Synthesis of ZnMn2O4 Nanoparticles by a Microwave-Assisted Colloidal Method and their Evaluation as a Gas Sensor of Propane and Carbon Monoxide
Abstract
:1. Introduction
2. Materials and Methods
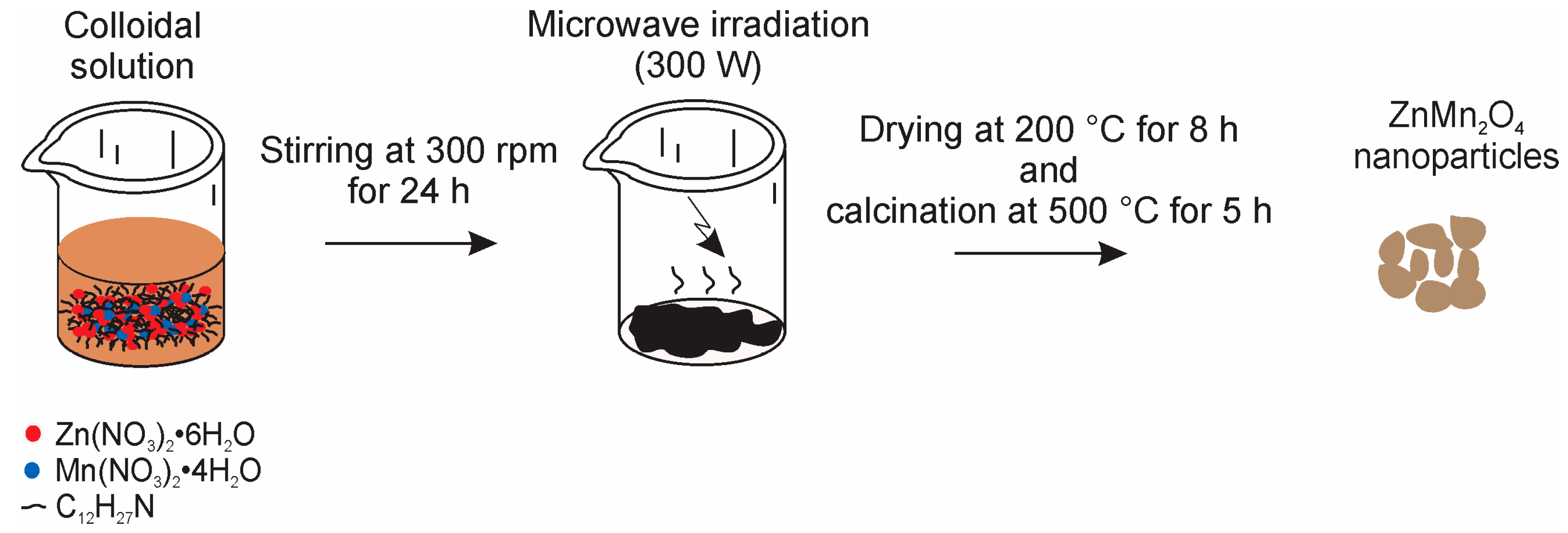
2.1. ZnMn2O4 Synthesis
2.2. Characterization of ZnMn2O4 Powders
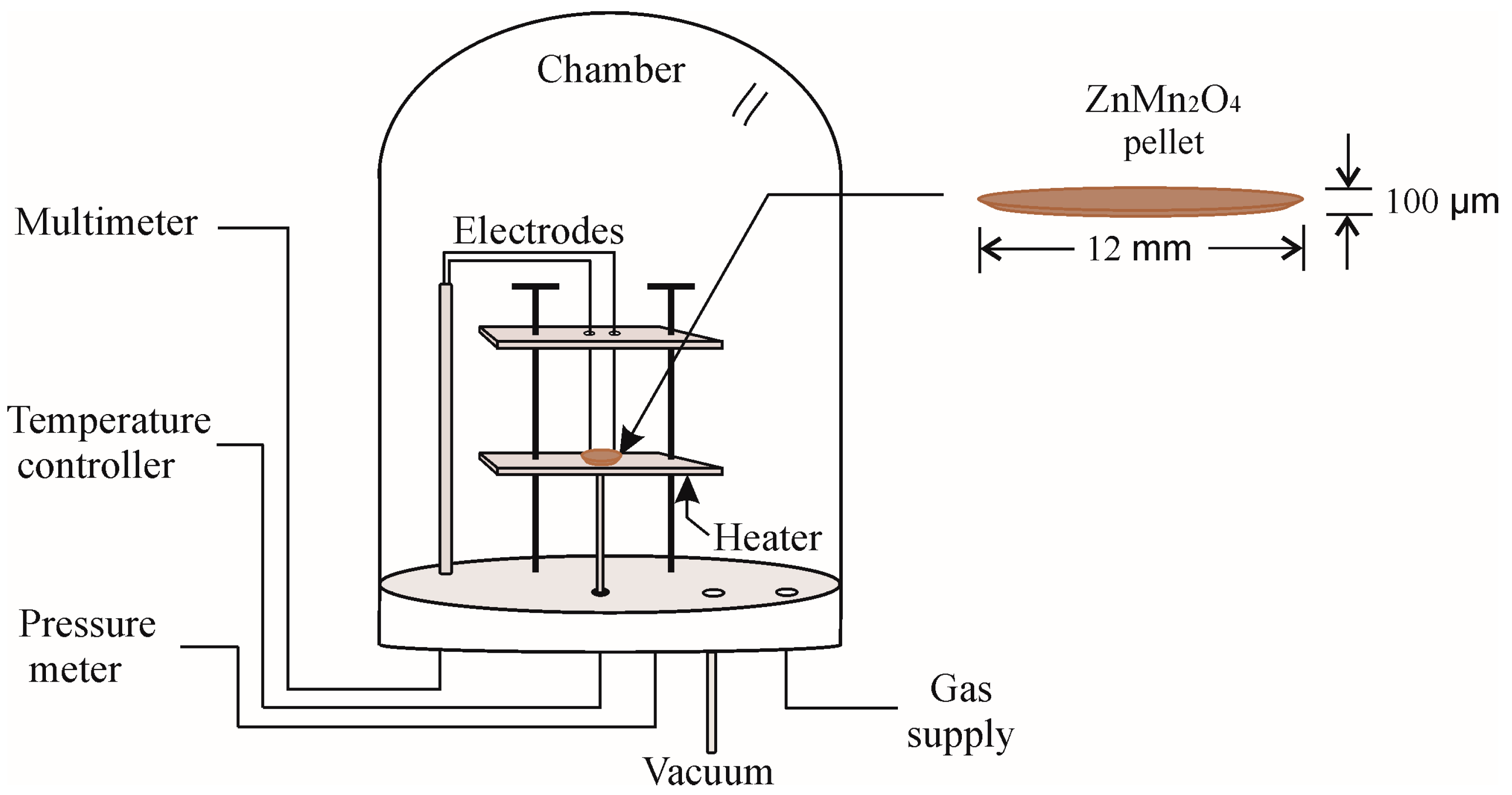
2.3. Gas Sensitivity Tests
3. Results and Discussion
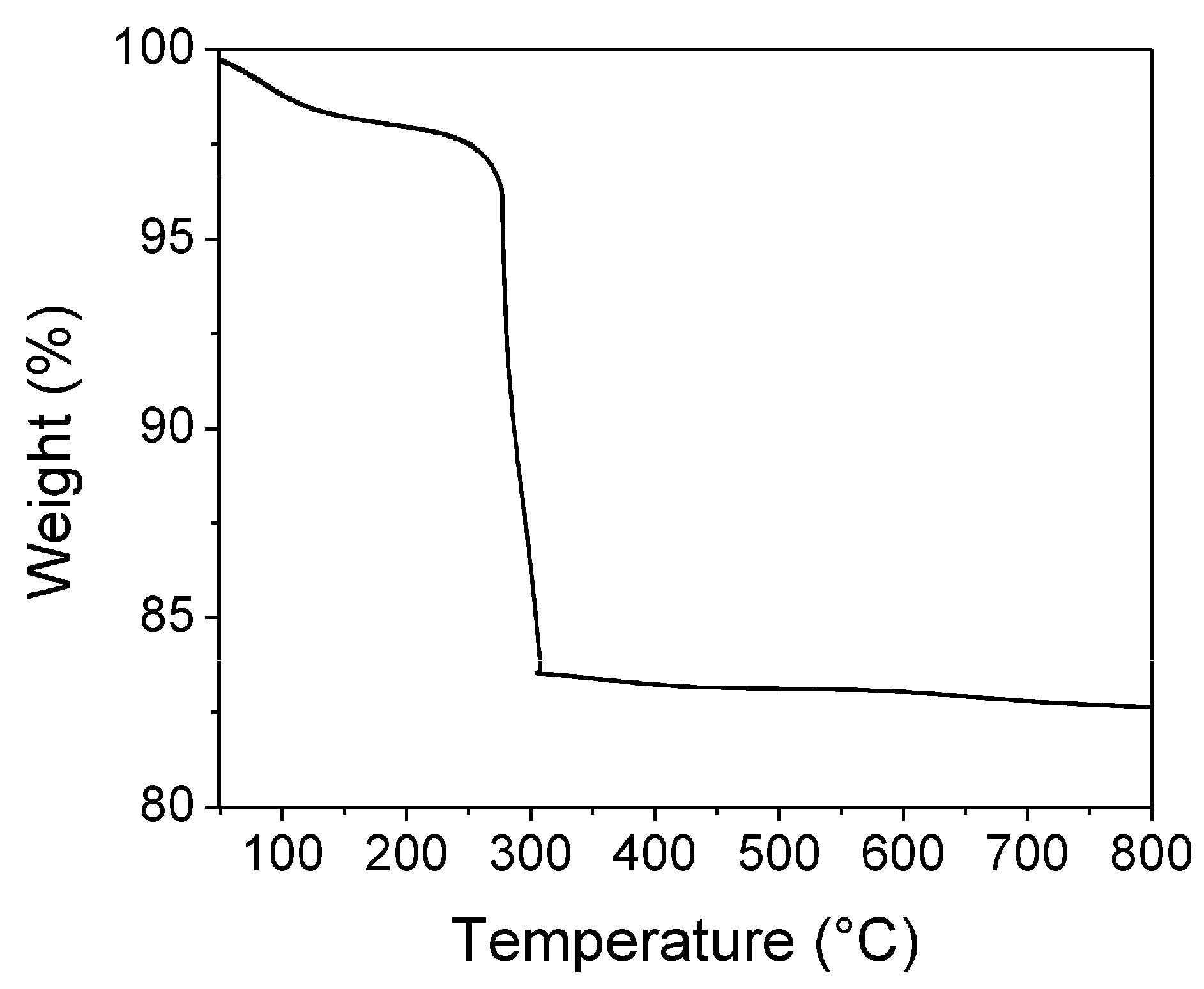
3.1. Thermogravimetric Analysis
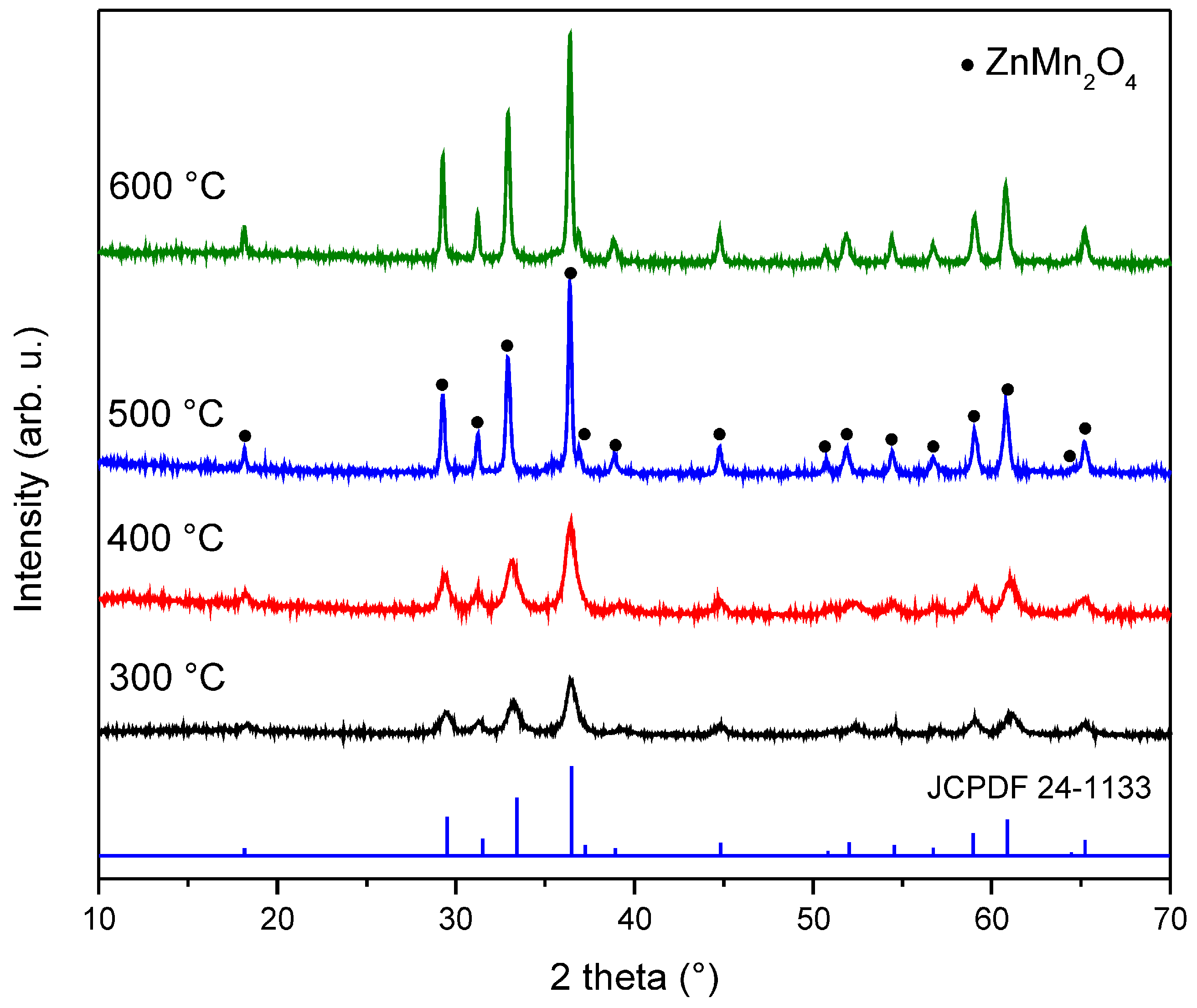
3.2. XRD Results
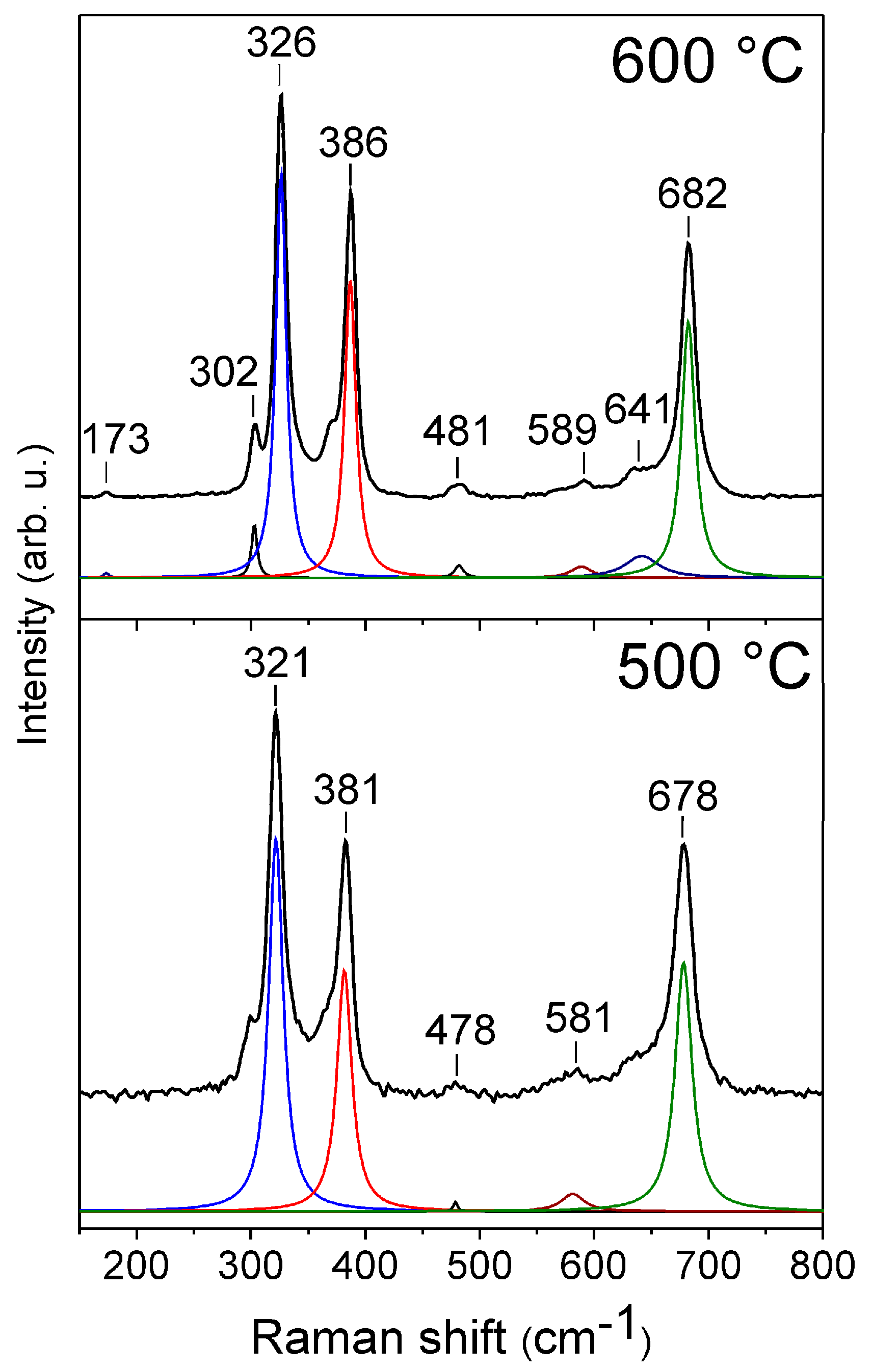
3.3. Raman Spectroscopy
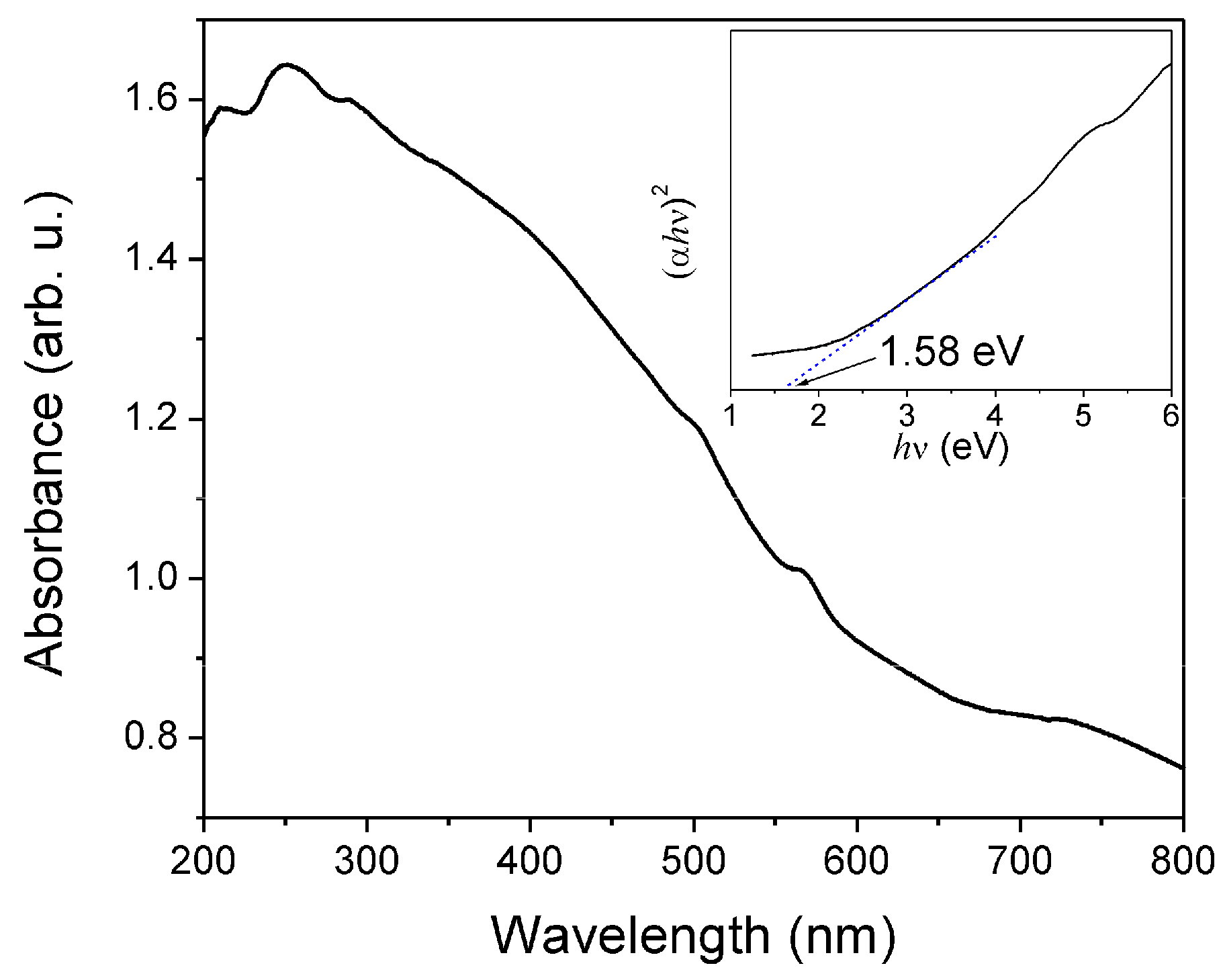
3.4. UV-Vis Analysis
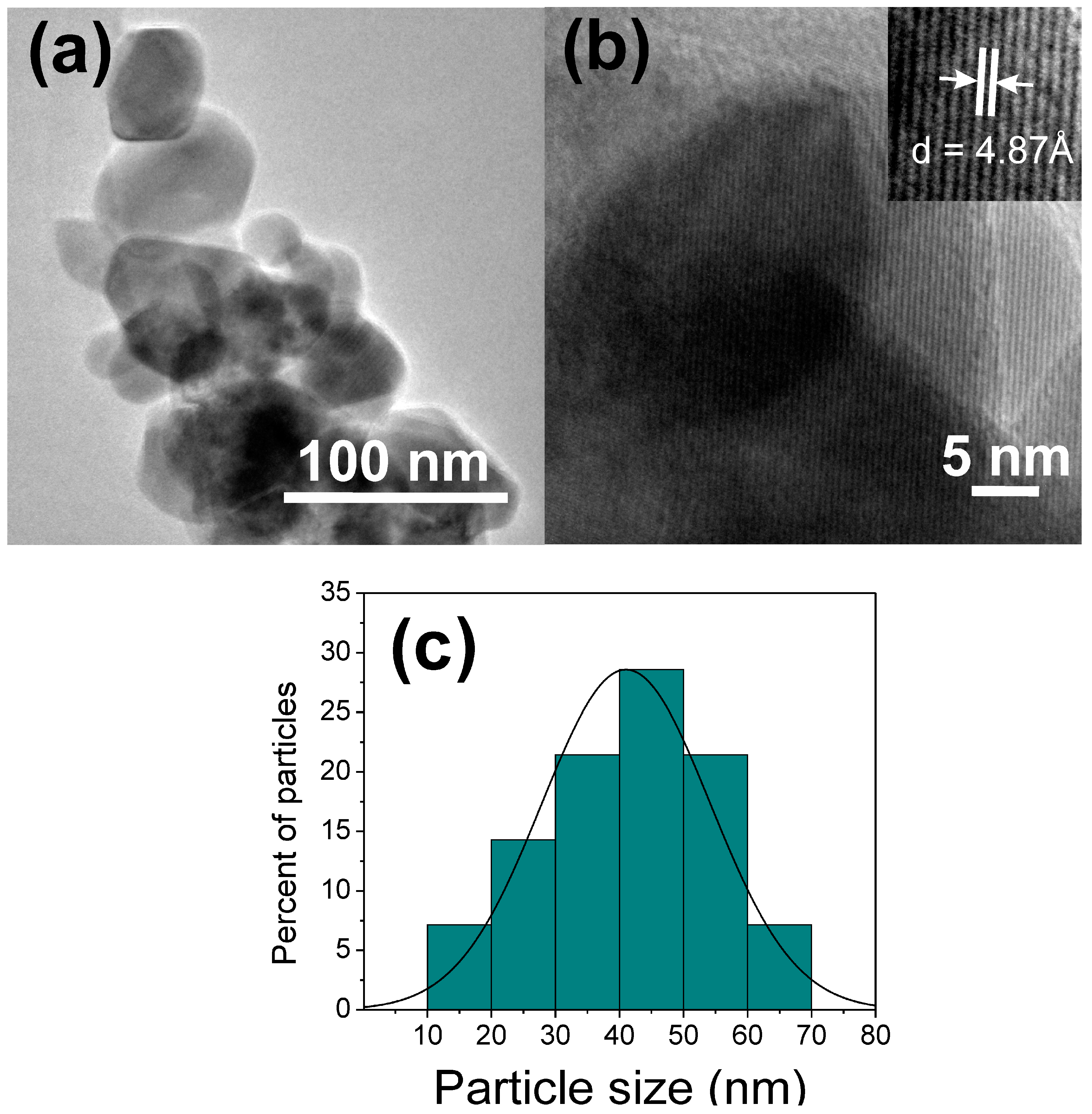
3.5. TEM Analysis
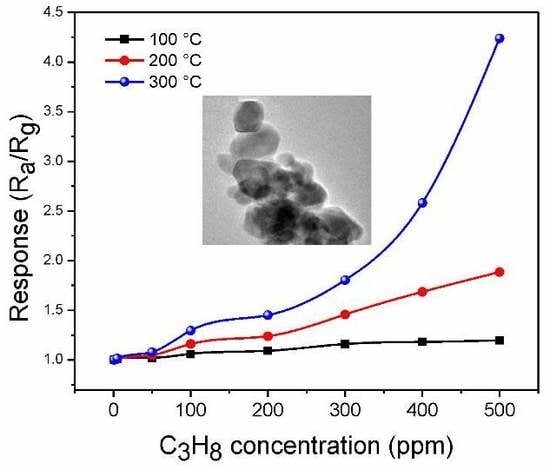
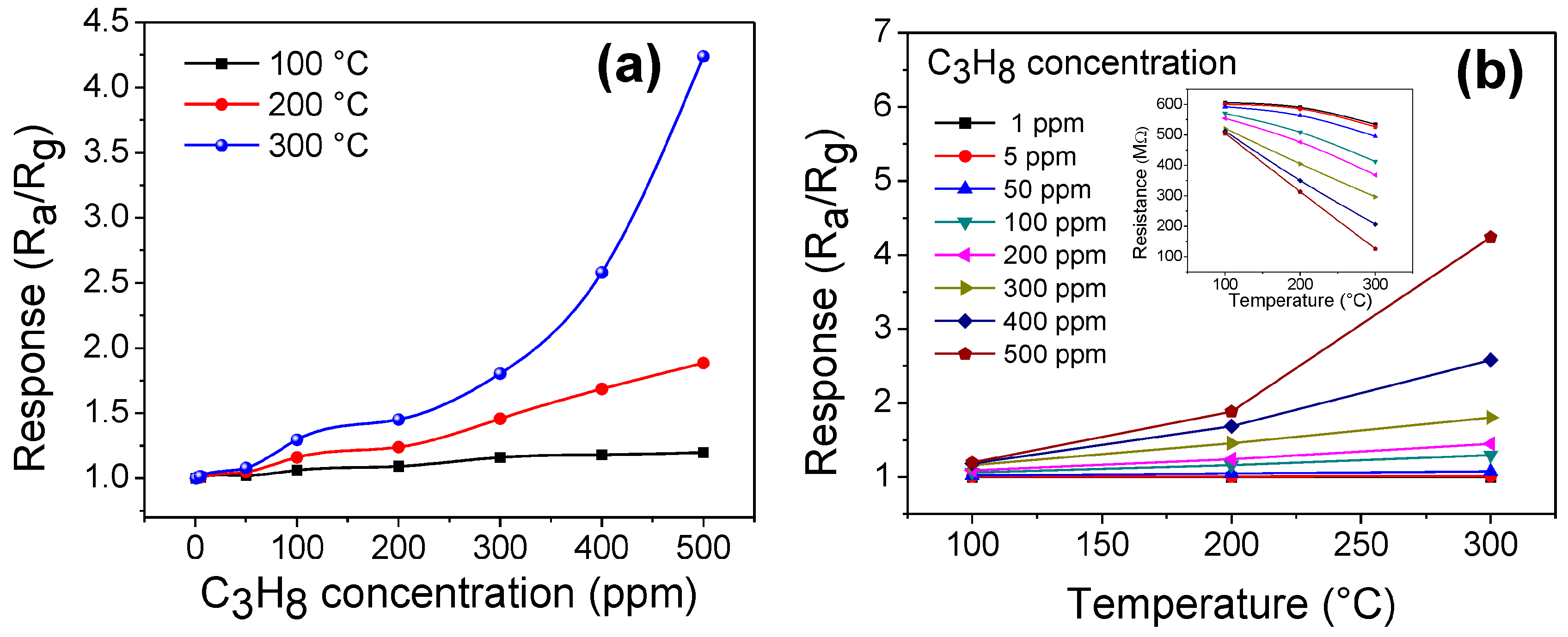
3.6. Gas Sensing Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Xu, C.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain size effects on gas sensitivity of porous SnO2-based elements. Sens. Actuators B Chem. 1991, 3, 147–155. [Google Scholar] [CrossRef]
- Kolmakov, A.; Klenov, D.O.; Lilach, Y.; Stemmer, S.; Moskovits, M. Enhanced gas sensing by individual SnO2 nanowires and nanobelts functionalized with Pd catalyst particles. Nano Lett. 2005, 5, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Kolmakov, A.; Zhang, Y.; Cheng, G.; Moskovits, M. Detection of CO and O2 using tin oxide nanowire sensors. Adv. Mater. 2003, 15, 997–1000. [Google Scholar] [CrossRef]
- Ahn, M.-W.; Park, K.-S.; Heo, J.-H.; Park, J.-G.; Kim, D.-W.; Choi, K.J.; Lee, J.-H.; Hong, S.-H. Gas sensing properties of defect-controlled ZnO-nanowire gas sensor. Appl. Phys. Lett. 2009, 93, 263103. [Google Scholar] [CrossRef]
- Shishiyanu, S.T.; Shishiyanu, T.S.; Lupan, O.I. Sensing characteristics of tin-doped ZnO thin films as NO2 gas sensor. Sens. Actuators B Chem. 2005, 107, 379–386. [Google Scholar] [CrossRef]
- Tang, H.; Prasad, K.; Sanjinés, R.; Lévy, F. TiO2 anatase thin films as gas sensors. Sens. Actuators B Chem. 1995, 26, 71–75. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Rodríguez Betancourtt, V.-M.; Guillén Bonilla, H.; Flores Martínez, M.; Guillén Bonilla, A.; Moran Lazaro, J.P.; Guillen Bonilla, J.T.; González, M.A.; Olvera Amador, M.d.l.L. Gas sensing properties of NiSb2O6 micro- and nanoparticles in propane and carbon monoxide atmospheres. J. Nanomater. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.M.; Guillén-Bonilla, J.T.; Sánchez-Martínez, A.; Gildo-Ortiz, L.; Santoyo-Salazar, J.; Morán-Lázaro, J.P.; Guillén-Bonilla, H.; Blanco-Alonso, O. A novel CO and C3H8 sensor made of CuSb2O6 nanoparticles. Ceram. Int. 2017, 43, 13635–13644. [Google Scholar] [CrossRef]
- Guillén-Bonilla, H.; Flores-Martínez, M.; Rodríguez-Betancourtt, V.M.; Guillén-Bonilla, A.; Reyes-Gómez, J.; Gildo-Ortiz, L.; Olvera-Amador, M.L.; Santoyo-Salazar, J. A novel gas sensor based on MgSb2O6 nanorods to indicate variations in carbon monoxide and propane concentrations. Sensors 2016, 16, 177. [Google Scholar] [CrossRef] [PubMed]
- Gildo-Ortiz, L.; Guillén-Bonilla, H.; Santoyo-Salazar, J.; Olvera, M.L.; Karthik, T.V.K.; Campos-González, E.; Reyes-Gómez, J. Low-temperature synthesis and gas sensitivity of perovskite-type LaCoO3 nanoparticles. J. Nanomater. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Niu, X.; Du, W.; Du, W. Preparation and gas sensing properties of ZnM2O4 (M = Fe, Co, Cr). Sens. Actuators B Chem. 2004, 99, 405–409. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Lv, M.; Chai, P.; Liu, Y.; Meng, J. Preparation of ferrite MFe2O4 (M = Co, Ni) ribbons with nanoporous structure and their magnetic properties. J. Phys. Chem. B 2008, 112, 11292–11297. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuators B Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Lingmin, Y.; Xinhui, F.; Lijun, Q.; Lihe, M.; Wen, Y. Dependence of morphologies for SnO2 nanostructures on their sensing property. Appl. Surf. Sci. 2011, 257, 3140–3144. [Google Scholar] [CrossRef]
- Gildo-Ortiz, L.; Reyes-Gómez, J.; Flores-Álvarez, J.M.; Guillén-Bonilla, H.; Olvera, M.d.l.L.; Betancourtt, V.R.; Verde-Gómez, Y.; Guillén-Cervantes, A.; Santoyo-Salazar, J. Synthesis, characterization and sensitivity tests of perovskite-type LaFeO3 nanoparticles in CO and propane atmospheres. Ceram. Int. 2016, 42, 18821–18827. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, L.; Wu, H.B.; Hoster, H.E.; Lou, X.W. Formation of ZnMn2O4 ball-in-ball hollow microspheres as a high-performance anode for lithium-ion batteries. Adv. Mater. 2012, 24, 4609–4613. [Google Scholar] [CrossRef] [PubMed]
- Courtel, F.M.; Duncan, H.; Abu-Lebdeh, Y.; Davidson, I.J. High capacity anode materials for Li-ion batteries based on spinel metal oxides AMn2O4 (A = Co, Ni, and Zn). J. Mater. Chem. 2011, 21, 10206–10218. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, H.B.; Zhu, T.; Lou, X.W. Facile preparation of ZnMn2O4 hollow microspheres as high-capacity anodes for lithium-ion batteries. J. Mater. Chem. 2012, 22, 827–829. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, Y.; Yin, J.; Li, Q.; Zhang, L. Low temperature synthesis of flower-like ZnMn2O4 superstructures with enhanced electrochemical lithium storage. J. Power Sources 2009, 194, 1089–1093. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Xiao, L.; Zhang, L. Nanocrystalline ZnMn2O4 as a novel lithium-storage material. Electrochem. Commun. 2008, 10, 1117–1120. [Google Scholar] [CrossRef]
- Bai, Z.; Fan, N.; Sun, C.; Ju, Z.; Guo, C.; Yang, J.; Qian, Y. Facile synthesis of loaf-like ZnMn2O4 nanorods and their excellent performance in Li-ion batteries. Nanoscale 2013, 5, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Sharma, Y. Synthesis and characterization of nanostructured ternary zinc manganese oxide as novel supercapacitor material. Mater. Chem. Phys. 2015, 149, 721–727. [Google Scholar] [CrossRef]
- Huang, T.; Zhao, C.; Qiu, Z.; Luo, J.; Hu, Z. Hierarchical porous ZnMn2O4 synthesized by the sucrose-assisted combustion method for high-rate supercapacitors. Ionics 2017, 23, 139–146. [Google Scholar] [CrossRef]
- Guo, N.; Wei, X.Q.; Deng, X.L.; Xu, X.J. Synthesis and property of spinel porous ZnMn2O4 microspheres. Appl. Surf. Sci. 2015, 356, 1127–1134. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, M.; Pang, H. Achieving high-performance supercapacitors by constructing porous zinc-manganese oxide microstructures. Energy Technol. 2015, 3, 820–824. [Google Scholar] [CrossRef]
- Guan, Y.; Feng, Y.; Mu, Y.; Fang, L.; Zhang, H.; Wang, Y. Ultra-tiny ZnMn2O4 nanoparticles encapsulated in sandwich-like carbon nanosheets for high-performance supercapacitors. Nanotechnology 2016, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guillemet-Fritsch, S.; Chanel, C.; Sarrias, J.; Bayonne, S.; Rousset, A.; Alcobe, X.; Martinez Sarriòn, M.L. Structure, thermal stability and electrical properties of zinc manganites. Solid State Ionics 2000, 128, 233–242. [Google Scholar] [CrossRef]
- Talebi, R. Simple synthesis and characterization of zinc manganite nanoparticles: Investigation of surfactants effect and its photocatalyst application. J. Mater. Sci.: Mater. Electron. 2017, 28, 3774–3779. [Google Scholar] [CrossRef]
- Deraz, N.M.; Abdeltawab, A.A.; Selim, M.M.; El-Shafey, O.; El-Asmy, A.A.; Al-Deyab, S.S. Precipitation–deposition assisted fabrication and characterization of nano-sized zinc manganite. J. Ind. Eng. Chem. 2014, 20, 2901–2904. [Google Scholar] [CrossRef]
- Juibari, N.M.; Eslami, A. Investigation of catalytic activity of ZnAl2O4 and ZnMn2O4 nanoparticles in the thermal decomposition of ammonium perchlorate. Therm. Anal. Calorim. 2017, 128, 115–124. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Robert, D.; Weber, J.-V. Photocatalytic activity of Cu2O/TiO2, Bi2O3/TiO2 and ZnMn2O4/TiO2 heterojunctions. Catal. Today 2005, 101, 315–321. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Trari, M. Photocatalytic hydrogen production from suspension of spinel powders AMn2O4 (A = Cu and Zn). Int. J. Hydrogen Energy 2002, 27, 357–362. [Google Scholar] [CrossRef]
- Nassar, M.Y.; El-Moety, E.A.; El-Shahat, M.F. Synthesis and characterization of a ZnMn2O4 nanostructure as a chemical nanosensor: A facile and new approach for colorimetric determination of omeprazole and lansoprazole drugs. RSC Adv. 2017, 7, 43798–43811. [Google Scholar] [CrossRef]
- Sorita, R.; Kawano, T. A highly selective CO sensor: Screening of electrode materials. Sens. Actuators B Chem. 1996, 36, 274–277. [Google Scholar] [CrossRef]
- Na, C.W.; Park, S.-Y.; Chung, J.-H.; Lee, J.-H. Transformation of ZnO Nanobelts into single-crystalline Mn3O4 nanowires. ACS Appl. Mater. Interfaces 2012, 4, 6565–6572. [Google Scholar] [CrossRef] [PubMed]
- Panmatarith, T.; Innoi, S. Vapour sensing characteristics of ZnO-ZnMn2O4 ceramics using the visual basic-based measurement system. Suranaree J. Sci. Technol. 2009, 16, 235–243. [Google Scholar]
- Courtel, F.M.; Abu-Lebdeh, Y.; Davidson, I.J. ZnMn2O4 nanoparticles synthesized by a hydrothermal method as an anode material for Li-ion batteries. Electrochim. Acta 2012, 71, 123–127. [Google Scholar] [CrossRef]
- Song, M.S.; Cho, Y.J.; Yoon, D.Y.; Nahm, S.; Oh, S.H.; Woo, K.; Ko, J.M.; Cho, W.I. Solvothermal synthesis of ZnMn2O4 as an anode material in lithium ion battery. Electrochim. Acta 2014, 137, 266–272. [Google Scholar] [CrossRef]
- Brahma, S.; Liu, C.-P.; Shivashankar, S.A. Microwave irradiation assisted, one pot synthesis of simple and complex metal oxide nanoparticles: A general approach. J. Phys. D: Appl. Phys. 2017, 50, 1–5. [Google Scholar] [CrossRef]
- Morán-Lázaro, J.P.; Blanco, O.; Rodríguez-Betancourtt, V.M.; Reyes-Gómez, J.; Michel, C.R. Enhanced CO2-sensing response of nanostructured cobalt aluminate synthesized using a microwave-assisted colloidal method. Sens. Actuators B Chem. 2016, 226, 518–524. [Google Scholar] [CrossRef]
- Blanco, O.; Morán-Lázaro, J.P.; Rodríguez-Betancourtt, V.M.; Reyes-Gómez, J.; Barrera, A. Colloidal synthesis of CoAl2O4 nanoparticles using dodecylamine and their structural characterization. Superficies y Vacío 2016, 29, 78–82. [Google Scholar]
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B Chem. 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Morán-Lázaro, J.P.; López-Urías, F.; Muñoz-Sandoval, E.; Blanco-Alonso, O.; Sanchez-Tizapa, M.; Carreon-Alvarez, A.; Guillén-Bonilla, H.; Olvera-Amador, M.d.l.L.; Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.M. Synthesis, characterization, and sensor applications of spinel ZnCo2O4 nanoparticles. Sensors 2016, 16, 2162. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Jiang, H.; Yang, M. Designed formation through a metal organic framework route of ZnO/ZnCo2O4 hollow core–shell nanocages with enhanced gas sensing properties. Nanoscale 2016, 8, 16349–16356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Feng, W.; Wang, C.; Hu, X.; Li, X.; Sun, P.; Shimanoe, K.; Yamazoe, N.; Lu, G. Porous ZnO/ZnCo2O4 hollow spheres: Synthesis, characterization, and applications in gas sensing. J. Mater. Chem. 2014, 2, 17683–17690. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, Y.; Liew, K.; Li, J. Catalytic performance of zirconium-modified Co/Al2O3 for Fischer–Tropsch synthesis. J. Mol. Catal. A: Chem. 2005, 231, 145–151. [Google Scholar] [CrossRef]
- Malavasi, L.; Galinetto, P.; Mozzati, M.C.; Azzoni, C.B.; Flor, G. Raman spectroscopy of AMn2O4 (A = Mn, Mg and Zn) spinels. Phys. Chem. Chem. Phys. 2002, 4, 3876–3880. [Google Scholar] [CrossRef]
- Tortosa, M.; Manjón, F.J.; Mollar, M.; Marí, B. ZnO-based spinels grown by electrodeposition. J. Phys. Chem. Solids 2012, 73, 1111–1115. [Google Scholar] [CrossRef]
- Samanta, K.; Dussan, S.; Katiyar, R.S.; Bhattacharya, P. Structural and optical properties of nanocrystalline Zn1−xMnxO. Appl. Phys. Lett. 2007, 90, 261903. [Google Scholar] [CrossRef]
- Li, H.; Song, B.; Wang, W.J.; Chen, X.L. Facile synthesis, thermal, magnetic, Raman characterizations of spinel structure ZnMn2O4. Mater. Chem. Phys. 2011, 130, 39–44. [Google Scholar] [CrossRef]
- Hadzic, B.; Romcevic, N.; Romcevic, M.; Kuryliszyn-Kudelska, I.; Dobrowolski, W.; Wróbel, R.; Narkiewicz, U.; Sibera, D. Raman study of surface optical phonons in ZnO(Mn) nanoparticles. J. Alloy. Compd. 2014, 585, 214–219. [Google Scholar] [CrossRef]
- Zhang, T.; Yue, H.; Qiu, H.; Zhu, K.; Zhang, L.; Wei, Y.; Du, F.; Chen, G.; Zhang, D. Synthesis of graphene-wrapped ZnMn2O4 hollow microspheres as high performance anode materials for lithium ion batteries. RSC Adv. 2015, 5, 99107–99114. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Zhao, Q.; Liu, S. Synthesis and optical property of one dimensional spinel ZnMn2O4 nanorods. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Javed, Q.; Wang, F.; Toufiq, A.M.; Rafiq, M.Y.; Iqbal, M.Z.; Kamran, M.A. Preparation, characterizations and optical property of single crystalline ZnMn2O4 nanoflowers via template-free hydrothermal synthesis. J. Nanosci. Nanotechnol. 2013, 13, 2937–2942. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, X.; Zhao, J. Fabrication, characterization and photocatalytic activity of cubic-like ZnMn2O4. Appl. Surf. Sci. 2013, 268, 274–277. [Google Scholar] [CrossRef]
- Misho, R.H.; Murad, W.A. Band gap measurements in thin films of hematite Fe2O3, pyrite FeS2 and troilite FeS prepared by chemical spray pyrolysis. Sol. Energy Mater. Sol. Cells 1992, 27, 335–345. [Google Scholar] [CrossRef]
- Rani, S.; Sanghi, S.; Agarwal, A.; Seth, V.P. Study of optical band gap and FTIR spectroscopy of Li2O·Bi2O3·P2O5 glasses. Spectrochim. Acta A 2009, 74, 673–677. [Google Scholar] [CrossRef] [PubMed]
- LaMer, V.K.; Dinegar, R.H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Vekilov, P.G. What determines the rate of growth of crystals from solution? Cryst. Growth Des. 2007, 7, 2796–2810. [Google Scholar] [CrossRef]
- Gildo-Ortiz, L.; Guillén-Bonilla, H.; Reyes-Gómez, J.; Rodríguez-Betancourtt, V.M.; Olvera-Amador, M.d.l.L.; Eguía-Eguía, S.I.; Guillén-Bonilla, A.; Santoyo-Salazar, J. Facile synthesis, microstructure, and gas sensing properties of NdCoO3 nanoparticles. J. Nanomater. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Kerlau, M.; Reichel, P.; Bârsan, N.; Weimar, U.; Delsarte-Guéguen, S.; Merdrignac-Conanec, O. Detection of propane by “GaON” thick-film gas sensors. Sens. Actuators B Chem. 2007, 122, 14–19. [Google Scholar] [CrossRef]
- Bahrami, B.; Khodadadi, A.; Kazemeini, M.; Mortazavi, Y. Enhanced CO sensitivity and selectivity of gold nanoparticles-doped SnO2 sensor in presence of propane and methane. Sens. Actuators B Chem. 2008, 133, 352–356. [Google Scholar] [CrossRef]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceram. 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Barsan, N.; Wiemar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Gómez-Pozos, H.; González-Vidal, J.L.; Alberto-Torres, G.; Olvera, M.L.; Castañeda, L. Physical characterization and effect of effective surface area on the sensing properties of tin dioxide thin solid films in a propane atmosphere. Sensors 2014, 14, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pozos, H.; González-Vidal, J.L.; Torres, G.A.; Rodríguez-Baez, J.; Maldonado, A.; Olvera, M.L.; Acosta, D.R.; Avendaño-Alejo, M.; Castañeda, L. Chromium and ruthenium-doped zinc oxide thin films for propane sensing applications. Sensors 2013, 13, 3432–3444. [Google Scholar] [CrossRef] [PubMed]
- Guillen-Bonilla, H.; Rodríguez-Betancourtt, V.M.; Guillén-Bonilla, J.T.; Reyes-Gómez, J.; Gildo-Ortiz, L.; Flores-Martínez, M.; Olvera-Amador, M.L.; Santoyo-Salazar, J. CO and C3H8 sensitivity behavior of zinc antimonate prepared by a microwave-assisted solution method. J. Nanomater. 2015, 16, 450. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morán-Lázaro, J.P.; Guillen-López, E.S.; López-Urias, F.; Muñoz-Sandoval, E.; Blanco-Alonso, O.; Guillén-Bonilla, H.; Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.M.; Sanchez-Tizapa, M.; Olvera-Amador, M.D.l.L. Synthesis of ZnMn2O4 Nanoparticles by a Microwave-Assisted Colloidal Method and their Evaluation as a Gas Sensor of Propane and Carbon Monoxide. Sensors 2018, 18, 701. https://doi.org/10.3390/s18030701
Morán-Lázaro JP, Guillen-López ES, López-Urias F, Muñoz-Sandoval E, Blanco-Alonso O, Guillén-Bonilla H, Guillén-Bonilla A, Rodríguez-Betancourtt VM, Sanchez-Tizapa M, Olvera-Amador MDlL. Synthesis of ZnMn2O4 Nanoparticles by a Microwave-Assisted Colloidal Method and their Evaluation as a Gas Sensor of Propane and Carbon Monoxide. Sensors. 2018; 18(3):701. https://doi.org/10.3390/s18030701
Chicago/Turabian StyleMorán-Lázaro, Juan Pablo, Erwin Said Guillen-López, Florentino López-Urias, Emilio Muñoz-Sandoval, Oscar Blanco-Alonso, Héctor Guillén-Bonilla, Alex Guillén-Bonilla, Verónica María Rodríguez-Betancourtt, Marciano Sanchez-Tizapa, and María De la Luz Olvera-Amador. 2018. "Synthesis of ZnMn2O4 Nanoparticles by a Microwave-Assisted Colloidal Method and their Evaluation as a Gas Sensor of Propane and Carbon Monoxide" Sensors 18, no. 3: 701. https://doi.org/10.3390/s18030701
APA StyleMorán-Lázaro, J. P., Guillen-López, E. S., López-Urias, F., Muñoz-Sandoval, E., Blanco-Alonso, O., Guillén-Bonilla, H., Guillén-Bonilla, A., Rodríguez-Betancourtt, V. M., Sanchez-Tizapa, M., & Olvera-Amador, M. D. l. L. (2018). Synthesis of ZnMn2O4 Nanoparticles by a Microwave-Assisted Colloidal Method and their Evaluation as a Gas Sensor of Propane and Carbon Monoxide. Sensors, 18(3), 701. https://doi.org/10.3390/s18030701