods for Sensing the Nitrogen Status in Plants: Advantages, Disadvantages and Recent Advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.D.; Xiong, S.S.; Liu, D.L. Applications of near infrared reflectance sensors technique (NIRS) to soil attributes research. Sens. Spectr. Anal. 2014, 34, 2639–2644. [Google Scholar]
- Bevilacqua, M.; Bucci, R.; Materazzi, S.; Marini, F. Application of near infrared (NIR) sensors coupled to chemometrics for dried egg-pasta characterization and egg content quantification. Food Chem. 2013, 140, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Filippi, J.J.; Belhassen, E.; Baldovini, N.; Brevard, H.; Meierhenrich, U.J. Qualitative and quantitative analysis of vetiver essential oils by comprehensive two-dimensional gas chromatography and comprehensive two-dimensional gas chromatography/mass spectrometry. J. Chromatogr. A 2013, 1288, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.C. Simultaneous determination of moisture, organic carbon, and total nitrogen by near infrared reflectance spectrophotometry. Soil Sci. Soc. Am. J. 1986, 50, 120–123. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, D.H.; Sudduth, K.A.; Chung, S.O.; Kitchen, N.R.; Drummond, S.T. Wavelength identification and diffuse reflectance estimation for surface and profile soil properties. Trans. ASABE 2009, 52, 683–695. [Google Scholar] [CrossRef]
- Cen, Y.L.; Song, T.; He, Y.; Bao, Y.D. Rapid detection method of soil organic matter contents using visible/near infrared diffuse reflectance spectral data. J. Zhejiang Univ. 2011, 37, 300–306. [Google Scholar]
- He, Y.; Huang, M.; García, A.; Hernández, A.; Song, H. Prediction of soil macronutrients content using near-infrared sensors. Comput. Electron. Agric. 2007, 58, 144–153. [Google Scholar] [CrossRef]
- Stoner, E.R.; Baumgardner, M.F. Characteristic variations in reflectance of surface soils. Soil Sci. Soc. Am. J. 1981, 45, 1161–1165. [Google Scholar] [CrossRef]
- Ramirez-Lopez, L.; Behrens, T.; Schmidt, K.; Stevens, A.; Demattê, J.A.M.; Scholten, T. The spectrum-based learner: A new local approach for modeling soil vis–NIR spectra of complex datasets. Geoderma 2013, 195–196, 268–279. [Google Scholar] [CrossRef]
- Brunet, D.; Barthès, B.G.; Chotte, J.L.; Feller, C. Determination of carbon and nitrogen contents in alfisols, oxisols and ultisols from africa and brazil using NIRS analysis: Effects of sample grinding and set heterogeneity. Geoderma 2007, 139, 106–117. [Google Scholar] [CrossRef]
- Barthès, B.; Brunet, D.; Ferrer, H.; Chotte, J.L.; Feller, C. Determination of total carbon and nitrogen content in a range of tropical soils using near infrared sensors: Influence of replication and sample grinding and drying. J. Near Infrared Sens. 2006, 14, 341–348. [Google Scholar] [CrossRef]
- Hermamez, A.H.; He, Y.; Pereira, A.G. Non-destructive measurement of acidity, soluble solids and firmness of satsuma mandarin using Vis/NIR-sensors techniques. J. Food Eng. 2006, 77, 313–319. [Google Scholar]
- Nie, P.C.; Dong, T.; He, Y.; Qu, F.F. Detection of soil nitrogen using near infrared sensors based on soil pretreatment and algorithms. Sensors 2017, 17, 1102. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Morón, A. Potential of near-infrared reflectance sensors and chemometrics to predict soil organic carbon fractions. Soil Tillage Res. 2006, 85, 78–85. [Google Scholar] [CrossRef]
- Nocita, M.; Kooistra, L.; Bachmann, M.; Müller, A.; Powell, M.; Weel, S. Predictions of soil surface and topsoil organic carbon content through the use of laboratory and field sensors in the Albany Thicket Biome of Eastern Cape Province of South Africa. Geoderma 2011, 167, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Nocita, M.; Stevens, A.; Noon, C.; Wesemael, B.V. Prediction of soil organic carbon for different levels of soil moisture using Vis-NIR sensors. Geoderma 2013, 199, 37–42. [Google Scholar] [CrossRef]
- Nocita, M.; Stevens, A.; Toth, G.; Panagos, P.; Wesemael, B.V.; Montanarella, L. Prediction of soil organic carbon content by diffuse reflectance sensors using a local partial least square regression approach. Soil Biol. Biochem. 2014, 68, 337–347. [Google Scholar] [CrossRef]
- Chen, S.K.; Subler, S.; Edwards, C.A. Effects of agricultural biostimulants on soil microbial activity and nitrogen dynamics. Appl. Soil Ecol. 2002, 19, 249–259. [Google Scholar] [CrossRef]
- Chen, S.K.; Edwards, C.A.; Subler, S. The influence of two agricultural biostimulants on nitrogen transformations, microbial activity, and plant growth in soil microcosms. Soil Biol. Biochem. 2003, 35, 9–19. [Google Scholar] [CrossRef]
- Cartes, P.; Jara, A.A.; Demanet, R.; de la Mora, M. Urease activity and nitrogen mineralization kinetics as affected by temperature and urea input rate in Southern Chilean Andisols. J. Soil Sci. Plant Nutr. 2009, 9, 69–82. [Google Scholar] [CrossRef]
- Fystro, G. The prediction of C and N content and their potential mineralisation in heterogeneous soil samples using Vis–NIR spectroscopy and comparative methods. Plant Soil 2002, 246, 139–149. [Google Scholar] [CrossRef]
- Bo, S. Effects of soil sample pretreatments and standardised rewetting as interacted with sand classes on Vis-NIR predictions of clay and soil organic carbon. Geoderma 2010, 158, 15–22. [Google Scholar]
- Mouazen, A.M.; Baerdemaeker, J.D.; Ramon, H. Towards development of on-line soil moisture content sensor using a fibre-type NIR spectrophotometer. Soil Tillage Res. 2005, 80, 171–183. [Google Scholar] [CrossRef]
- Kuang, B.; Mouazen, A.M. Non-biased prediction of soil organic carbon and inorganic nitrogen with Vis–NIR spectroscopy, as affected by soil moisture content and texture. Biosyst. Eng. 2013, 114, 249–258. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Xiao, S.; Nie, P.; Dong, T.; Qu, F.; Lin, L. Research on the optimum water content of detecting soil nitrogen using near infrared sensor. Sensors 2017, 17, 2045. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, J.; Yao, X.; Cao, W.; Zhu, Y. Laboratory assessment of three quantitative methods for estimating the organic matter content of soils in china based on visible/near-infrared reflectance spectra. Geoderma 2013, 202, 161–170. [Google Scholar] [CrossRef]
- Nduwamungu, C.; Ziadi, N.; Tremblay, G.F.; Parent, L.É. Near-infrared reflectance spectroscopy prediction of soil properties: Effects of sample cups and preparation. Soil Sci. Soc. Am. J. 2009, 73, 1896–1903. [Google Scholar] [CrossRef]
- Ntziachristos, V.; Bremer, C.; Weissleder, R. Fluorescence imaging with near-infrared light: New technological advances that enable in vivo molecular imaging. Eur. Radiol. 2003, 13, 195–208. [Google Scholar] [PubMed]
- Sarkhel, S.; Desiraju, G.R. N-H…O, O-H…O, and C-H…O hydrogen bonds in protein-ligand complexes: Strong and weak interactions in molecular recognition. Proteins 2004, 54, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, K.; Valle, B.L. The physical basis of analytical atomic absorption spectrometry. The pertinence of the beer-lambert law. Anal. Chem. 1963, 35, 942–946. [Google Scholar] [CrossRef]
- Zou, X.B.; Zhao, J.W.; Povey, M.J.; Holmes, M.; Mao, H.P. Variables selection methods in near-infrared spectroscopy. Anal. Chim. Acta 2010, 667, 14–32. [Google Scholar]
- Chen, J.; Jönsson, P.; Tamura, M.; Gu, Z.; Matsushita, B.; Eklundh, L. A simple method for reconstructing a high-quality NDVI time-series data set based on the Savitzky–Golay filter. Remote Sens. Environ. 2004, 91, 332–344. [Google Scholar] [CrossRef]
- Lindberg, W.; Persson, J.A.; Wold, S. Partial least-squares method for spectrofluorimetric analysis of mixtures of humic acid and lignin sulfonate. Anal. Chem. 1983, 55, 643–648. [Google Scholar] [CrossRef]
- Zhan, X.R.; Zhu, X.R.; Shi, X.Y.; Zhang, Z.Y.; Qiao, Y.J. Determination of hesperidin in tangerine leaf by near-infrared spectroscopy with SPXY algorithm for sample subset partitioning and Monte Carlo cross validation. Spectrosc. Spectr. Anal. 2009, 29, 964–968. [Google Scholar]
- Araújo, M.C.U.; Saldanha, T.C.B.; Galvão, R.K.H.; Yoneyama, T.; Chame, H.C.; Visani, V. The successive projections algorithm for variable selection in spectroscopic multicomponent analysis. Chemom. Intell. Lab. Syst. 2001, 57, 65–73. [Google Scholar] [CrossRef]
- Soares, S.F.C.; Galvão, R.K.H.; Araújo, M.C.U.; Silva, E.C.D.; Pereira, C.F.; Leite, F.C. A modification of the successive projections algorithm for spectral variable selection in the presence of unknown interferents. Anal. Chim. Acta 2011, 689, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Diniz, P.H.G.D.; Gomes, A.A.; Pistonesi, M.F.; Band, B.S.F.; Araújo, M.C.U.D. Simultaneous classification of teas according to their varieties and geographical origins by using NIR sensors and SPA-LDA. Food Anal. Methods 2014, 7, 1712–1718. [Google Scholar]
- Yun, Y.H.; Wang, W.T.; Deng, B.C.; Lai, G.B.; Liu, X.B.; Ren, D.B.; Xu, Q.S. Using variable combination population analysis for variable selection in multivariate calibration. Anal. Chim. Acta 2015, 862, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Krakowska, B.; Custers, D.; Deconinck, E.; Daszykowski, M. The Monte Carlo validation framework for the discriminant partial least squares model extended with variable selection methods applied to authenticity studies of Viagra® based on chromatographic impurity profiles. Analyst 2016, 141, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- D’Acqui, L.P.; Pucci, A.; Janik, L.J. Soil properties prediction of western mediterranean islands with similar climatic environments by means of mid-infrared diffuse reflectance spectroscopy. Eur. J. Soil Sci. 2010, 61, 865–876. [Google Scholar] [CrossRef]
- Sudduth, K.A.; Hummel, J.W. Soil Organic Matter, CEC, and Moisture Sensing with a Portable NIR Spectrophotometer. Trans. ASAE 1993, 36, 1571–1582. [Google Scholar]
- Libowitzky, E. Correlation of O-H stretching frequencies and O-H…O hydrogen bond lengths in minerals. Korrelation von O-H-Streckfrequenzen und O-R…O-Wasserstoffbrückenlängen in Mineralen. Monatshefte Für Chemie 1999, 130, 1047–1059. [Google Scholar]
- Dalton, D.A.; Evans, H.J.; Hanus, F.J. Stimulation by nickel of soil microbial urease activity and urease and hydrogenase activities in soybeans grown in a low-nickel soil. Plant Soil 1985, 88, 245–258. [Google Scholar] [CrossRef]
- Alahmadi, Y.J.; Gholami, A.; Fridgen, T.D. The protonated and sodiated dimers of proline studied by IRMPD spectroscopy in the N-H and O-H stretching region and computational methods. Phys. Chem. Chem. Phys. 2014, 16, 26855–26863. [Google Scholar] [CrossRef] [PubMed]
- Ketterings, Q.M.; Bigham, J.M. Soil color as an indicator of slash-and-burn fire severity and soil fertility in Sumatra, Indonesia. Soil Sci. Soc. Am. J. 2000, 64, 1826–1833. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Z.; Zhang, B.; Song, K.; Li, X.; Li, J.; Li, F.; Duan, H. Spatial distribution of soil organic carbon and analysis of related factors in croplands of the black soil region, Northeast China. Agric. Ecosyst. Environ. 2006, 113, 73–81. [Google Scholar] [CrossRef]
- Knadel, M.; Deng, F.; Alinejadian, A.; Jonge, L.W.D.; Moldrup, P.; Greve, M.H. The effects of moisture conditions-from wet to hyper dry-on visible near-infrared spectra of Danish reference soils. Soil Sci. Soc. Am. J. 2014, 78, 422–433. [Google Scholar] [CrossRef]
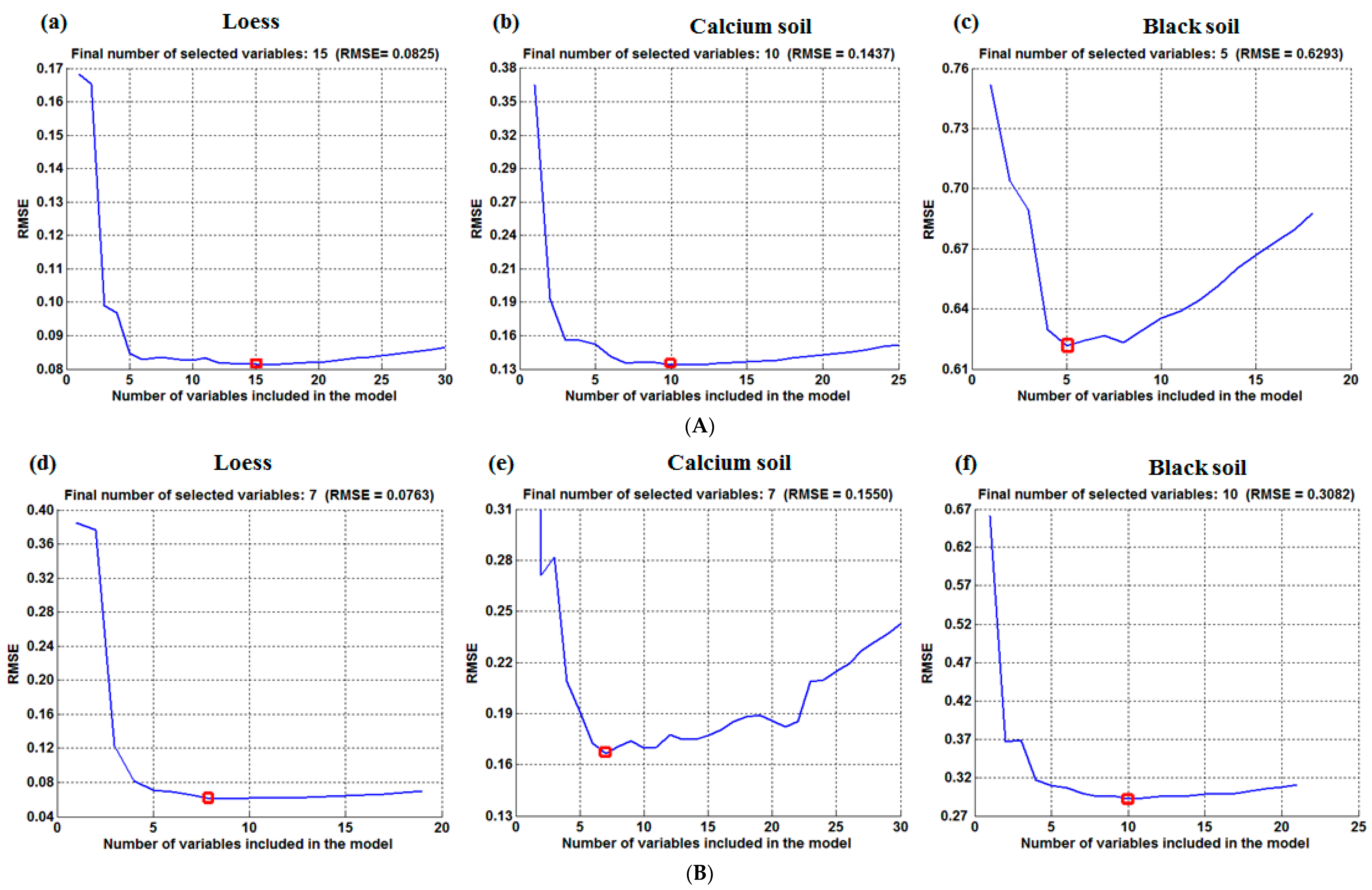
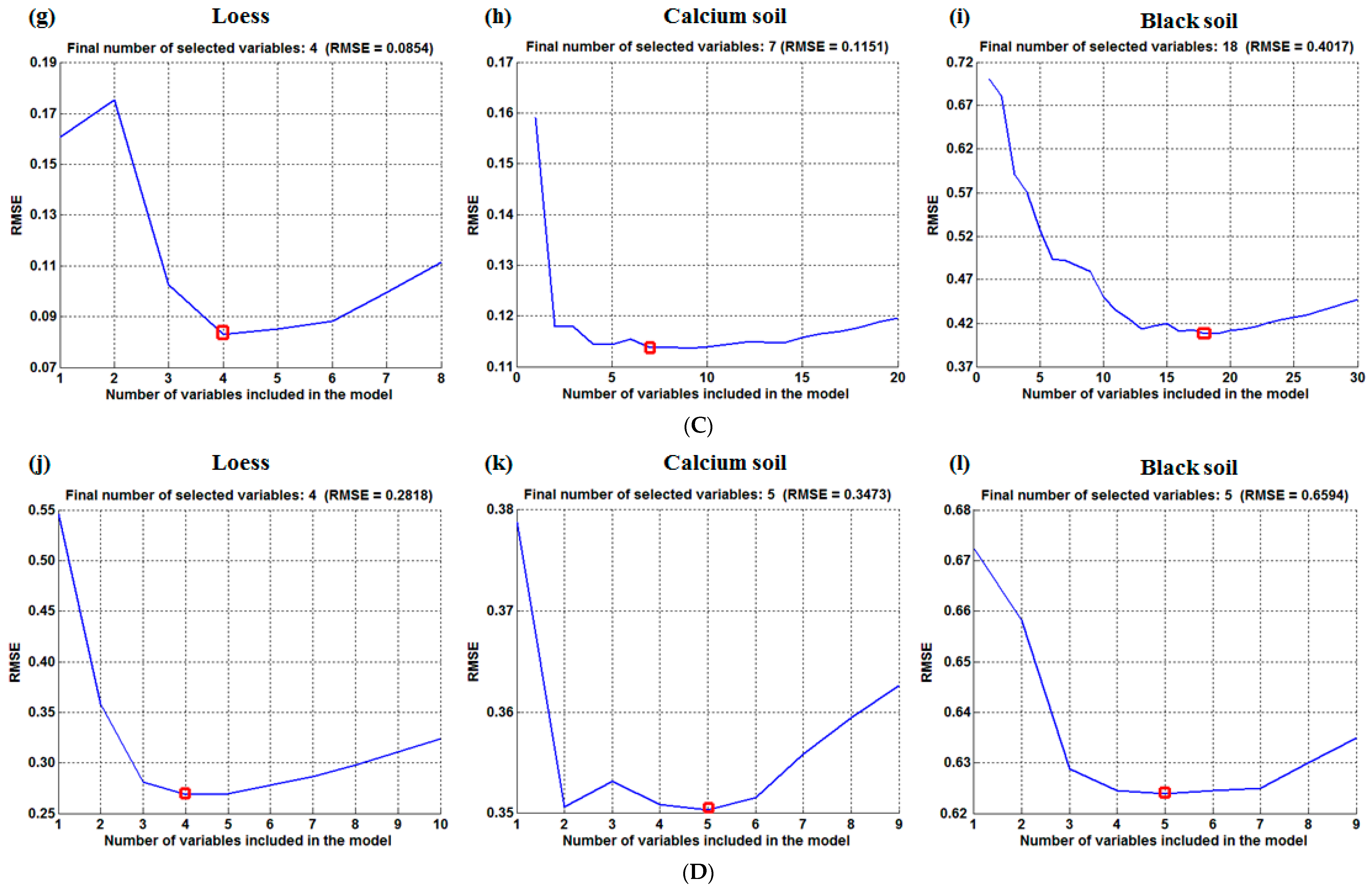


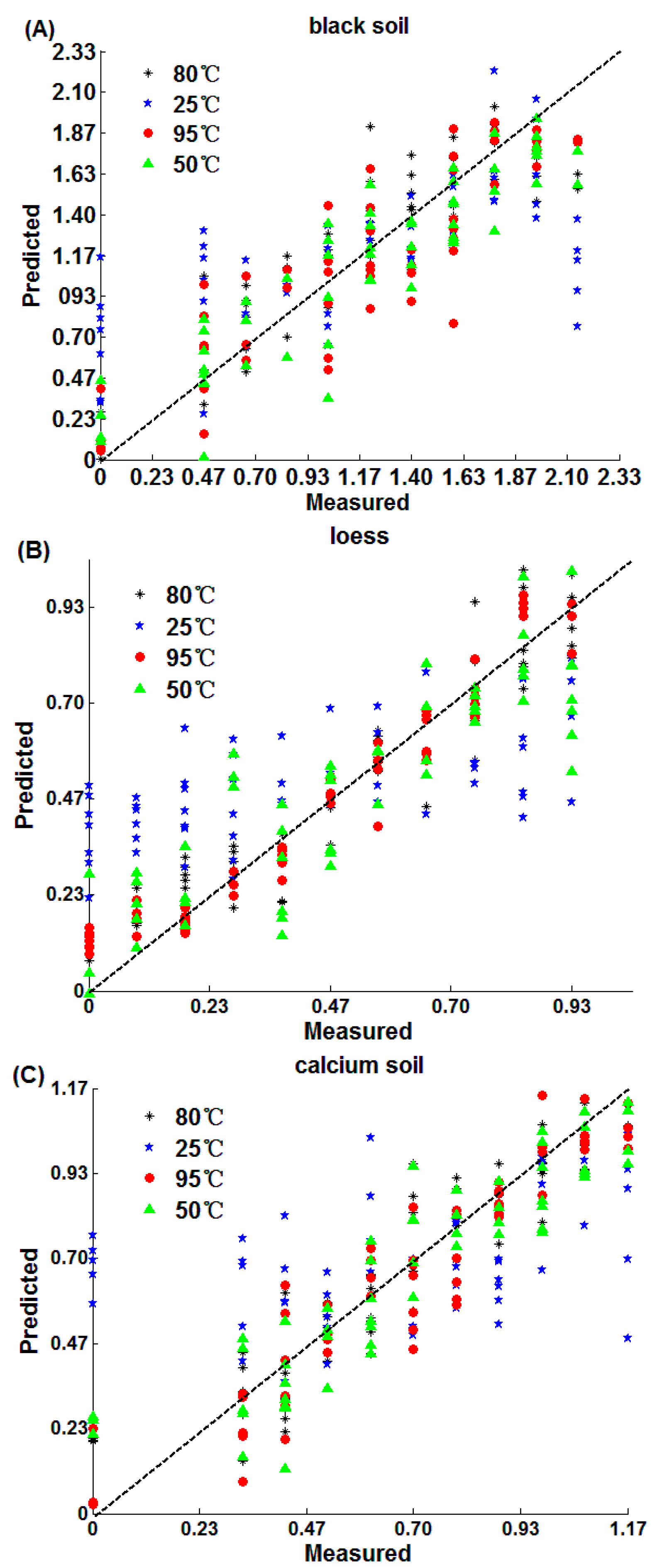
| Soil Type | Temperature | Variable Number | Wavelength (nm) |
|---|---|---|---|
| Loess | 50 °C | 15 | 915, 1428, 1695, 1694, 1693, 1692, 1487, 1550, 1683, 1676, 1673, 1675, 1686, 1582, 1650 |
| 80 °C | 7 | 1160, 1660, 1582, 1682, 1675, 1489,1428 | |
| 95 °C | 4 | 1160, 1428, 1675, 1486 | |
| 25 °C | 4 | 1424, 1488, 1694, 1428 | |
| Calcium soil | 50 °C | 10 | 1651, 1154, 1438, 910,1301, 979, 1450, 1675, 1246, 1677 |
| 80 °C | 7 | 1651, 1675, 1678, 979, 1677, 1058, 1244 | |
| 95 °C | 7 | 1552, 1675, 1487, 1491, 1673, 921, 1650 | |
| 25 °C | 5 | 1651, 1675, 1146, 979, 1167 | |
| Black soil | 50 °C | 5 | 1423, 928, 1654, 1496, 1694 |
| 80 °C | 10 | 928, 1654, 1681, 1682, 1694, 1496, 1423, 915, 1684, 1662 | |
| 95 °C | 18 | 1650, 1680, 1682, 1694, 915, 1684, 1050, 1429, 1491, 1662, 928, 925, 910, 916, 918, 1662, 1675, 1690 | |
| 25 °C | 5 | 1423, 925, 1681, 1496, 1694 |
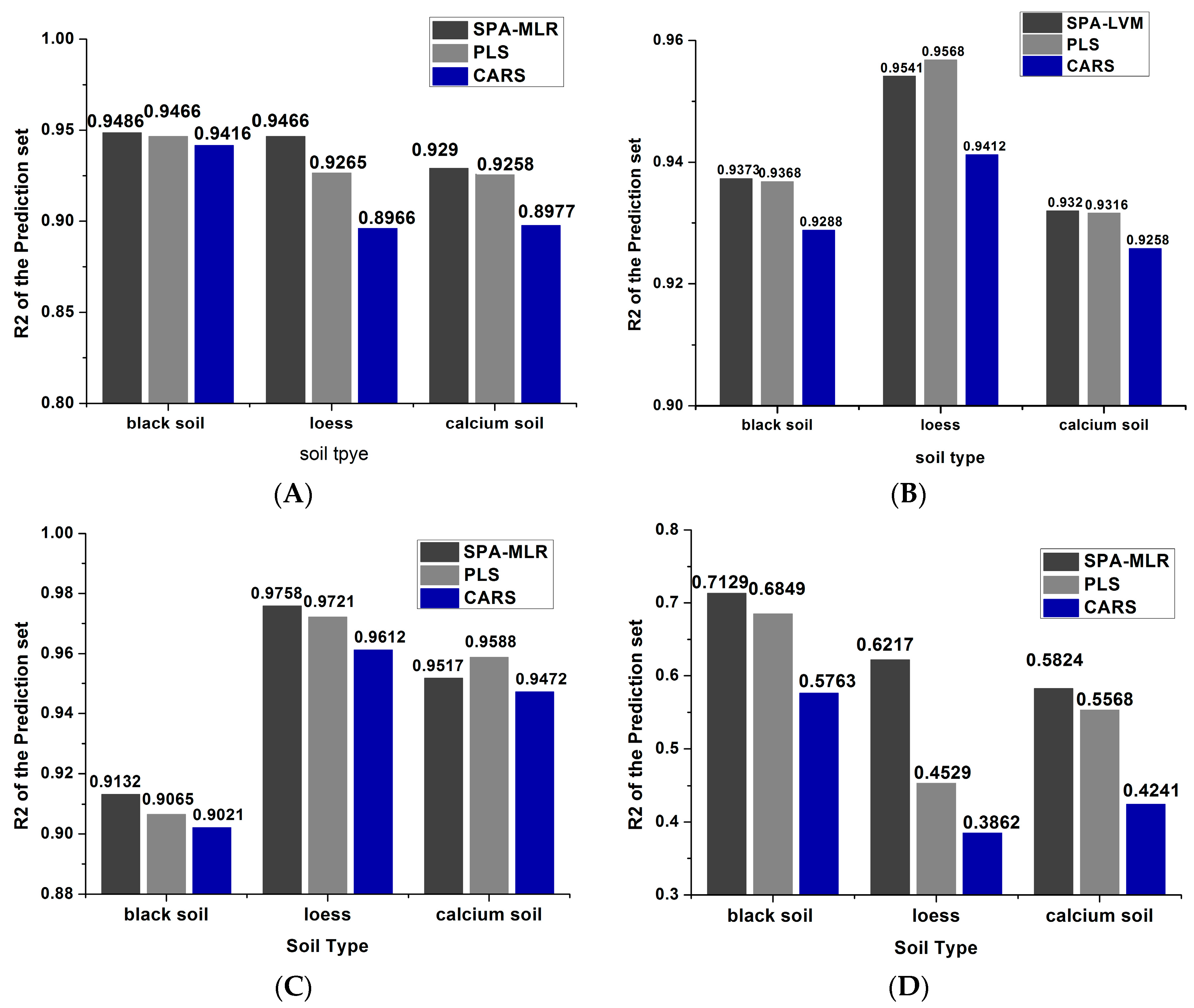
| Group | Soil Type | Calibration Set | Prediction Set | |||||
|---|---|---|---|---|---|---|---|---|
| N1 | Rc | RMSEC (g/kg) | N2 | Rp | RMSEP (g/kg) | RPD | ||
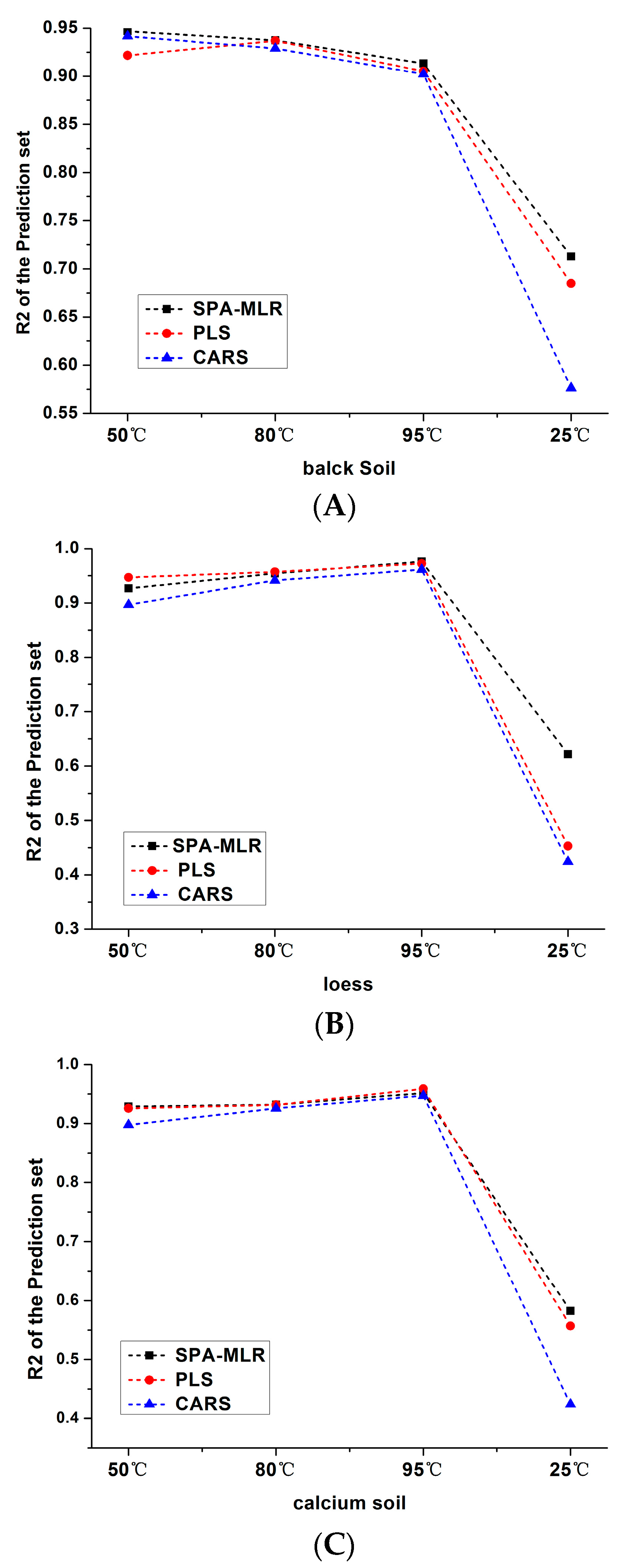
| 1 (50 °C) | Black soil | 118 | 0.9725 | 0.11 | 58 | 0.9486 | 0.22 | 2.82 |
| Loess | 118 | 0.9649 | 0.072 | 58 | 0.9265 | 0.13 | 2.34 | |
| Calcium soil | 118 | 0.9681 | 0.039 | 58 | 0.9290 | 0.120 | 2.63 | |
| 2 (80 °C) | Black soil | 118 | 0.9203 | 0.251 | 58 | 0.9373 | 0.234 | 2.55 |
| Loess | 118 | 0.9727 | 0.067 | 58 | 0.9541 | 0.090 | 3.20 | |
| Calcium soil | 118 | 0.9492 | 0.108 | 58 | 0.9320 | 0.162 | 2.12 | |
| 3 (95 °C) | Black soil | 118 | 0.9692 | 0.156 | 58 | 0.9132 | 0.282 | 2.16 |
| Loess | 118 | 0.9660 | 0.075 | 58 | 0.9758 | 0.070 | 4.35 | |
| Calcium soil | 118 | 0.9670 | 0.087 | 58 | 0.9517 | 0.103 | 3.24 | |
| 4 (25 °C) | Black soil | 118 | 0.6061 | 0.486 | 58 | 0.7129 | 0.418 | 1.25 |
| Loess | 118 | 0.5473 | 0.247 | 58 | 0.6217 | 0.246 | 1.20 | |
| Calcium soil | 118 | 0.4391 | 0.302 | 58 | 0.5824 | 0.365 | 0.94 | |
| Group | Soil Type | Calibration Set | Prediction Set | |||||
|---|---|---|---|---|---|---|---|---|
| N1 | Rc | RMSEC (g/kg) | N2 | Rp | RMSEP (g/kg) | RPD | ||
| 1 (50 °C) | Black soil | 118 | 0.9525 | 0.198 | 58 | 0.9216 | 0.228 | 2.72 |
| Loess | 118 | 0.9609 | 0.077 | 58 | 0.9466 | 0.112 | 2.71 | |
| Calcium soil | 118 | 0.9881 | 0.057 | 58 | 0.9258 | 0.128 | 2.69 | |
| 2 (80 °C) | Black soil | 118 | 0.9417 | 0.216 | 58 | 0.9368 | 0.217 | 2.82 |
| Loess | 118 | 0.9935 | 0.033 | 58 | 0.9568 | 0.090 | 3.31 | |
| Calcium soil | 118 | 0.9173 | 0.132 | 58 | 0.9316 | 0.119 | 2.75 | |
| 3 (95 °C) | Black soil | 118 | 0.9906 | 0.086 | 58 | 0.9065 | 0.273 | 2.22 |
| Loess | 118 | 0.9739 | 0.066 | 58 | 0.9721 | 0.067 | 4.34 | |
| Calcium soil | 118 | 0.9269 | 0.129 | 58 | 0.9588 | 0.094 | 3.89 | |
| 4 (25 °C) | Black soil | 118 | 0.7773 | 0.391 | 58 | 0.6849 | 0.480 | 1.26 |
| Loess | 118 | 0.3507 | 0.267 | 58 | 0.4529 | 0.287 | 1.09 | |
| Calcium soil | 118 | 0.5332 | 0.286 | 58 | 0.5568 | 0.258 | 1.34 | |
| Soil Type | Temperature | Selected Variables Number | Principal Component Number |
|---|---|---|---|
| Loess | 50 °C | 20 | 5 |
| 80 °C | 40 | 6 | |
| 95 °C | 19 | 3 | |
| 25 °C | 29 | 3 | |
| Calcium soil | 50 °C | 18 | 3 |
| 80 °C | 26 | 5 | |
| 95 °C | 20 | 5 | |
| 25 °C | 14 | 3 | |
| Black soil | 50 °C | 21 | 5 |
| 80 °C | 11 | 5 | |
| 95 °C | 42 | 6 | |
| 25 °C | 32 | 5 |
| Group | Soil Type | Calibration Set | Prediction Set | |||||
|---|---|---|---|---|---|---|---|---|
| N1 | Rc | RMSEC (g/kg) | N2 | Rp | RMSEP (g/kg) | RPD | ||
| 1 (50 °C) | Black soil | 118 | 0.9625 | 0.163 | 58 | 0.9416 | 0.185 | 2.95 |
| Loess | 118 | 0.9009 | 0.1875 | 58 | 0.8966 | 0.1885 | 1.61 | |
| Calcium soil | 118 | 0.9281 | 0.1549 | 58 | 0.8977 | 0.1414 | 2.43 | |
| 2 (80 °C) | Black soil | 118 | 0.9205 | 0.25 | 58 | 0.9288 | 0.237 | 2.68 |
| Loess | 118 | 0.93 | 0.106 | 58 | 0.9412 | 0.105 | 2.90 | |
| Calcium soil | 118 | 0.9117 | 0.136 | 58 | 0.9258 | 0.119 | 2.79 | |
| 3 (95 °C) | Black soil | 118 | 0.9731 | 0.146 | 58 | 0.9021 | 0.277 | 2.24 |
| Loess | 118 | 0.9609 | 0.077 | 58 | 0.9612 | 0.079 | 3.92 | |
| Calcium soil | 118 | 0.9381 | 0.118 | 58 | 0.9472 | 0.112 | 3.07 | |
| 4 (25 °C) | Black soil | 118 | 0.5458 | 0.551 | 58 | 0.5763 | 0.476 | 1.31 |
| Loess | 118 | 0.5615 | 0.247 | 58 | 0.3862 | 0.265 | 1.15 | |
| Calcium soil | 118 | 0.3698 | 0.322 | 58 | 0.4241 | 0.304 | 1.13 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, P.; Dong, T.; He, Y.; Xiao, S. Research on the Effects of Drying Temperature on Nitrogen Detection of Different Soil Types by Near Infrared Sensors. Sensors 2018, 18, 391. https://doi.org/10.3390/s18020391
Nie P, Dong T, He Y, Xiao S. Research on the Effects of Drying Temperature on Nitrogen Detection of Different Soil Types by Near Infrared Sensors. Sensors. 2018; 18(2):391. https://doi.org/10.3390/s18020391
Chicago/Turabian StyleNie, Pengcheng, Tao Dong, Yong He, and Shupei Xiao. 2018. "Research on the Effects of Drying Temperature on Nitrogen Detection of Different Soil Types by Near Infrared Sensors" Sensors 18, no. 2: 391. https://doi.org/10.3390/s18020391






