First Evaluation of Infrared Thermography as a Tool for the Monitoring of Udder Health Status in Farms of Dairy Cows
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Farms
2.2. Milk Sampling, Milk Sample Analysis and Definition of Udder Health Status
2.3. Thermographic Images Collection
- It identified the pixel with the maximum intensity (PImax, Figure 1C), calculating its coordinates inside the image and its value (equal to the maximum recorded temperature in the thermographic image).
- It calculated a range of intensities to use as thresholds, according to the following formulas:These values were selected considering both, the average USSTs that have been observed in previous experiments [2,10,11,16,19] and the increments that have been reported in case of subclinical and clinical mastitis. The range of intensities calculated was applied as a filter [33], to the thermographic image, in order to detect the udder of the cow (Figure 1B).
- On the filtered image, it applied a grid made by image subsections of 4 × 4 pixels.
- On each image subsection, it calculated the pixel average intensity (i.e., the recorded average temperature of the image subsection evaluated).
- On the resulting set of pixel average intensities, it selected the maximum value and it considered that number as the recorded maximum temperature of the thermographic image evaluated (i.e., the Tmax), taken as possible index of the udder health status in accordance with results of previous scientific studies [2,17]).
- It calculated a “temperature proximity area” (APT, Figure 1C,D) considering the coordinates of PImax as a starting point and a set of connected pixels which intensities were different from zero after applying the following filter:where the indicator T (tolerance) was set up at a level of 15 [34]. This last value was selected considering the increments of USST already found in scientific literature [2,10,11,16,19]. Furthermore, a connectivity mode of 4 pixels was used for the recursive application of the above reported filter [35]. This value specified at the algorithm whether a pixel should be considered in the following cycle. In detail, it imposed at the software application to take into consideration the pixels that were at the cardinal points (i.e., North, East, South and West) of the pixel under evaluation for the filtering operations that followed.
2.4. Statistical Analysis
3. Result
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zaninelli, M.; Rossi, L.; Costa, A.; Tangorra, F.M.; Agazzi, A.; Savoini, G. Monitoring of goats’ health status by on-line analysis of milk electrical conductivity. Large Anim. Rev. 2015, 21, 81–86. [Google Scholar]
- Hovinen, M.; Siivonen, J.; Taponen, S.; Hänninen, L.; Pastell, M.; Aisla, A.; Pyörälä, S. Detection of Clinical Mastitis with the Help of a Thermal Camera. J. Dairy Sci. 2008, 91, 4592–4598. [Google Scholar] [CrossRef] [PubMed]
- Pyörälä, S.; Taponen, S. Coagulase-negative staphylococci-emerging mastitis pathogens. Vet. Microbiol. 2009, 134, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Zaninelli, M.; Rossi, L.; Costa, A.; Tangorra, F.M.; Agazzi, A.; Savoini, G. Signal spectral analysis to characterize gland milk electrical conductivity in dairy goats. Ital. J. Anim. Sci. 2015, 14, 362–367. [Google Scholar] [CrossRef]
- Zaninelli, M.; Tangorra, F.M.; Costa, A.; Rossi, L.; Dell’Orto, V.; Savoini, G. Improved fuzzy logic system to evaluate milk electrical conductivity signals from on-line sensors to monitor dairy goat mastitis. Sensors 2016, 16, 1079. [Google Scholar] [CrossRef] [PubMed]
- Zaninelli, M.; Agazzi, A.; Costa, A.; Tangorra, F.M.; Rossi, L.; Savoini, G. Evaluation of the fourier frequency spectrum peaks of milk electrical conductivity signals as indexes to monitor the dairy goats’ health status by on-line sensors. Sensors 2015, 15, 20698–20716. [Google Scholar] [CrossRef] [PubMed]
- Samara, E.M.; Ayadi, M.; Aljumaah, R.S. Feasibility of utilising an infrared-thermographic technique for early detection of subclinical mastitis in dairy camels (Camelus dromedarius). J. Dairy Res. 2014, 81, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Radostits, O.M.; Gay, C.C.; Hinchcliff, K.W.; Constable, P.D. Clinical findings of bovine mastitis. In Veterinary Medicine: A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses; Saunders Ltd.: Philadelphia, PA, USA, 2007; pp. 563–574. [Google Scholar]
- Paulrud, C.O.; Clausen, S.; Andersen, P.E.; Bjerring, M.; Rasmussen, M.D. Infrared thermography to evaluate milking induced alterations in teat tissue fluid circulation. J. Dairy Sci. 2002, 85, 84. [Google Scholar]
- Polat, B.; Colak, A.; Cengiz, M.; Yanmaz, L.E.; Oral, H.; Bastan, A.; Kaya, S.; Hayirli, A. Sensitivity and specificity of infrared thermography in detection of subclinical mastitis in dairy cows. J. Dairy Sci. 2010, 93, 3525–3532. [Google Scholar] [CrossRef] [PubMed]
- Colak, A.; Polat, B.; Okumus, Z.; Kaya, M.; Yanmaz, L.E.; Hayirli, A. Short Communication: Early detection of mastitis using infrared thermography in dairy cows. J. Dairy Sci. 2008, 91, 4244–4248. [Google Scholar] [CrossRef] [PubMed]
- Bortolami, A.; Fiore, E.; Gianesella, M.; Corrò, M.; Catania, S.; Morgante, M. Evaluation of the udder health status in subclinical mastitis affected dairy cows through bacteriological culture, Somatic Cell Count and thermographic imaging. Pol. J. Vet. Sci. 2015, 18, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Maldagues, X. Nondestructive Testing Handbook, 3rd Edition: Volume 3. Infrared and Thermal Testing; Moore, P., Ed.; The American Society for Nondestructive Testing: Columbus, OH, USA, 2001. [Google Scholar]
- Poikalainen, V.; Praks, J.; Veermäe, I.; Kokin, E. Infrared temperature patterns of cow’s body as an indicator for health control at precision cattle farming. Agron. Res. 2012, 10, 187–194. [Google Scholar]
- Zaninelli, M.; Redaelli, V.; Tirloni, E.; Bernardi, C.; Dell’Orto, V.; Savoini, G. First results of a detection sensor for the monitoring of laying hens reared in a commercial organic egg production farm based on the use of infrared technology. Sensors 2016, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.L.; Schaefer, A.L.; Tong, A.K.W.; Lacasse, P. Use of infrared thermography for early detection of mastitis in cows. In Agri-Food 2000; Agricultural Institute of Canada: Winnipeg, MB, Canada, 2000. [Google Scholar]
- Metzner, M.; Sauter-Louis, C.; Seemueller, A.; Petzl, W.; Zerbe, H. Infrared thermography of the udder after experimentally induced Escherichia coli mastitis in cows. Vet. J. 2015, 204, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.J.; Kennedy, A.D.; Scott, S.L.; Kyle, B.L.; Schaefer, A.L. Daily variation in the udder surface temperature of dairy cows measured by infrared thermography: Potential for mastitis detection. Can. J. Anim. Sci. 2003, 83, 687–693. [Google Scholar] [CrossRef]
- Pezeshki, A.; Stordeur, P.; Wallemacq, H.; Schynts, F.; Stevens, M.; Boutet, P.; Peelman, L.J.; Spiegeleer, B.D.; Duchateau, L.; Bureau, F.; et al. Variation of inflammatory dynamics and mediators in primiparous cows after intramammary challenge with Escherichia coli. Vet. Res. 2011, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nursing and Midwifery Council (NMC). Current Concepts of Bovine Mastitis, 5th ed.; NMC (National Mastitis Council), Ed.; National Mastitis Council Inc.: New Prague, MN, USA, 2016. [Google Scholar]
- Nursing and Midwifery Council (NMC). Laboratory and Field Hand-book on Bovine Mastitis, 3th ed.; NMC (National Mastitis Council), Ed.; National Mastitis Council Inc.: Arlington, VA, USA, 2017. [Google Scholar]
- De Vliegher, S.; Laevens, H.; Opsomer, G.; Muêlenaere, E.; De Kruif, A. De Somatic cell counts in dairy heifers during early lactations. Flem. Vet. J. 2001, 70, 212–215. [Google Scholar]
- Chagunda, M.G.; Larsen, T.; Bjerring, M.; Ingvartsen, K.L. L-lactate dehydrogenase and N-acetyl-β-D-glucosaminidase activities in bovine milk as indicators of non-specific mastitis. J. Dairy Res. 2006, 73, 431. [Google Scholar] [CrossRef] [PubMed]
- Talukder, S.; Kerrisk, K.L.; Ingenhoff, L.; Thomson, P.C.; Garcia, S.C.; Celi, P. Infrared technology for estrus detection and as a predictor of time of ovulation in dairy cows in a pasture-based system. Theriogenology 2017, 81, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Montanholi, Y.R.; Swanson, K.C.; Schenkel, F.S.; McBride, B.W.; Caldwell, T.R.; Miller, S.P. On the determination of residual feed intake and associations of infrared thermography with efficiency and ultrasound traits in beef bulls. Livest. Sci. 2017, 125, 22–30. [Google Scholar] [CrossRef]
- Montanholi, Y.R.; Odongo, N.E.; Swanson, K.C.; Schenkel, F.S.; McBride, B.W.; Miller, S.P. Application of infrared thermography as an indicator of heat and methane production and its use in the study of skin temperature in response to physiological events in dairy cattle (Bos taurus). J. Therm. Biol. 2008, 33, 468–475. [Google Scholar] [CrossRef]
- Weschenfelder, A.V.; Saucier, L.; Maldague, X.; Rocha, L.M.; Schaefer, A.L.; Faucitano, L. Use of infrared ocular thermography to assess physiological conditions of pigs prior to slaughter and predict pork quality variation. Meat Sci. 2013, 95, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Castro-Costa, A.; Caja, G.; Salama, A.A.K.; Rovai, M.; Flores, C.; Aguiló, J. Thermographic variation of the udder of dairy ewes in early lactation and following an Escherichia coli endotoxin intramammary challenge in late lactation. J. Dairy Sci. 2014, 97, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Menegassi, S.R.O.; Stumpf, M.T.; Kolling, G.J.; Dias, E.; et al. Infrared thermography in animal production: An overview. Comput. Electron. Agric. 2016, 123, 10–16. [Google Scholar] [CrossRef]
- Sathiyabarathi, M.; Jeyakumar, S.; Manimaran, A.; Jayaprakash, G.; Pushpadass, H.A.; Sivaram, M.; Ramesha, K.P.; Das, D.N.; Kataktalware, M.A.; Prakash, M.A.; et al. Infrared thermography: A potential noninvasive tool to monitor udder health status in dairy cows. Vet. World 2016, 9, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Alsaaod, M.; Syring, C.; Dietrich, J.; Doherr, M.G.; Gujan, T.; Steiner, A. A field trial of infrared thermography as a non-invasive diagnostic tool for early detection of digital dermatitis in dairy cows. Vet. J. 2014, 199, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Zaninelli, M.; Costa, A.; Tangorra, F.M.; Rossi, L.; Agazzi, A.; Savoini, G. Preliminary evaluation of a nest usage sensor to detect double nest occupations of laying hens. Sensors 2015, 15, 2680–2693. [Google Scholar] [CrossRef] [PubMed]
- Zaninelli, M.; Redaelli, V.; Luzi, F.; Bontempo, V.; Dell’Orto, V.; Savoini, G. A Monitoring System for Laying Hens That Uses a Detection Sensor Based on Infrared Technology and Image Pattern Recognition. Sensors 2017, 17, 1195. [Google Scholar] [CrossRef] [PubMed]
- Xiaobo, M.; Jing, Y. Research on object-background segmentation of color image based on LabVIEW. In Proceedings of the 2011 IEEE International Conference on Cyber Technology in Automation, Control, and Intelligent Systems (CYBER), Kunming, China, 20–23 March 2011; pp. 190–194. [Google Scholar]
- Relf, C.G. Image Acquisition and Processing with LabVIEW; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Team R Core Development. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]
- Wilkinson, G.N.; Rogers, C.E. Symbolic descriptions of factorial models for analysis of variance. Appl. Stat. 1973, 22, 392–399. [Google Scholar] [CrossRef]
- Chambers, J.M. Linear models. In Statistical Models in S; Chambers, J.M., Hastie, T.J., Eds.; Chapman and Hall/CRC: New York, NY, USA, 1992. [Google Scholar]
- Sing, T.; Sander, O.; Beerenwinkel, N.; Lengauer, T. ROCR: Visualizing classifier performance in R. Bioinformatics 2005, 21, 3940–3941. [Google Scholar] [CrossRef] [PubMed]
- Barth, K. Basic investigations to evaluate a highly sensitive infrared-thermograph-technique to detect udder inflammation in cows. Milchwissenschaft 2000, 55, 607–609. [Google Scholar]
- Martins, R.F.S.; do Prado Paim, T.; de Abreu Cardoso, C.; Stéfano Lima Dallago, B.; de Melo, C.B.; Louvandini, H.; McManus, C. Mastitis detection in sheep by infrared thermography. Res. Vet. Sci. 2013, 94, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Porcionato, M.A.F.; Canata, T.F.; De Oliveira, C.E.L.; Santos, M.V. Dos Udder Thermography of Gyr Cows for Subclinical Mastitis Detection/Termografia Do Úbere De Vacas Gir Para Detecção De Mastite Subclínica. Rev. Bras. Eng. Biossistemas 2009, 3, 251. [Google Scholar] [CrossRef]
- Metzner, M.; Sauter-Louis, C.; Seemueller, A.; Petzl, W.; Klee, W. Infrared thermography of the udder surface of dairy cattle: Characteristics, methods, and correlation with rectal temperature. Vet. J. 2014, 199, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Willits, S. Infrared Thermography for Screening and Early Detection of Mastitis Infections in Working Dairy Herds. InfraMation Proc. ITC 2005, 42, 1–5. [Google Scholar]


| Items | Linear Coefficients | ||
|---|---|---|---|
| Estimate | Standard Error | Significance | |
| Intercept | 33.6 | 0.98 | p < 0.01 |
| SCC (log) | 0.881 | 0.0430 | p < 0.05 |
| APT | −0.000995 | 0.0000395 | p < 0.05 |
| SCC × APT | 0.000369 | 0.0000201 | p < 0.05 |
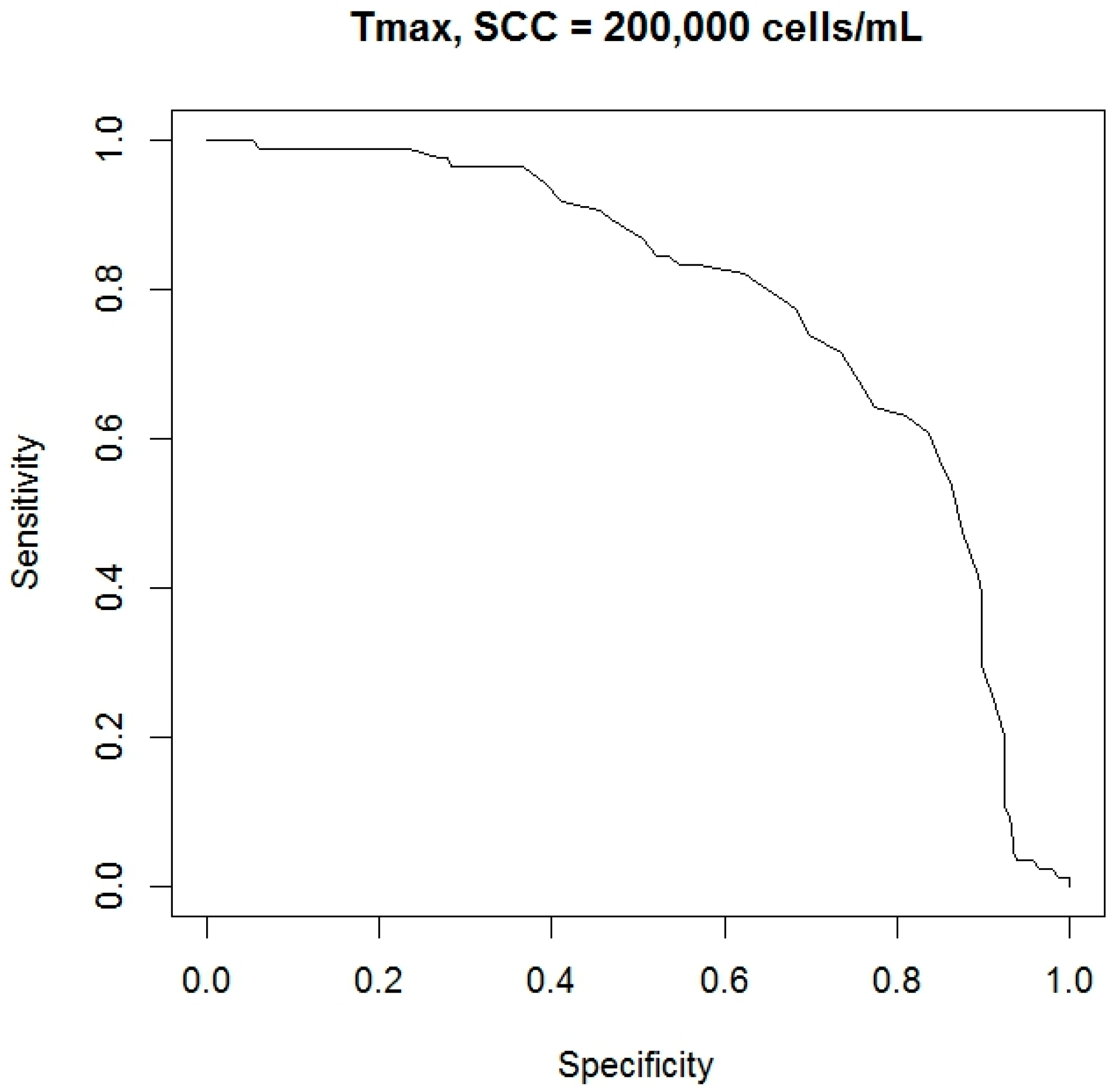
| SCC Threshold (Cells/mL) | AUC (Area) | Sensitivity (%) | Specificity (%) | Cutoff Level (°C) |
|---|---|---|---|---|
| 200,000 | 0.805 | 78.6 | 77.9 | 35.1 |
| 400,000 | 0.811 | 71.4 | 71.6 | 35.3 |
| SCC Threshold (Cells/mL) | Udder Health State (Healthy/Not Healthy) | Cases (n) | Tmax (°C, Means ± S.E.) | SCC (×103 Cells/mL, Means ± S.E.) | APT (# Pixels, Means ± S.E.) |
|---|---|---|---|---|---|
| 200,000 | healthy | 113 | 34.19 ± 0.17 | 62.64 ± 4.53 | 2460 ± 90 |
| not healthy | 42 | 35.79 ± 0.15 | 592.38 ± 71.40 | 1476 ± 151 | |
| 400,000 | healthy | 134 | 34.40 ± 0.16 | 92.62 ± 7.33 | 2397 ± 85 |
| not healthy | 21 | 36.08 ± 0.22 | 930.81 ± 96.58 | 898 ± 79 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaninelli, M.; Redaelli, V.; Luzi, F.; Bronzo, V.; Mitchell, M.; Dell’Orto, V.; Bontempo, V.; Cattaneo, D.; Savoini, G. First Evaluation of Infrared Thermography as a Tool for the Monitoring of Udder Health Status in Farms of Dairy Cows. Sensors 2018, 18, 862. https://doi.org/10.3390/s18030862
Zaninelli M, Redaelli V, Luzi F, Bronzo V, Mitchell M, Dell’Orto V, Bontempo V, Cattaneo D, Savoini G. First Evaluation of Infrared Thermography as a Tool for the Monitoring of Udder Health Status in Farms of Dairy Cows. Sensors. 2018; 18(3):862. https://doi.org/10.3390/s18030862
Chicago/Turabian StyleZaninelli, Mauro, Veronica Redaelli, Fabio Luzi, Valerio Bronzo, Malcolm Mitchell, Vittorio Dell’Orto, Valentino Bontempo, Donata Cattaneo, and Giovanni Savoini. 2018. "First Evaluation of Infrared Thermography as a Tool for the Monitoring of Udder Health Status in Farms of Dairy Cows" Sensors 18, no. 3: 862. https://doi.org/10.3390/s18030862





