A Fiber Optic Interferometric Sensor Platform for Determining Gas Diffusivity in Zeolite Films
Abstract
:1. Introduction
2. Experimental Section
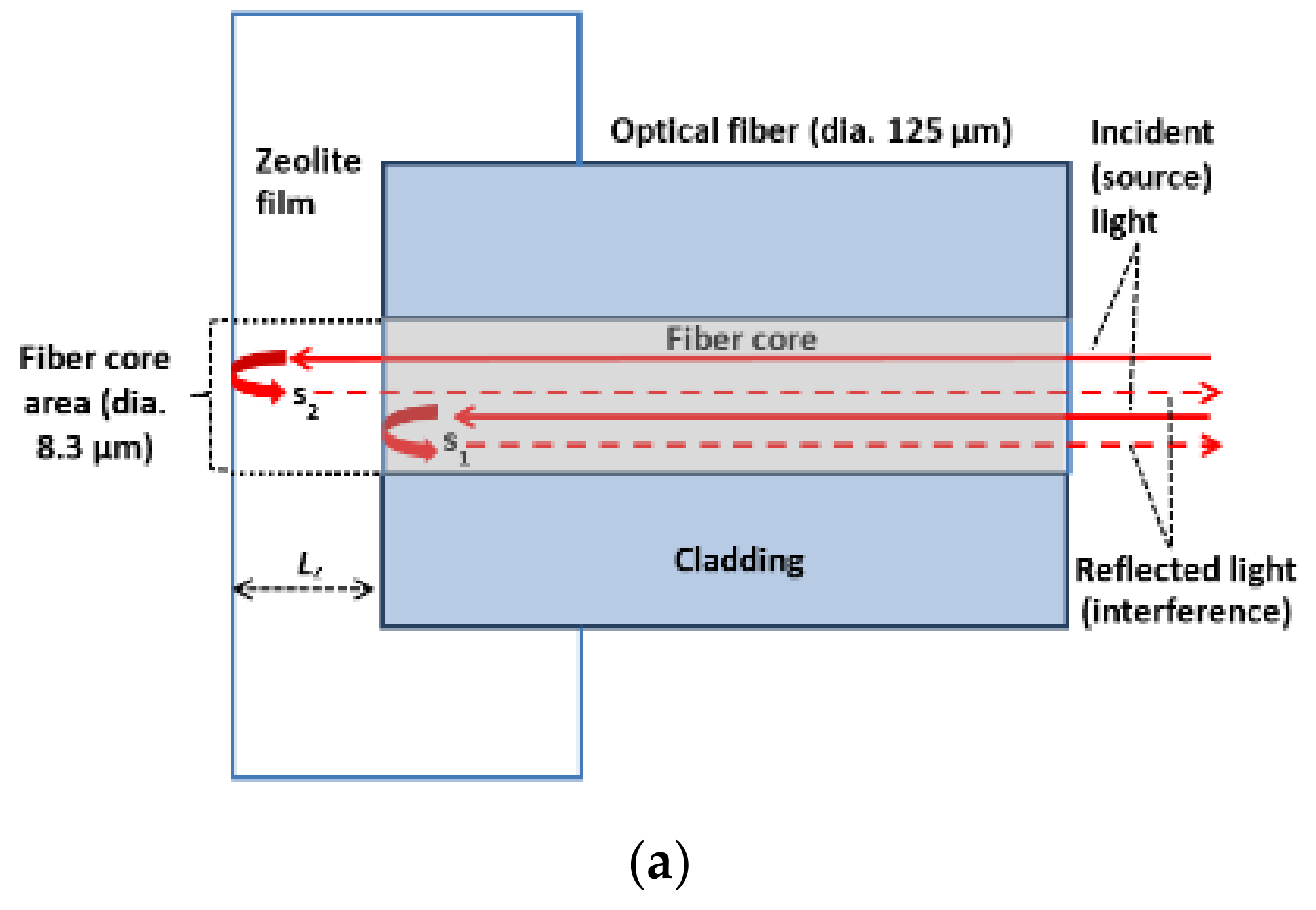
2.1. Sensor Structure and Operation Principle
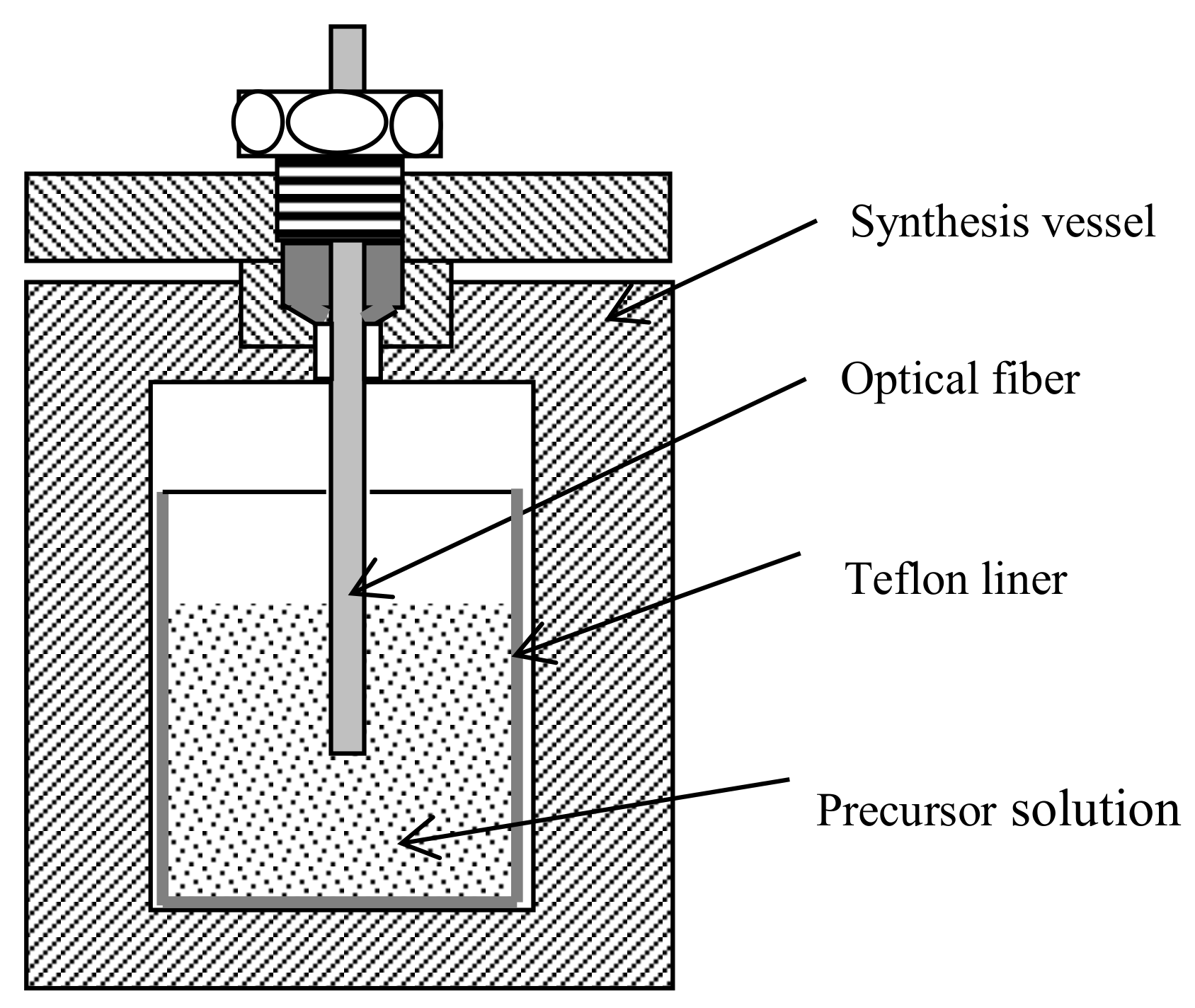
2.2. Sensor Fabrication
2.3. Apparatus and Measurement Procedure
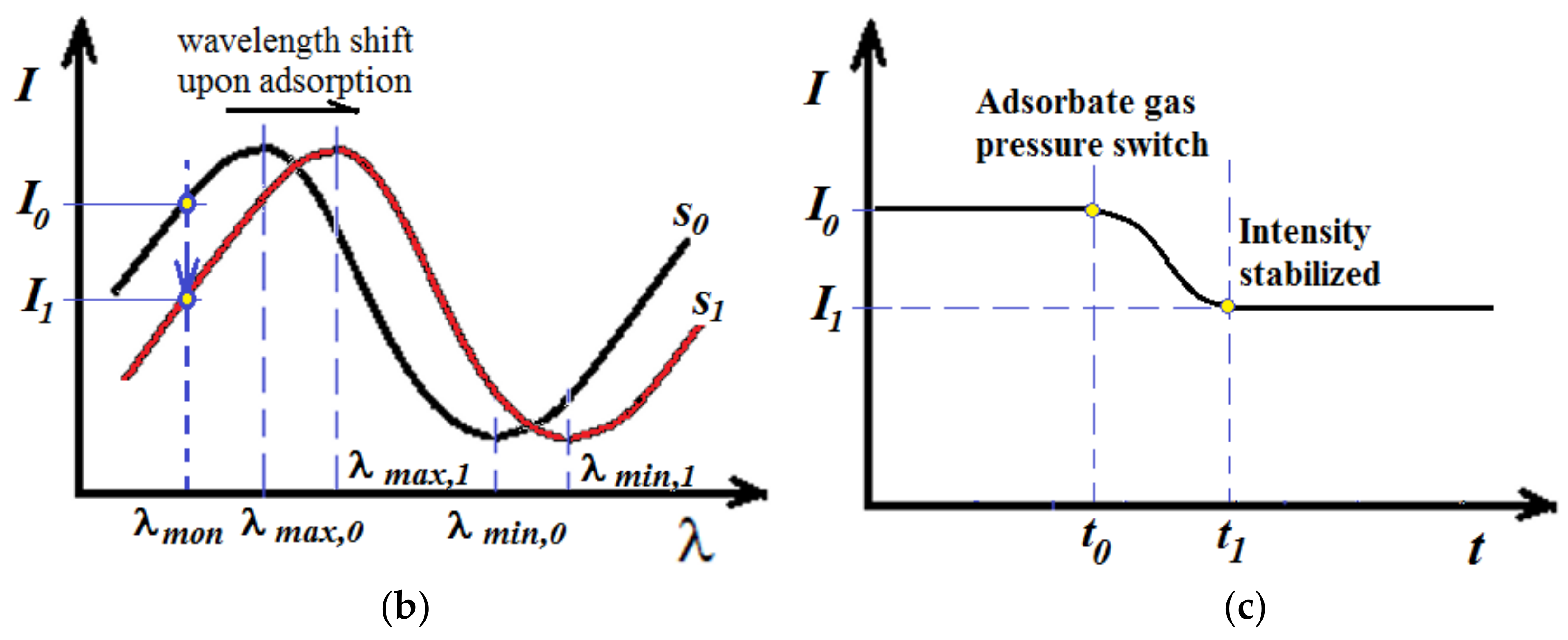
2.4. Determination of Optical Thickness of the Zeolite Film
2.5. Determination of Diffusivity
3. Results and Discussion
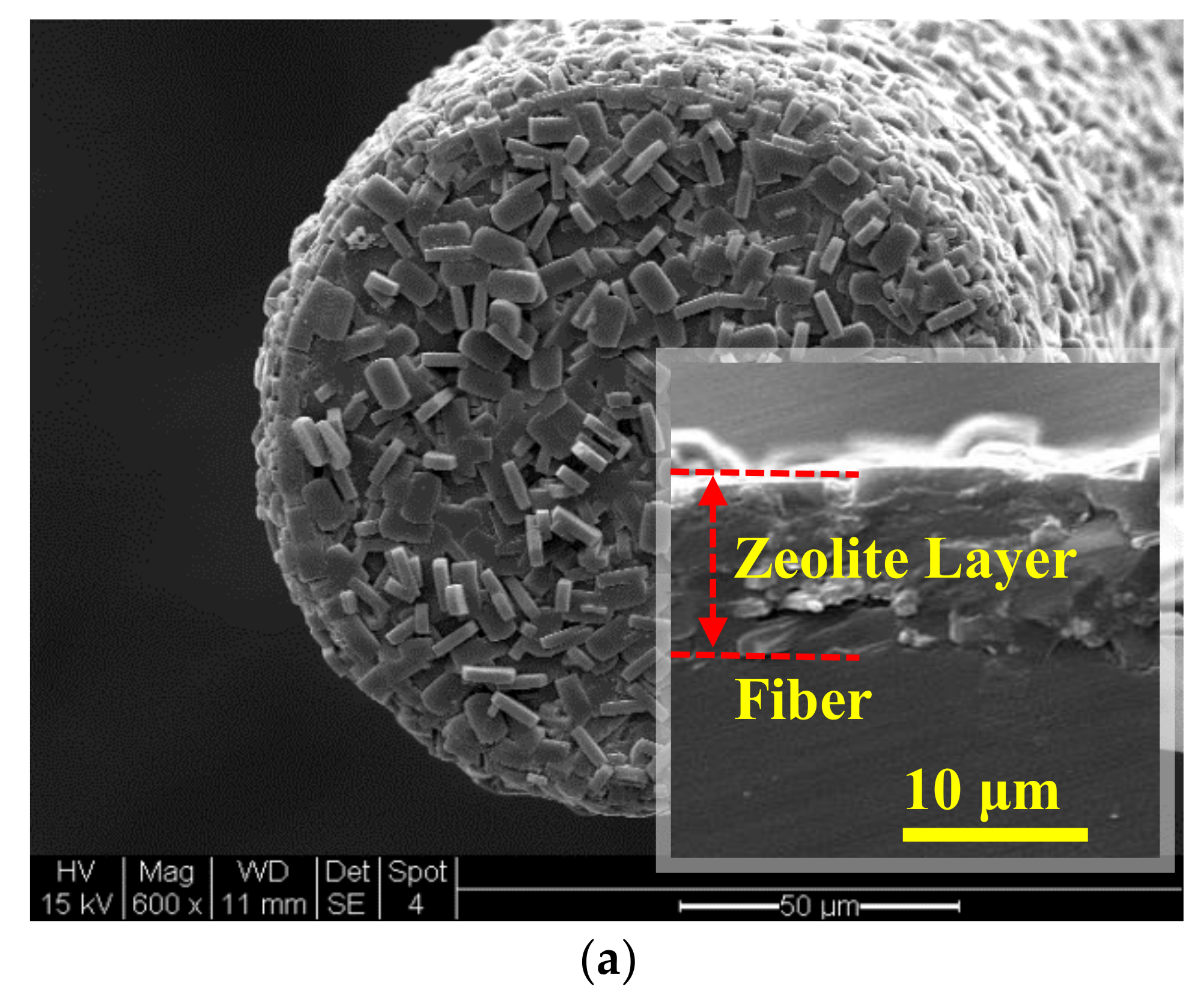
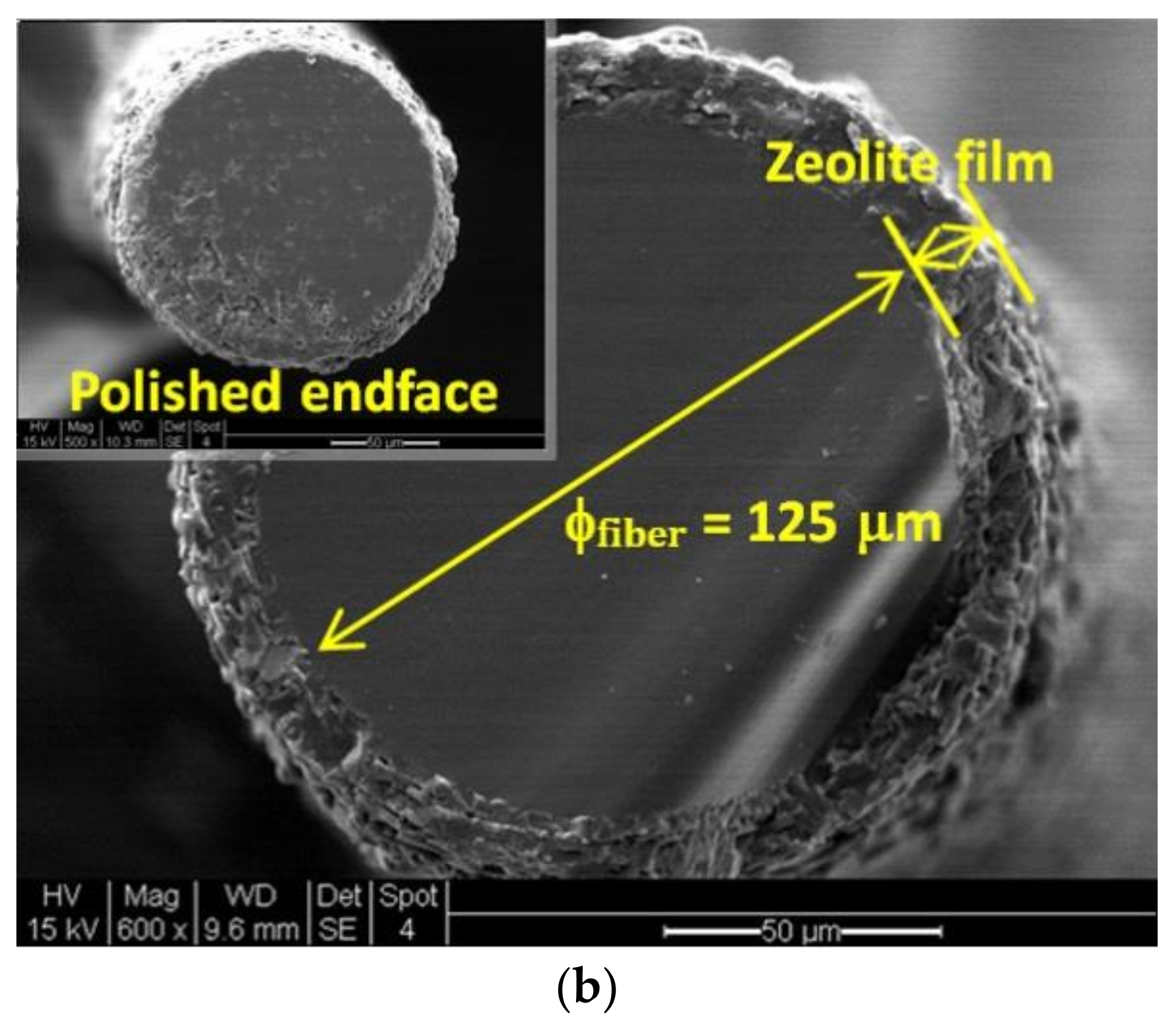
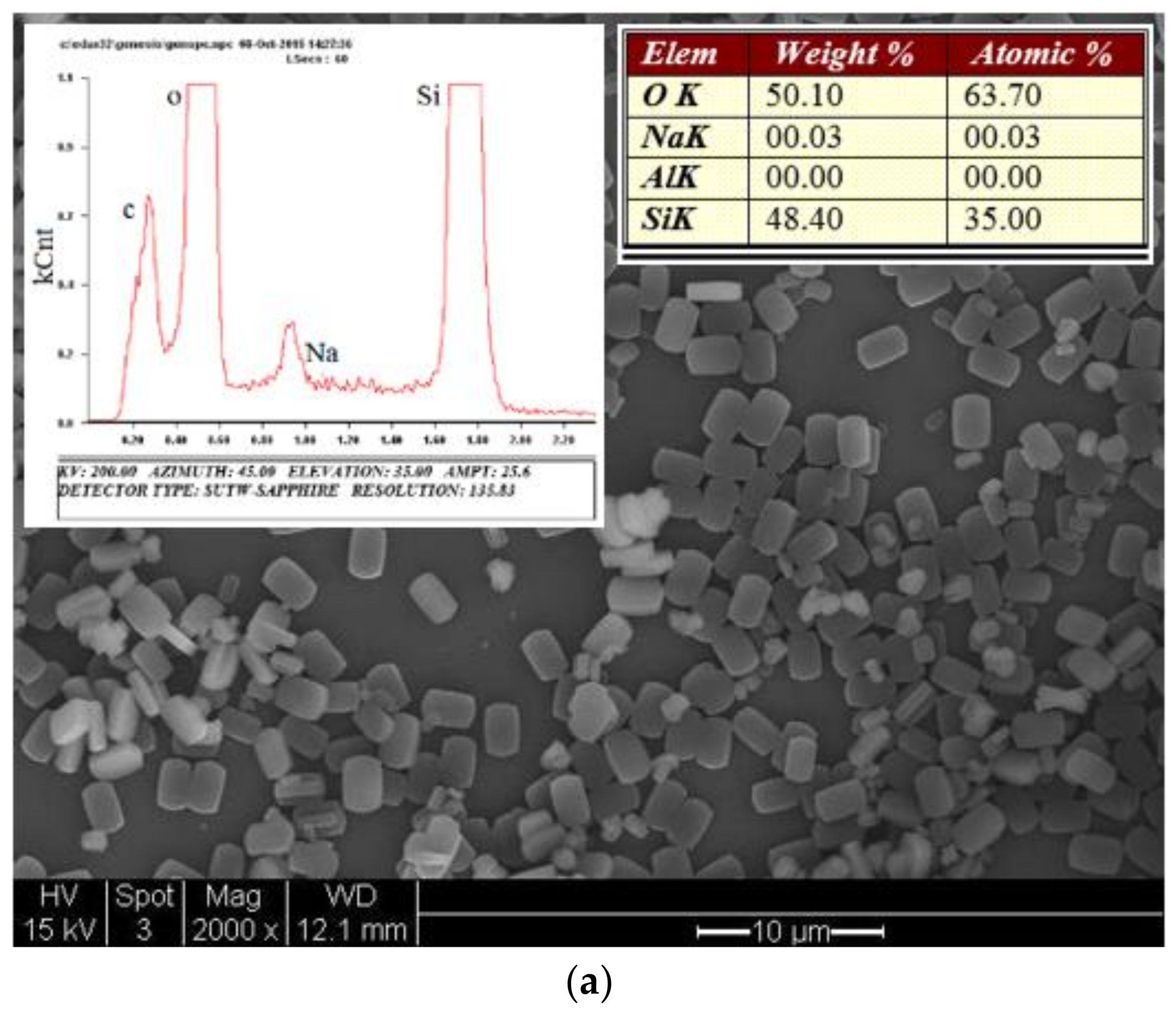
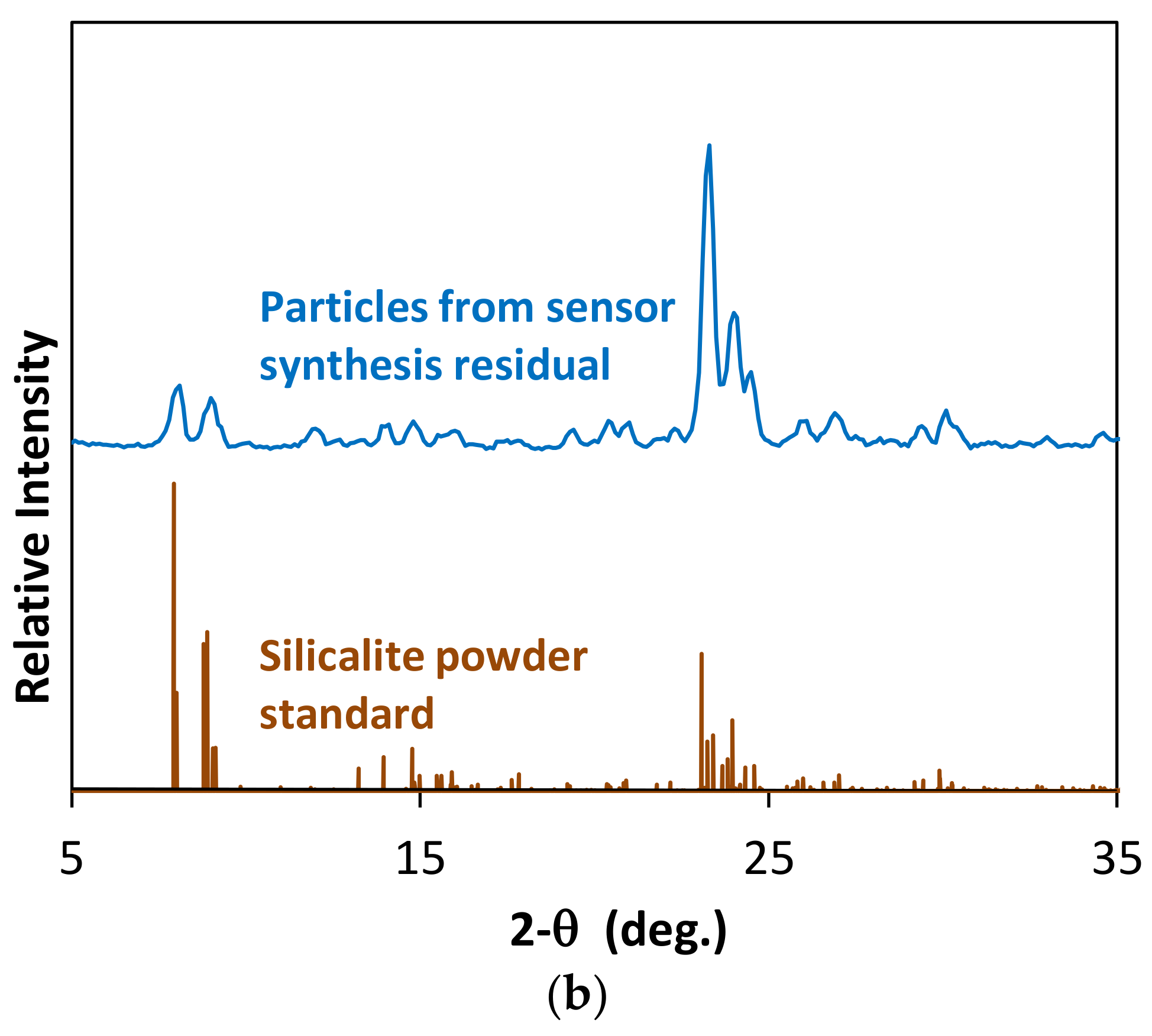
3.1. Zeolite Film-Coated FOI Sensor
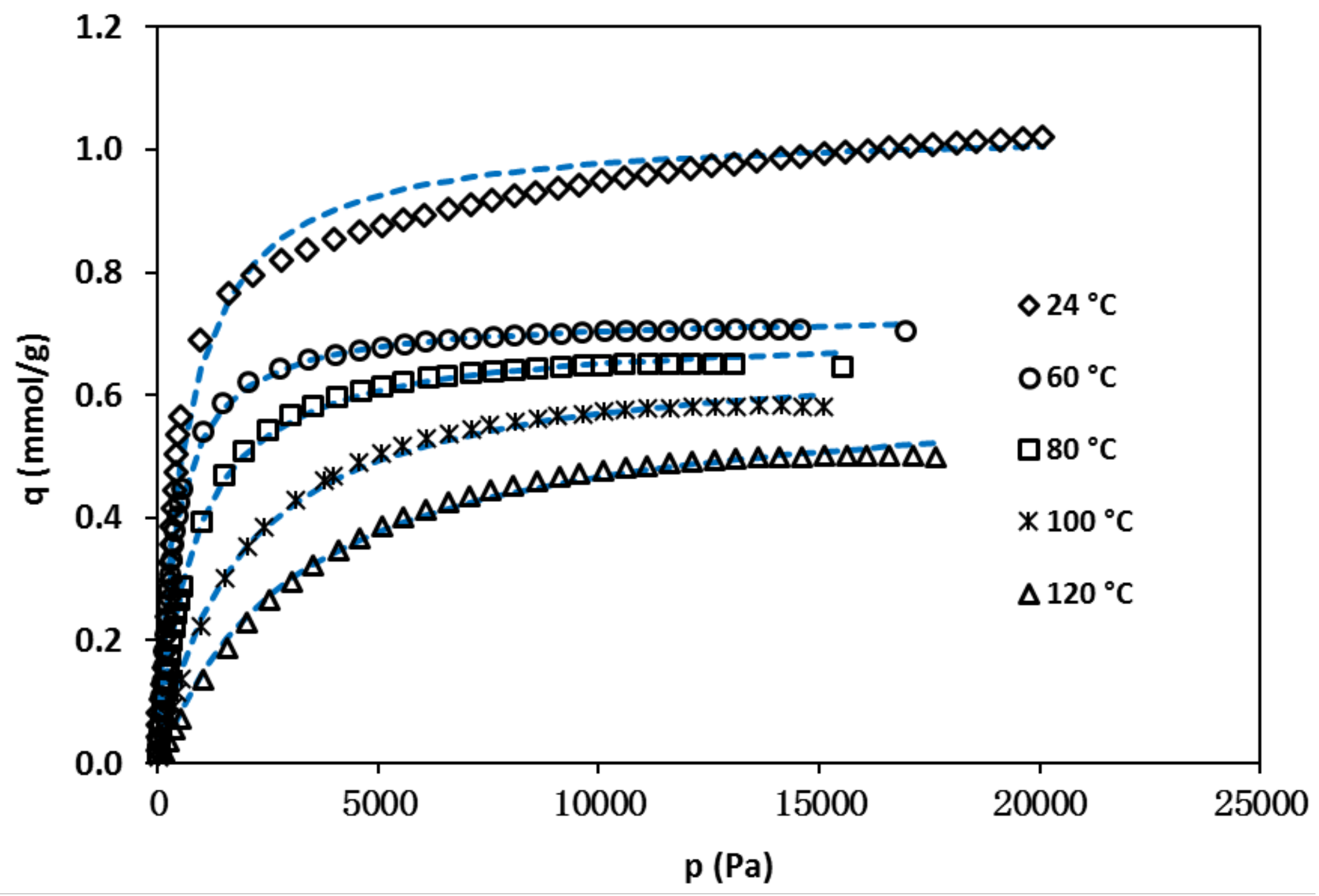
3.2. Isobutane Isotherms
3.3. Diffusivity Determination by Optical Measurements
3.3.1. Diffusivity and Concentration Dependence
3.3.2. Determination of Temperature Dependence
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kärger, J.; Ruthven, D.M. Diffusion in Zeolites and Other Microporous Solids; Wiley: New York, NY, USA, 1992. [Google Scholar]
- Kärger, J.; Ruthven, D.M.; Theodorou, D.N. Diffusion in Nanoporous Materials; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 1329. [Google Scholar]
- Xu, Z.; Michos, I.; Cao, Z.; Jing, W.; Gu, X.; Kinkel, K.R.; Murad, S.; Dong, J. Proton-Selective Ion Transport in ZSM-5 Zeolite Membrane. J. Phys. Chem. C 2016, 120, 26386–26392. [Google Scholar] [CrossRef]
- Talu, O.; Sun, M.S.; Shah, D.B. Diffusivities of n-alkanes in silicalite by steady-state single-crystal membrane technique. AIChE J. 1998, 44, 681–694. [Google Scholar] [CrossRef]
- Ruthven, D.M. Diffusion in Zeolites. In Zeolites: A Refined Tool for Designing Catalytic Sites; Elesvier Science: Armsterdam, The Netherlands, 1995; Volume 97, pp. 223–234. [Google Scholar]
- Kärger, J.; Ruthven, D.M. Diffusion in nanoporous materials: Fundamental principles, insights and challenges. New J. Chem. 2016, 40, 4027–4048. [Google Scholar] [CrossRef]
- Chmelik, C.; Varmla, A.; Heinke, L.; Shah, D.B.; Kärger, J.; Kremer, F.; Wilczok, U.; Schmidt, W. Effect of surface modification on uptake rates of isobutane in MFI crystals: An infrared Microscopy study. Chem. Mater. 2007, 19, 6012–6019. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, M.; Xiao, H.; Dong, J. Interferometric study on the adsorption-dependent refractive index of silicalite thin films grown on optical fibers. Chem. Mater. 2006, 18, 4–6. [Google Scholar] [CrossRef]
- Kortunov, P.; Chmelik, C.; Kärger, J.; Rakoczy, R.A.; Ruthven, D.M.; Traa, Y.; Vasenkov, S.; Weitkamp, J. Sorption kinetics and intracrystalline diffusion of methanol in ferrierite: An example of disguised kinetics. Adsorpt.-J. Int. Adsorpt. Soc. 2005, 11, 235–244. [Google Scholar] [CrossRef]
- Papadokostaki, G.K.; Theodorou, D.N. Diffusion Fundamentals; Leipzig, Germany, 2005; Volume 2, pp. 1–25. [Google Scholar]
- Papadopoulos, G.K.; Theodorou, D.N.; Vasenkov, S.; Kärger, J. Mesoscopic simulations of the diffusivity of ethane in beds of NaX zeolite crystals: Comparison with pulsed field gradient NMR measurements. J. Chem. Phys. 2007, 126, 94702. [Google Scholar] [CrossRef] [PubMed]
- Bremard, C.; Bougeard, D. Raman-Scattering in Zeolites and Molecular-Sieves. Adv. Mater. 1995, 7, 10–25. [Google Scholar] [CrossRef]
- Hecht, E. Optics, 4th ed.; Addison Wesley: Boston, MA, USA, 2002. [Google Scholar]
- Xiao, H.; Zhang, J.; Dong, J.; Luo, M.; Lee, R.; Romero, V. Synthesis of MFI zeolite films on optical fibers for detection of chemical vapors. Opt. Lett. 2005, 30, 1270–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, J.; Luo, M.; Xiao, H.; Murad, S.; Normann, R.A. Zeolite-Fiber Integrated Optical Chemical Sensors for Detection of Dissolved Organics in Water. Langmuir 2005, 21, 8609–8612. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Hui, J.; Sun, C.; Dong, J.; Zhang, L.; Xiao, H. Nanoporous Zeolite Thin Film-Based Fiber Intrinsic Fabry-Perot Interferometric Sensor for Detection of Dissolved Organics in Water. Sensors 2006, 6, 835–847. [Google Scholar] [CrossRef]
- Schemmert, U.; Kärger, J.; Krause, C.; Rakoczy, R.A.; Weitkamp, J. Monitoring the Evolution of Intracrystalline Concentration. Europhys. Lett. 1999, 46, 204–210. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Bennett, J.M.; Grose, R.W.; Cohen, J.P.; Patton, R.L.; Kirchner, R.M.; Smith, J.V. Silicalite, a new hydrophobic crystalline silica molecular sieve. Nature 1978, 271, 512–516. [Google Scholar] [CrossRef]
- Zhu, W.; Kapteijn, F.; Moulijn, J.A. Equilibrium adsorption of light alkanes in silicalite-1 by the inertial microbalance technique. Adsorpt.-J. Int. Adsorpt. Soc. 2000, 6, 159–167. [Google Scholar] [CrossRef]
- Krishna, R.; Paschek, D. Molecular simulations of adsorption and siting of light alkanes in silicalite-1. Phys. Chem. Chem. Phys. 2001, 3, 453–462. [Google Scholar] [CrossRef]
- Paravar, A.R.; Hayhurst, D.T. Direct Measurement of Diffusivity for Butane across a Single Large Silicalite Crystal. In Proceedings of the Sixth International Zeolite Conference, Reno, USA, 10–15 July 1983; Olson, D., Bisio, A., Eds.; Butterworths: Guildford, UK, 1984; pp. 217–224. [Google Scholar]
- Bakker, W.J.W.; van den Broeke, L.J.P.; Kapteijn, F.; Moulijn, J.A. Temperature dependence of one-component permeation through a silicalite-1 membrane. AiChE J. 1997, 43, 2203–2214. [Google Scholar] [CrossRef]
- Shah, D.B.; Chokchalacha, S.; Hayhurst, D.T. Measurements of Transport Rates of C-4 Hydrocarbons across a Single-Crystal Silicalite Membrane. J. Chem. Soc.-Farad. Trans. 1993, 89, 3161–3167. [Google Scholar] [CrossRef]
- Zhu, W.; Malekian, A.; Eic, M.; Kapteijn, F.; Moulijn, J.A. Concentration-dependent diffusion of isobutane in silicalite-1 studied with the ZLC technique. Chem. Eng. Sci. 2004, 59, 3827–3835. [Google Scholar] [CrossRef]
- Millot, B.; Methivier, A.; Jobic, H.; Moueddeb, H.; Dalmon, J.A. Permeation of linear and branched alkanes in ZSM-5 supported membranes. Microporous Mesoporous Mater. 2000, 38, 85–95. [Google Scholar] [CrossRef]
- Jiang, M.; Eic, M.; Miachon, S.; Dalmon, J.A.; Kocirik, M. Diffusion of n-butane, isobutane and ethane in a MFI-zeolite membrane investigated by gas permeation and ZLC measurements. Sep. Purif. Technol. 2001, 25, 287–295. [Google Scholar] [CrossRef]
- Millot, B.; Methivier, A.; Jobic, H.; Moueddeb, H.; Bee, M. Diffusion of isobutane in ZSM-5 zeolite: A comparison of quasi-elastic neutron scattering and supported membrane results. J. Phys. Chem. B 1999, 103, 1096–1101. [Google Scholar] [CrossRef]
- Majchrowicz, D.; Hirsch, M.; Wierzba, P.; Bechelany, M.; Viter, R.; Jedrzejewska-Szczerska, M. Application of Thin ZnO ALD Layers in Fiber-Optic Fabry-Pérot Sensing Interferometers. Sensors 2016, 16, 416. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.; Majchrowicz, D.; Wierzba, P.; Weber, M.; Bechelany, M.; Jedrzejewska-Szczerska, M. Low-Coherence Interferometric Fiber-Optic Sensors with Potential Applications as Biosensors. Sensors 2017, 17, 261. [Google Scholar] [CrossRef] [PubMed]

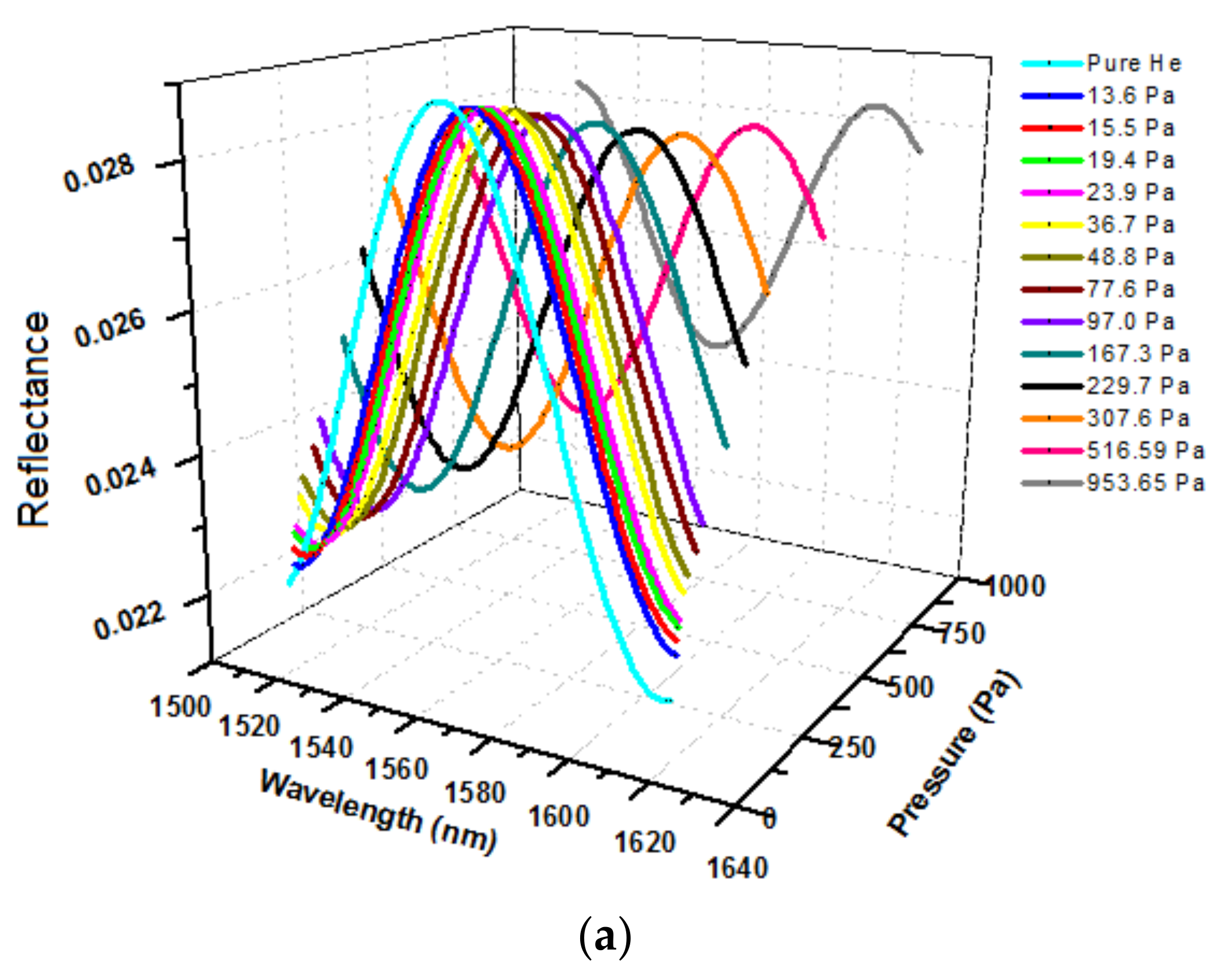
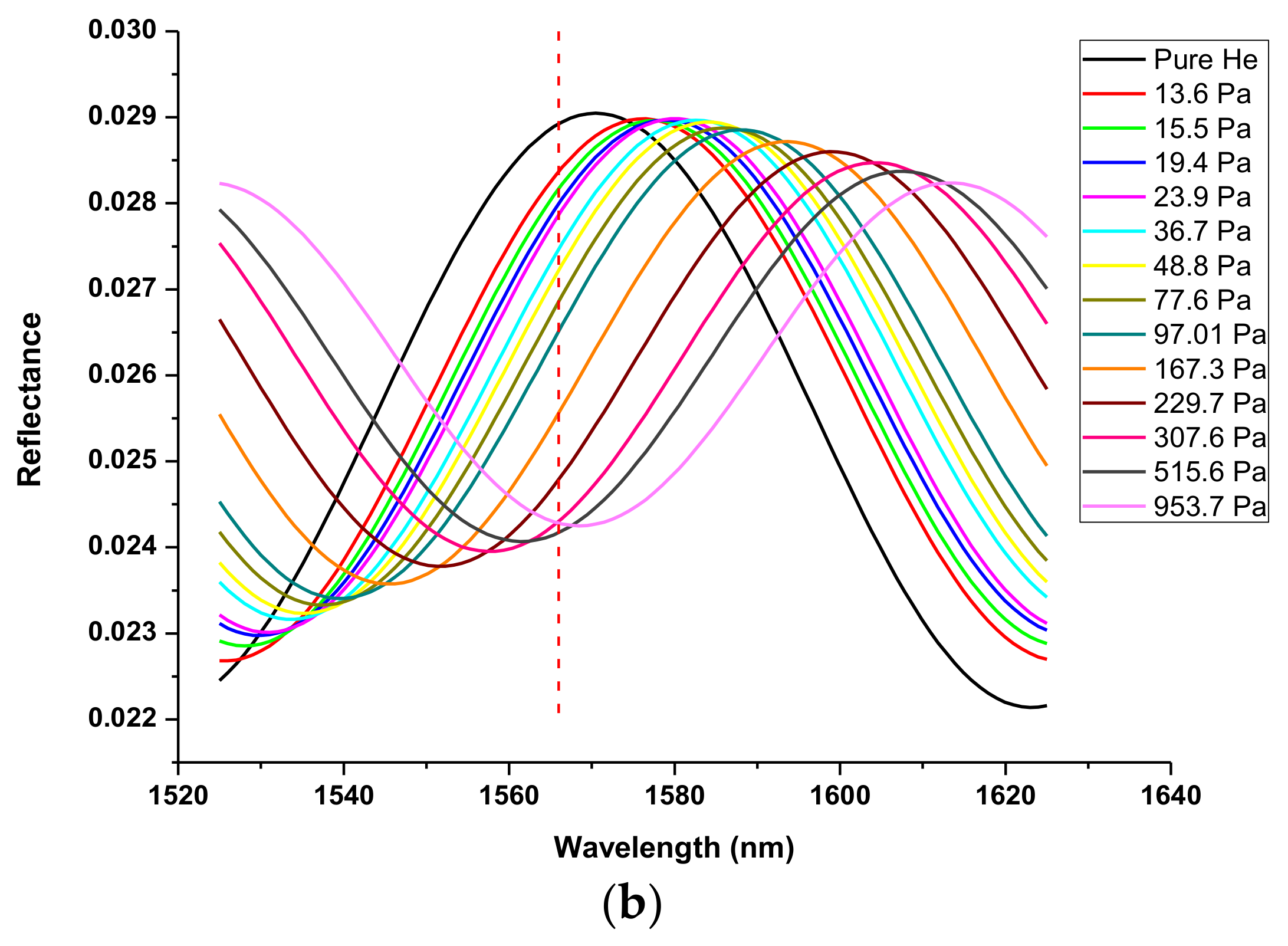

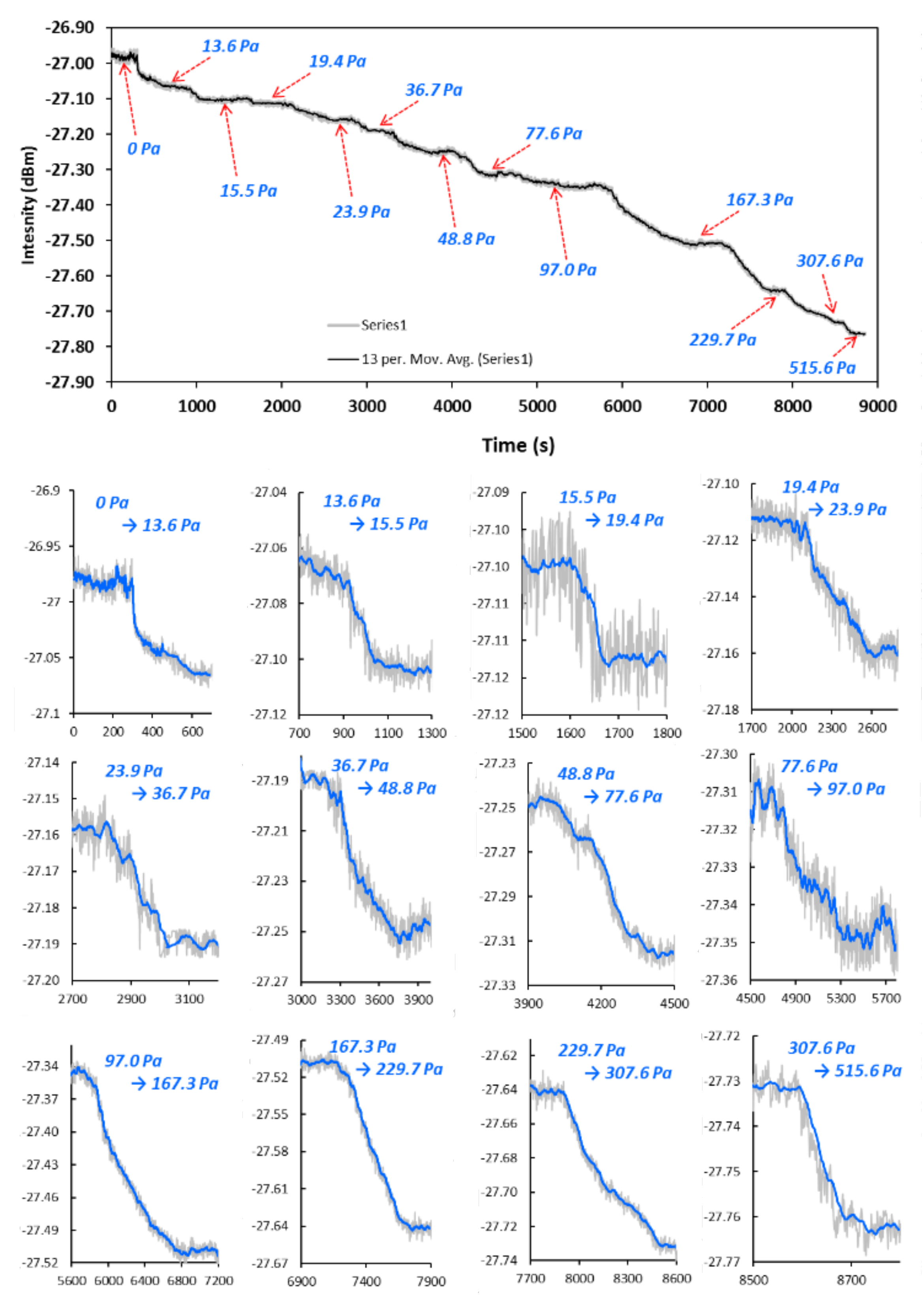

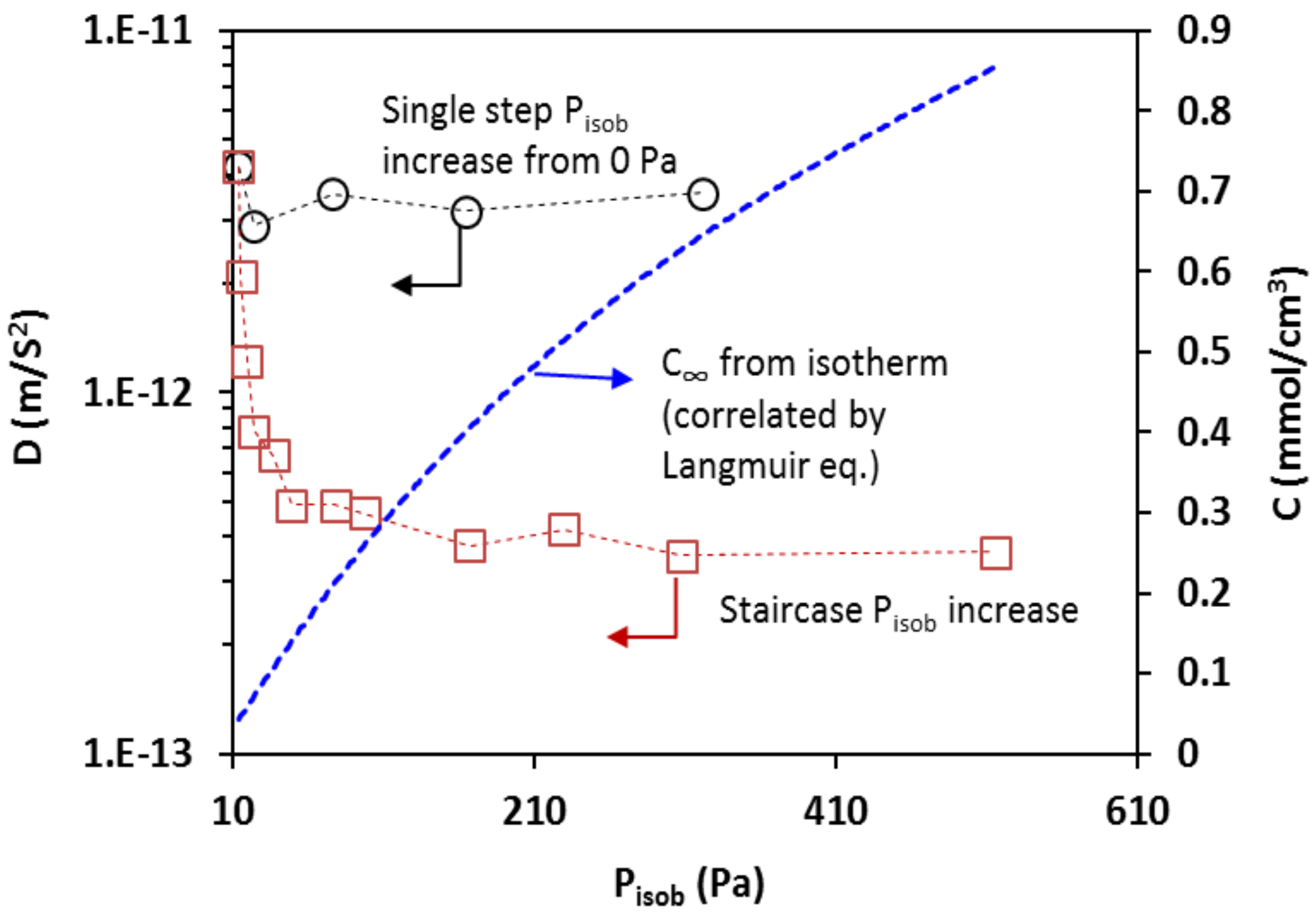
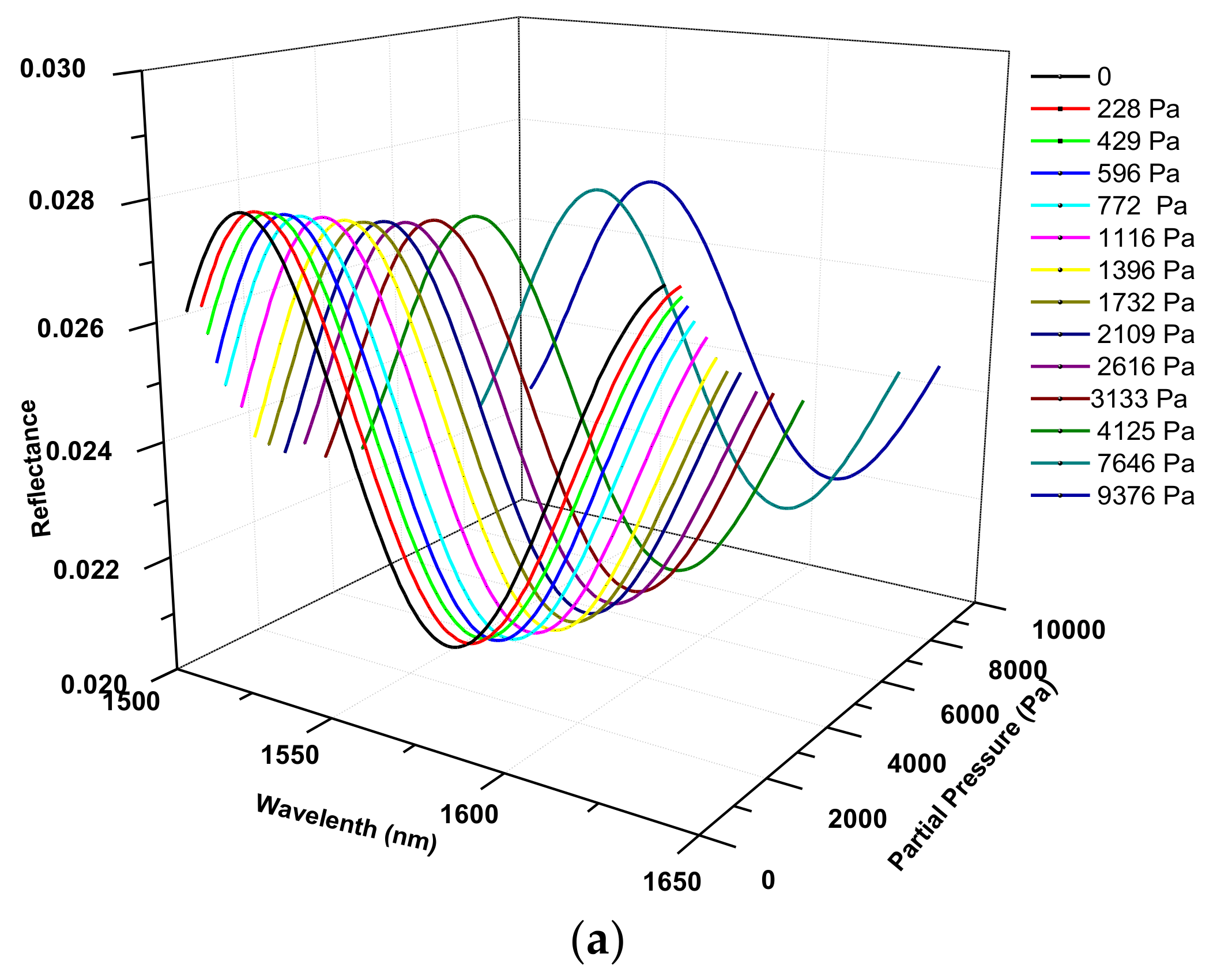
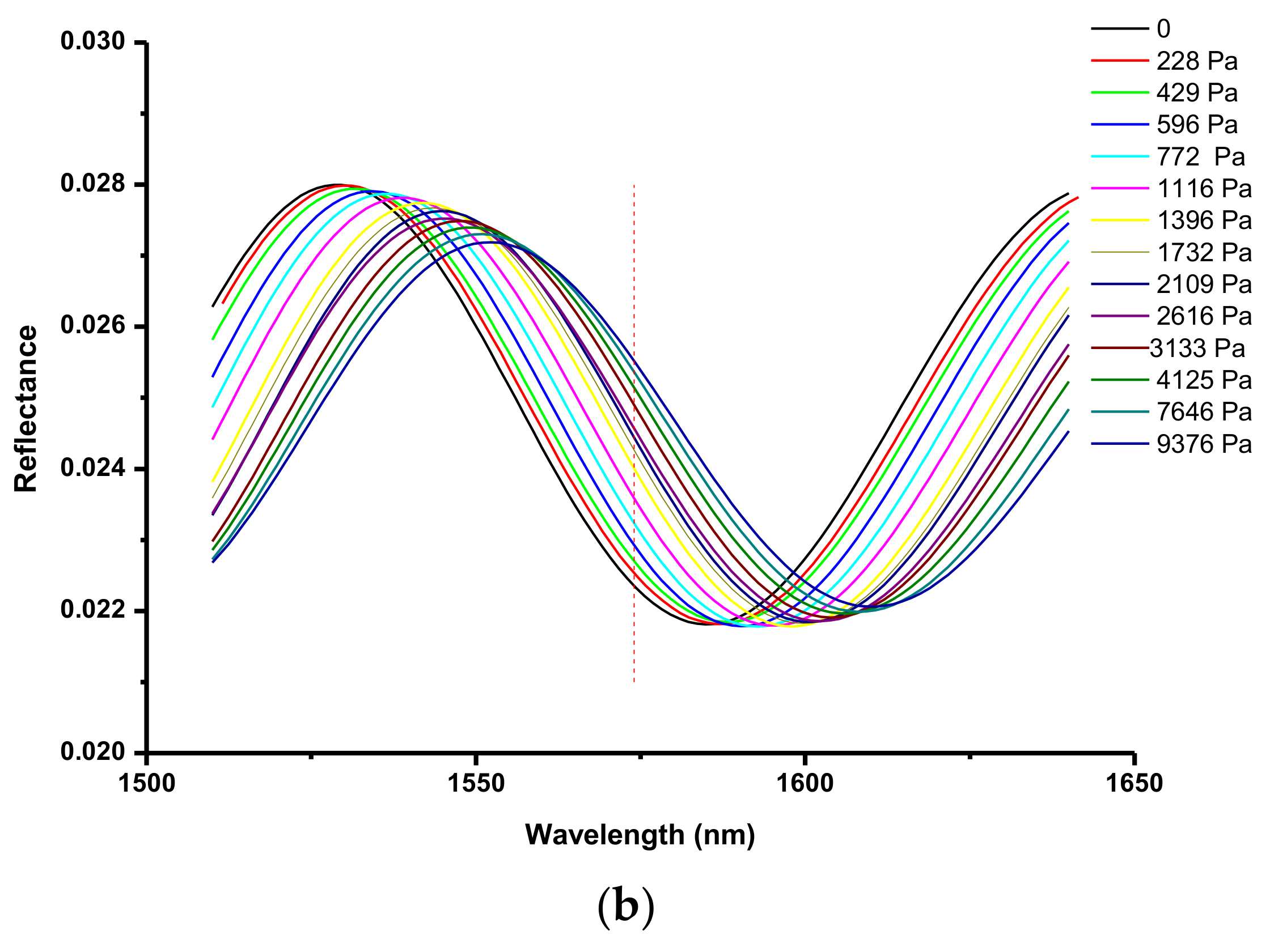
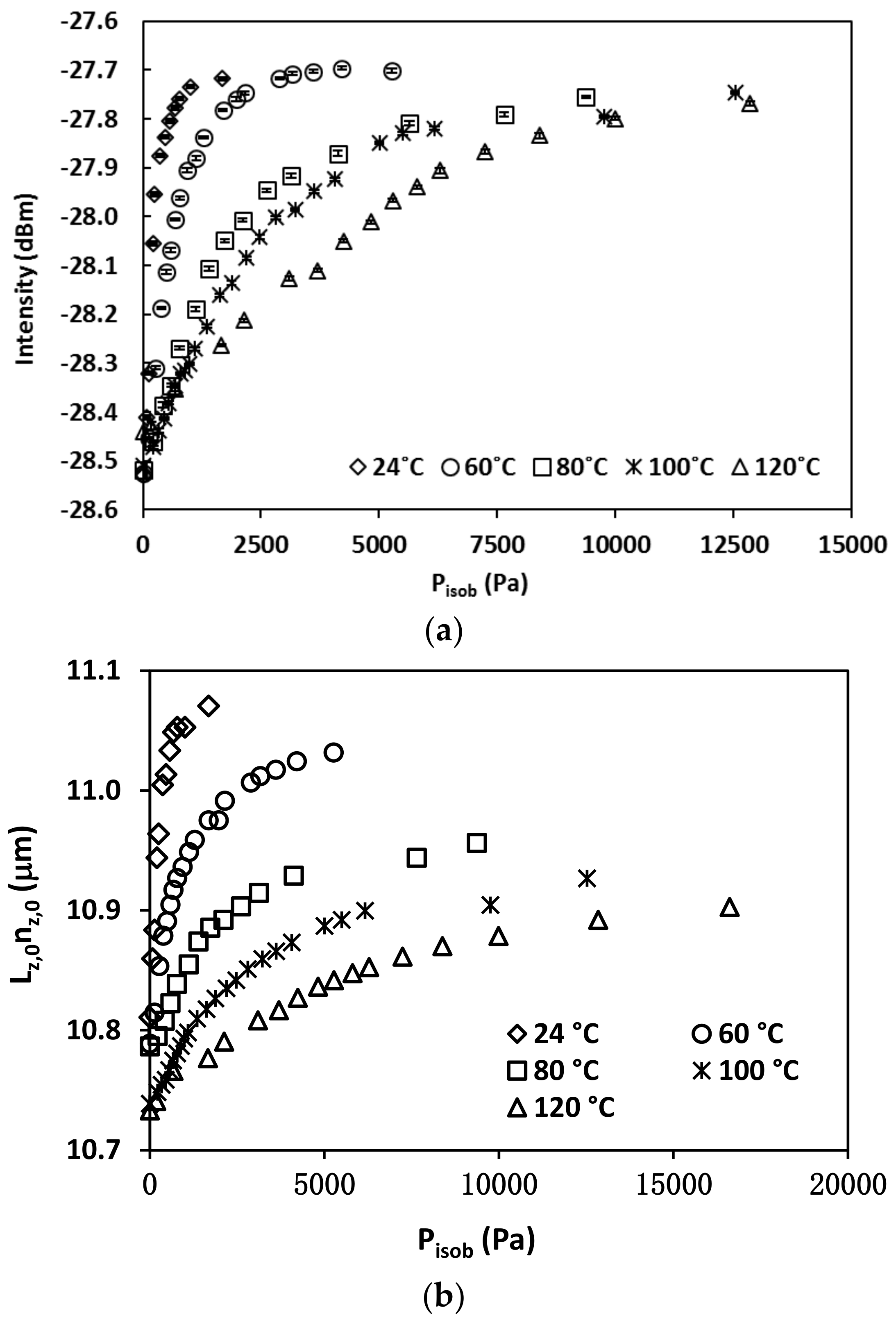
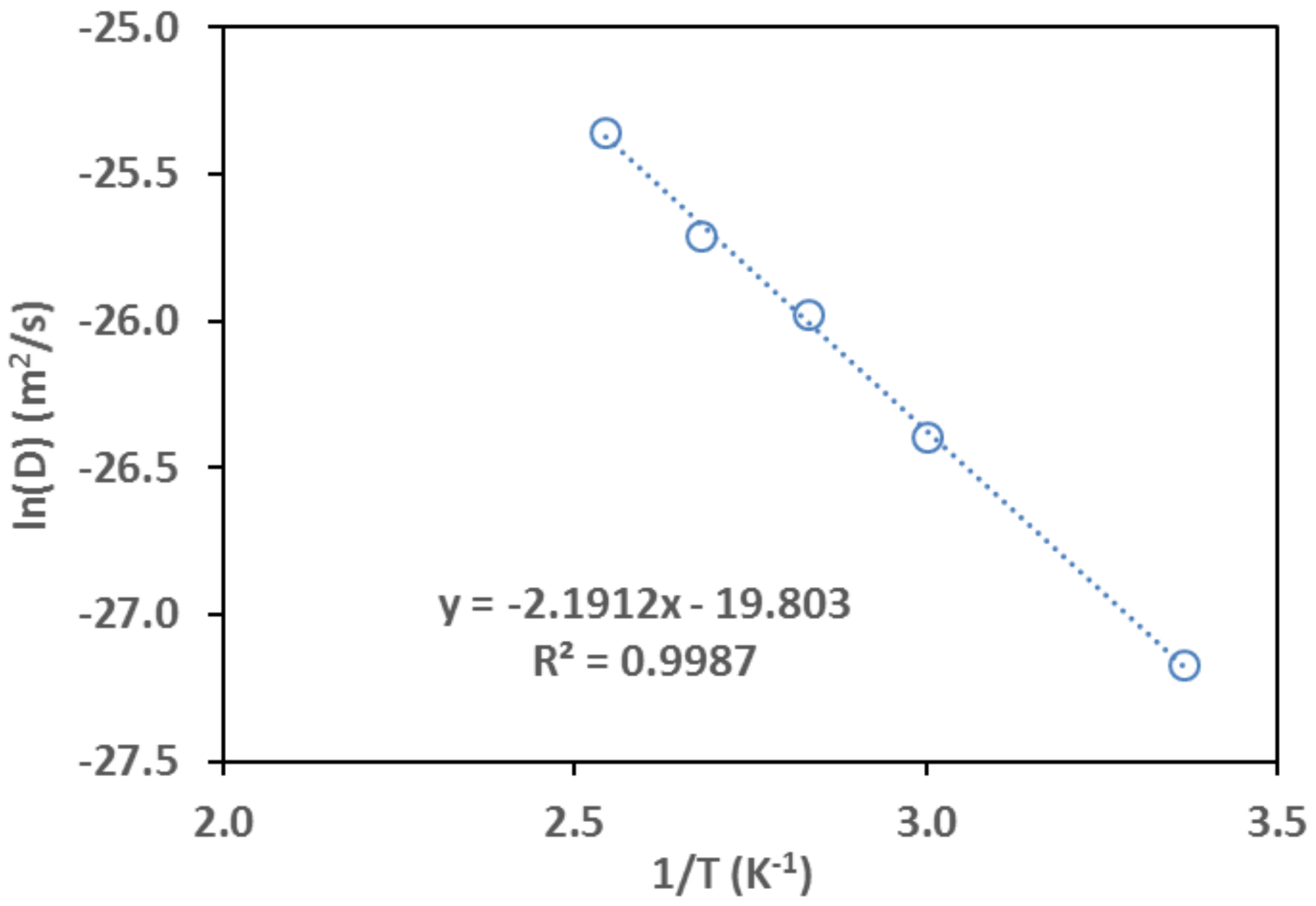
| T, °C | , 10−3 mol/g | , 10−3 mol/cm3 | , 10−3 Pa−1 |
|---|---|---|---|
| 24 | 1.034 | 1.8198 | 1.702 |
| 60 | 0.7309 | 1.2864 | 1.562 |
| 80 | 0.7019 | 1.2353 | 1.271 |
| 100 | 0.6743 | 1.1868 | 0.541 |
| 120 | 0.6143 | 1.0811 | 0.317 |
| Zeolite | T, K | D, 10−12 m2/s | Method | Source |
|---|---|---|---|---|
| Silicalite | 297 | 1.20–4.20 | FOI (1) | This work |
| Silicalite | 297 | 2.89–4.20 | FOI (2) | This work |
| Silicalite | 297 | 1.90 | MBR | [21] |
| Silicalite | 303 | 2.50 | MBR | [22] |
| Silicalite | 303 | 1.10 | MBR | [23] |
| Silicalite | 298 | 1.37–2.29 | FTIR | [7] |
| Silicalite | 303 | 1.49 | ZLC | [24] |
| ZSM-5 (Si/Al = 36) | 303 | 7.00 | MBR | [25] |
| ZSM-5 (Si/Al = 35) | 293 | 0.14 | ZLC | [26] |
| Zeolite | , 10−9 m2/s | Ed, kJ/mol | Method | Source |
|---|---|---|---|---|
| Silicalite | 2.51 | 18.22 | FOI | this study |
| Silicalite | 4.30 | 32.2 | ZLC | [24] |
| Silicalite | 3.29 | 24.3 | ZLC | [26] |
| ZSM-5 (Si/Al = 35) | 6.70 | 31.6 | MBR | [25] |
| ZSM-5 (Si/Al = 36) | 1.50 | 15.1 | MBR | [22] |
| ZSM-5 (Si/Al = 36) | NA | 24.2 | CHG | [27] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Xu, Z.; Zeng, S.; Jing, W.; Trontz, A.; Dong, J. A Fiber Optic Interferometric Sensor Platform for Determining Gas Diffusivity in Zeolite Films. Sensors 2018, 18, 1090. https://doi.org/10.3390/s18041090
Yang R, Xu Z, Zeng S, Jing W, Trontz A, Dong J. A Fiber Optic Interferometric Sensor Platform for Determining Gas Diffusivity in Zeolite Films. Sensors. 2018; 18(4):1090. https://doi.org/10.3390/s18041090
Chicago/Turabian StyleYang, Ruidong, Zhi Xu, Shixuan Zeng, Wenheng Jing, Adam Trontz, and Junhang Dong. 2018. "A Fiber Optic Interferometric Sensor Platform for Determining Gas Diffusivity in Zeolite Films" Sensors 18, no. 4: 1090. https://doi.org/10.3390/s18041090




