Label-Free Impedance Sensing of Aflatoxin B1 with Polyaniline Nanofibers/Au Nanoparticle Electrode Array
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Apparatus
2.3. Preparation of Au-Polyaniline (Au-PANI) on ITO Disk Electrodes
2.4. Surface Engineering of Impedimetric AFB1 Sensor
2.5. Aflatoxin B1 Spiking Test for Real Sample Analysis
3. Results and Discussion
3.1. Formation and SEM Characterization of Au-PANI Nanofibers on ITO Disk Electrode
3.2. Electrochemical Impedance Characterization of the Au-PANI Electrode
3.3. Optical Characterization of Au-PANI Electrodes
3.4. EIS Characterization of the Au-PANI Layer for Surface Immobilization
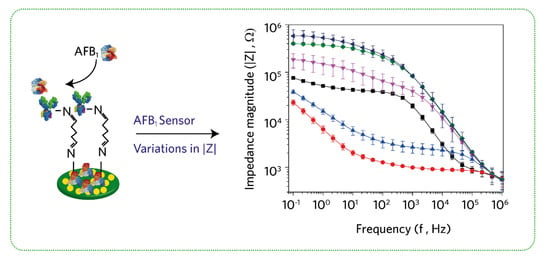
3.5. Impedance-Based AFB1 Sensor Characterization
3.6. Selectivity, Stability, and Reproducibility of the Developed AFB1 Sensor
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Supriya, C.; Akhila, B.; Reddy, K.P.; Girish, B.P.; Reddy, P.S. Effects of maternal exposure to aflatoxin b1 during pregnancy on fertility output of dams and developmental, behavioral and reproductive consequences in female offspring using a rat model. Toxicol. Mech. Methods 2016, 26, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Bossou, Y.M.; Serssar, Y.; Allou, A.; Vitry, S.; Momas, I.; Seta, N.; Menotti, J.; Achard, S. Impact of mycotoxins secreted by aspergillus molds on the inflammatory response of human corneal epithelial cells. Toxins 2017, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Sergeyeva, T.; Yarynka, D.; Piletska, E.; Lynnik, R.; Zaporozhets, O.; Brovko, O.; Piletsky, S.; El’skaya, A. Fluorescent sensor systems based on nanostructured polymeric membranes for selective recognition of aflatoxin b1. Talanta 2017, 175, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Soriano, J.M.; Molto, J.C.; Marin, R.; Manes, J. Determination of aflatoxins in peanuts by matrix solid-phase dispersion and liquid chromatography. J. Chromatogr. A 2003, 1011, 49–54. [Google Scholar] [CrossRef]
- Lin, L.M.; Zhang, J.; Wang, P.; Wang, Y.S.; Chen, J.P. Thin-layer chromatography of mycotoxins and comparison with other chromatographic methods. J. Chromatogr. A 1998, 815, 3–20. [Google Scholar] [CrossRef]
- Zheng, Z.M.; Humphrey, C.W.; King, R.S.; Richard, J.L. Validation of an elisa test kit for the detection of total aflatoxins in grain and grain products by comparison with HPLC. Mycopathologia 2005, 159, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Yagati, A.K.; Pyun, J.C.; Min, J.; Cho, S. Label-free and direct detection of c-reactive protein using reduced graphene oxide-nanoparticle hybrid impedimetric sensor. Bioelectrochemistry 2016, 107, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Brett, C. Electrochemical impedance spectroscopy for characterization of electrochemical sensors and biosensors. ECS Trans. 2008, 13, 67–80. [Google Scholar]
- Varshney, M.; Li, Y.B.; Srinivasan, B.; Tung, S. A label-free, microfluidics and interdigitated array microelectrode-based impedance biosensor in combination with nanoparticles immunoseparation for detection of Escherichia coli o157:H7 in food samples. Sens. Actuators B Chem. 2007, 128, 99–107. [Google Scholar] [CrossRef]
- Rengaraj, S.; Cruz-Izquierdo, Á.; Scott, J.L.; Di Lorenzo, M. Impedimetric paper-based biosensor for the detection of bacterial contamination in water. Sens. Actuators B Chem. 2018, 265, 50–58. [Google Scholar] [CrossRef]
- Singh, C.; Ali, M.A.; Kumar, V.; Ahmad, R.; Sumana, G. Functionalized MOS2 nanosheets assembled microfluidic immunosensor for highly sensitive detection of food pathogen. Sens. Actuators B Chem. 2018, 259, 1090–1098. [Google Scholar] [CrossRef]
- Zejli, H.; Goud, K.Y.; Marty, J.L. Label free aptasensor for ochratoxin a detection using polythiophene-3-carboxylic acid. Talanta 2018, 185, 513–519. [Google Scholar] [CrossRef]
- Smolko, V.; Shurpik, D.; Porfireva, A.; Evtugyn, G.; Stoikov, I.; Hianik, T. Electrochemical aptasensor based on poly(neutral red) and carboxylated pillar[5]arene for sensitive determination of aflatoxin m1. Electroanal 2018, 30, 486–496. [Google Scholar] [CrossRef]
- Geleta, G.S.; Zhao, Z.; Wang, Z. A novel reduced graphene oxide/molybdenum disulfide/polyaniline nanocomposite-based electrochemical aptasensor for detection of aflatoxin b1. Analyst 2018, 143, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, A.; Khan, R. A highly sensitive amperometric immunosensor probe based on gold nanoparticle functionalized poly(3,4-ethylenedioxythiophene) doped with graphene oxide for efficient detection of aflatoxin b-1. Synthet. Met. 2018, 235, 136–144. [Google Scholar] [CrossRef]
- Yagati, A.K.; Park, J.; Cho, S. Reduced graphene oxide modified the interdigitated chain electrode for an insulin sensor. Sensors 2016, 16, 109. [Google Scholar] [CrossRef] [PubMed]
- Yagati, A.K.; Cho, S. Effect of the relative area of exposed electrode to the insulating area on the impedance measurement of electrolyte. J. Nanosci. Nanotechnol. 2016, 16, 8733–8736. [Google Scholar] [CrossRef]
- Yagati, A.K.; Min, J.; Cho, S. Electrosynthesis of ergo-np nanocomposite films for bioelectrocatalysis of horseradish peroxidase towards H2O2. J. Electrochem. Soc. 2014, 161, G133–G140. [Google Scholar] [CrossRef]
- Nemati, M.; Hosseini, S.M.; Bagheripour, E.; Madaeni, S.S. Surface modification of cation exchange membranes by graft polymerization of paa-co-pani/mwcnts nanoparticles. Korean J. Chem. Eng. 2016, 33, 1037–1046. [Google Scholar] [CrossRef]
- Silva, C.H.B.; Galiote, N.A.; Huguenin, F.; Teixeira-Neto, E.; Constantino, V.R.L.; Temperini, M.L.A. Spectroscopic, morphological and electrochromic characterization of layer-by-layer hybrid films of polyaniline and hexaniobate nanoscrolls. J. Mater. Chem. 2012, 22, 14052–14060. [Google Scholar] [CrossRef]
- Amaya, T.; Isaji, T.; Abe, M.; Hirao, T. Synthesis of polyaniline and transition metal nanoparticles hybrids. J. Inorg. Organomet. Polym. 2015, 25, 145–152. [Google Scholar] [CrossRef]
- Pillalamarri, S.K.; Blum, F.D.; Tokuhiro, A.T.; Bertino, M.F. One-pot synthesis of polyaniline—Metal nanocomposites. Chem. Mater. 2005, 17, 5941–5944. [Google Scholar] [CrossRef]
- Yagati, A.K.; Choi, Y.; Park, J.; Choi, J.W.; Jun, H.S.; Cho, S. Silver nanoflower-reduced graphene oxide composite based micro-disk electrode for insulin detection in serum. Biosens. Bioelectron. 2016, 80, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, P.; Bagheri, H.; Afkhami, A.; Ardakani, Y.H.; Madrakian, T. Fabrication of a novel aptasensor based on three-dimensional reduced graphene oxide/polyaniline/gold nanoparticle composite as a novel platform for high sensitive and specific cocaine detection. Anal. Chim. Acta 2017, 996, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Y.; Yang, T.; Zhang, W.; Jiang, C.; Jiao, K. Enhanced sensitivity for deoxyribonucleic acid electrochemical impedance sensor: Gold nanoparticle/polyaniline nanotube membranes. Anal. Chim. Acta 2008, 616, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Lang, H.; Bahadur, D. Polyaniline-iron oxide nanohybrid film as multi-functional label-free electrochemical and biomagnetic sensor for catechol. Anal. Chim. Acta 2013, 795, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjya, D.; Mukhopadhyay, I. Controlled growth of polyaniline fractals on hopg through potentiodynamic electropolymerization. Langmuir 2012, 28, 5893–5899. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Park, S.M. Electrochemistry of conductive polymers—XXVI. Effects of electrolytes and growth methods on polyaniline morphology. J. Electrochem. Soc. 2002, 149, E26–E34. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Cabral-Miranda, G.; Reyes-Sandoval, A.; Bachmann, M.F.; Sales, M.G.F. Detecting circulating antibodies by controlled surface modification with specific target proteins: Application to malaria. Biosens. Bioelectron. 2017, 91, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.; Spinella, K.; Poturnayova, A.; Snejdarkova, M.; Mosiello, L.; Hianik, T. Detection of aflatoxin b-1 by aptamer-based biosensor using pamam dendrimers as immobilization platform. Food Control 2015, 52, 9–18. [Google Scholar] [CrossRef]
- Gutierrez, R.A.V.; Hedström, M.; Mattiasson, B. Bioimprinting as a tool for the detection of aflatoxin b1 using a capacitive biosensor. Biotechnol. Rep. 2016, 11, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. A label-free electrochemical impedance immunosensor for the sensitive detection of aflatoxin b-1. Anal. Methods 2015, 7, 2354–2359. [Google Scholar] [CrossRef]
- Li, Z.M.; Ye, Z.Z.; Fu, Y.C.; Xiong, Y.H.; Li, Y.B. A portable electrochemical immunosensor for rapid detection of trace aflatoxin b-1 in rice. Anal. Methods 2016, 8, 548–553. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Stepanova, V.; Sitdikov, R.; Stoikov, I.; Nikolelis, D.; Hianik, T. Electrochemical aptasensor based on polycarboxylic macrocycle modified with neutral red for aflatoxin b1 detection. Electroanal 2014, 26, 2100–2109. [Google Scholar] [CrossRef]
- Yu, L.L.; Zhang, Y.; Hu, C.Y.; Wu, H.; Yang, Y.Y.; Huang, C.S.; Jia, N.Q. Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of afb(1) in olive oil. Food Chem. 2015, 176, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Wang, Z.Y.; Sun, X.L.; Fang, Y.J.; Chen, P.P. A sensitive and highly stable electrochemical impedance immunosensor based on the formation of silica gel-ionic liquid biocompatible film on the glassy carbon electrode for the determination of aflatoxin b-1 in bee pollen. Talanta 2010, 80, 1632–1637. [Google Scholar]
- Foguel, M.V.; Giordano, G.F.; de Sylos, C.M.; Carlos, I.Z.; Ferreira, A.A.P.; Benedetti, A.V.; Yamanaka, H. A low-cost label-free afb(1) impedimetric immunosensor based on functionalized cd-trodes. Chemosensors 2016, 4, 17. [Google Scholar] [CrossRef]
- Srivastava, S.; Ali, M.A.; Umrao, S.; Parashar, U.K.; Srivastava, A.; Sumana, G.; Malhotra, B.D.; Pandey, S.S.; Hayase, S. Graphene oxide-based biosensor for food toxin detection. Appl. Biochem. Biotechnol. 2014, 174, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, W.H.; Xiong, Y.H.; Xu, Y.; Li, C.M. Multifunctionalized reduced graphene oxide-doped polypyrrole/pyrrolepropylic acid nanocomposite impedimetric immunosensor to ultra-sensitively detect small molecular aflatoxin b-1. Biosens. Bioelectron. 2015, 63, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.F.; Cao, L.X.; Li, Q.C.; Ma, K.; Yan, P.S.; Kirk, D.W. Fabrication and modeling of an ultrasensitive label free impedimetric immunosensor for aflatoxin b-1 based on poly(o-phenylenediamine) modified gold 3D nano electrode ensembles. RSC Adv. 2015, 5, 55209–55217. [Google Scholar] [CrossRef]
- Kolosova, A.Y.; Shim, W.B.; Yang, Z.Y.; Eremin, S.A.; Chung, D.H. Direct competitive elisa based on a monoclonal antibody for detection of aflatoxin b-1. Stabilization of elisa kit components and application to grain samples. Anal. Bioanal. Chem. 2006, 384, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.N.; Takabayashi, C.R.; Ono, M.A.; Saito, G.H.; Itano, E.N.; Kawamura, O.; Hirooka, E.Y.; Ono, E.Y.S. Immunoassay based on monoclonal antibody for aflatoxin detection in poultry feed. Food Chem. 2012, 132, 2211–2216. [Google Scholar] [CrossRef]
- Braga, S.M.L.F.M.; de Medeiros, F.D.; Oliveira, E.D.; Macedo, R.O. Development and validation of a method for the quantitative determination of aflatoxin contaminants in maytenus ilicifolia by hplc with fluorescence detection. Phytochem. Anal. 2005, 16, 267–271. [Google Scholar] [CrossRef] [PubMed]






| Electrode | RS [Ω] | CPE | Rct [Ω] | |
|---|---|---|---|---|
| T [×10−9 Ω−1·sP] | P | |||
| Bare-ITO | 918.7 ± 30.01 | 0.027 ± 0.005 | 0.893 ± 0.021 | 54,852 ± 1803.3 |
| PANI-ITO | 925.2 ± 20.83 | 67.02 ± 4.911 | 0.585 ± 0.021 | 45,597 ± 129.8 |
| Au-PANI-ITO | 922.7 ± 18.84 | 84.69 ± 5.99 | 0.680 ± 0.025 | 27,154 ± 571.7 |
| Sensing Technique | Transducing Matrix | Linear Range (ng/mL) | Limit of Detection (ng/mL) | Reference |
|---|---|---|---|---|
| EIS, CV | Aptamers on dendrimers | 0.1–10 | 0.4 | [31] |
| Bio imprinting | Au electrode | 1–1000 | 0.00197 | [32] |
| EIS | AFB1/BSA/Au | 0.08–100 | 0.05 | [33] |
| EIS | SPIM a Au electrode | -NA- | 5.0 | [34] |
| EIS | Aptamer/GCE b | 0.1–100 | 0.05 | [35] |
| EIS | MWCNTs c/RTIL d | 0.1–10 | 0.03 | [36] |
| EIS | Ab e-SGIL f-GCE | 0.1–10 | 0.01 | [37] |
| EIS | Au-CD-trodes g | 1.56–31.2 | 0.11 | [38] |
| EIS | GO h/Au | 0.5–5 | 0.23 | [39] |
| EIS | PPy i/PPa j/rGO k | 0.0001–0.01 | 0.0001 | [40] |
| EIS | PoPD l/3DNEEs m | 0.04–8 | 0.019 | [41] |
| ELISA n | monoclonal antibodies | 0.1–10 | 0.05 | [42] |
| ELISA | monoclonal antibody | 0.05–10 | 0.036 | [43] |
| HPLC o | immunoaffinity columns | 4–20 | 3.5 | [44] |
| EIS | Ab/BSA/Au-PANI/ITO | 0.1–100 | 0.05 | This work |
| Sample | Added (ng/mL) | Found (ng/mL) | Recovery (%) | RSD (%) (n = 3) |
|---|---|---|---|---|
| Corn | 0.1 | 0.094 | 94.5 | 5.25 |
| 1 | 0.98 | 98.2 | 2.56 | |
| 100 | 103.7 | 103.7 | 3.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagati, A.K.; Chavan, S.G.; Baek, C.; Lee, M.-H.; Min, J. Label-Free Impedance Sensing of Aflatoxin B1 with Polyaniline Nanofibers/Au Nanoparticle Electrode Array. Sensors 2018, 18, 1320. https://doi.org/10.3390/s18051320
Yagati AK, Chavan SG, Baek C, Lee M-H, Min J. Label-Free Impedance Sensing of Aflatoxin B1 with Polyaniline Nanofibers/Au Nanoparticle Electrode Array. Sensors. 2018; 18(5):1320. https://doi.org/10.3390/s18051320
Chicago/Turabian StyleYagati, Ajay Kumar, Sachin Ganpat Chavan, Changyoon Baek, Min-Ho Lee, and Junhong Min. 2018. "Label-Free Impedance Sensing of Aflatoxin B1 with Polyaniline Nanofibers/Au Nanoparticle Electrode Array" Sensors 18, no. 5: 1320. https://doi.org/10.3390/s18051320
APA StyleYagati, A. K., Chavan, S. G., Baek, C., Lee, M.-H., & Min, J. (2018). Label-Free Impedance Sensing of Aflatoxin B1 with Polyaniline Nanofibers/Au Nanoparticle Electrode Array. Sensors, 18(5), 1320. https://doi.org/10.3390/s18051320