Thermochromic Polymer Film Sensors for Detection of Incipient Thermal Damage in Carbon Fiber–Epoxy Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
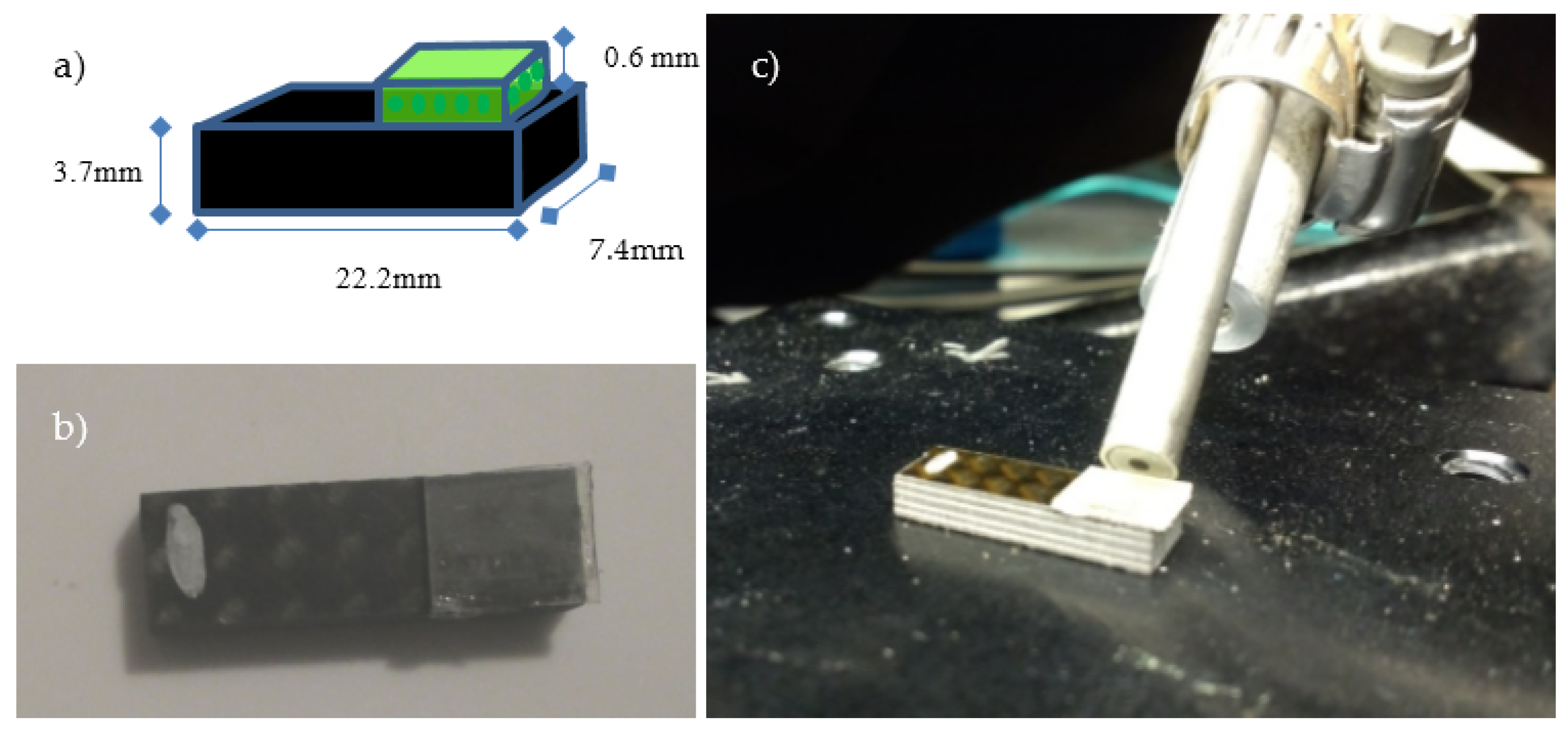
2.2. Sample Preparation
2.3. Thermal Exposure
2.4. Non-Destructive Evaluation
2.5. Mechanical Testing
2.6. Spectral Analysis and Imaging
3. Results
3.1. ITD of CFRE and Ultrasonic Testing Detection Threshold
3.2. Mechanical Testing
3.3. Fluorescent Activation of Sensors
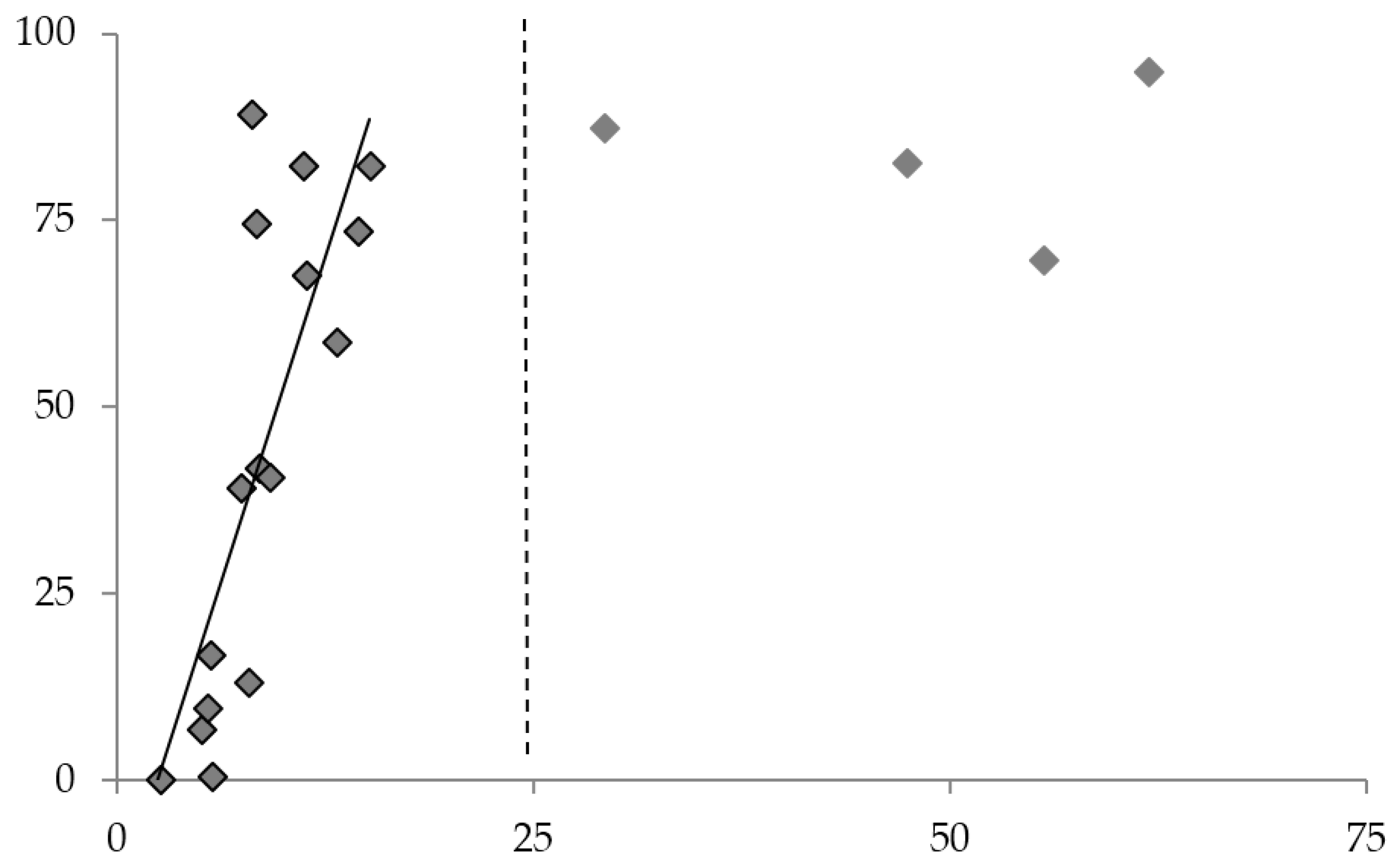
3.4. Correlation between Sensor Activation and Mechanical Property Loss
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
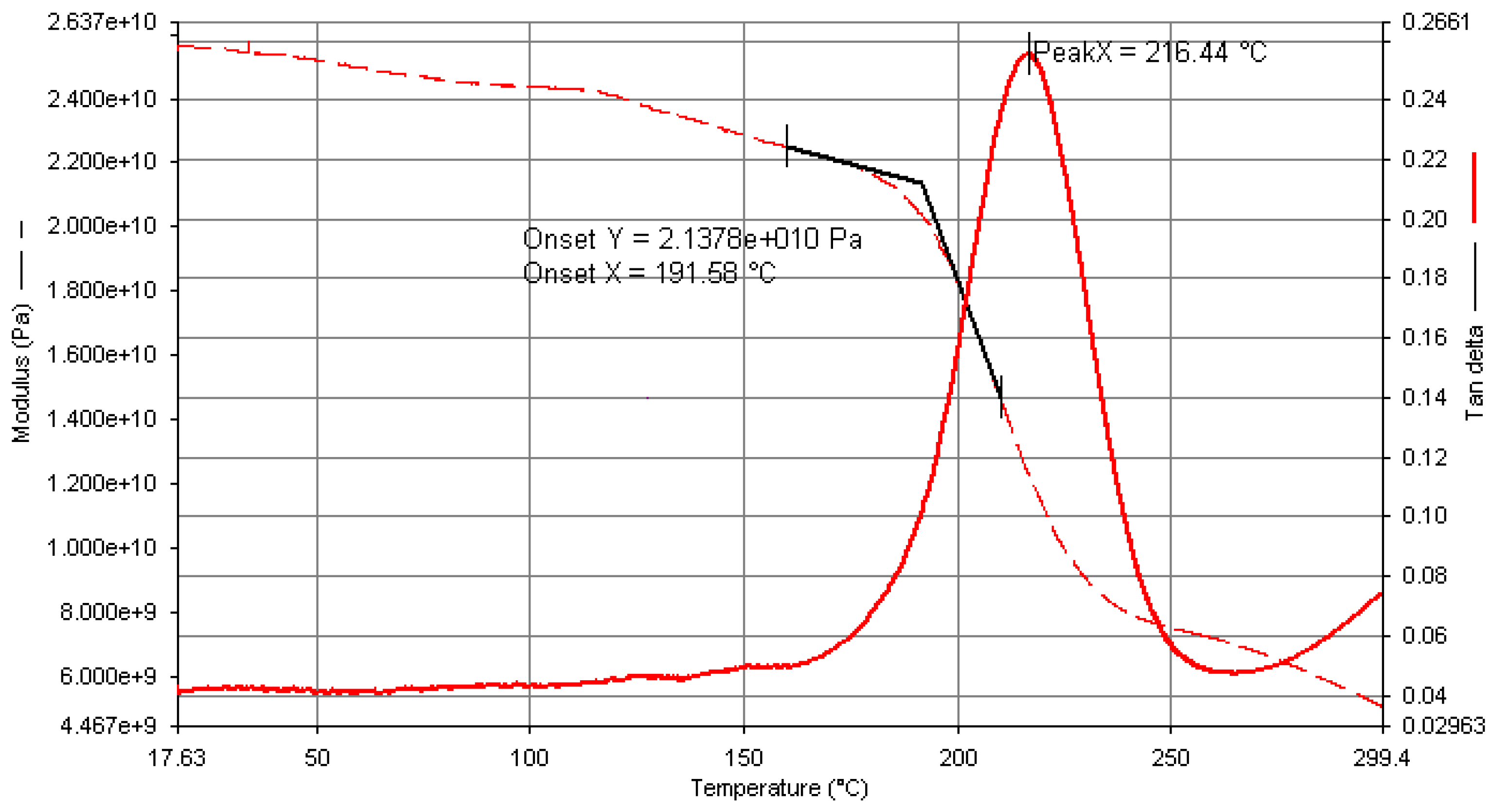
Appendix A. Determination of Glass Transition Temperature for CFRE Panel

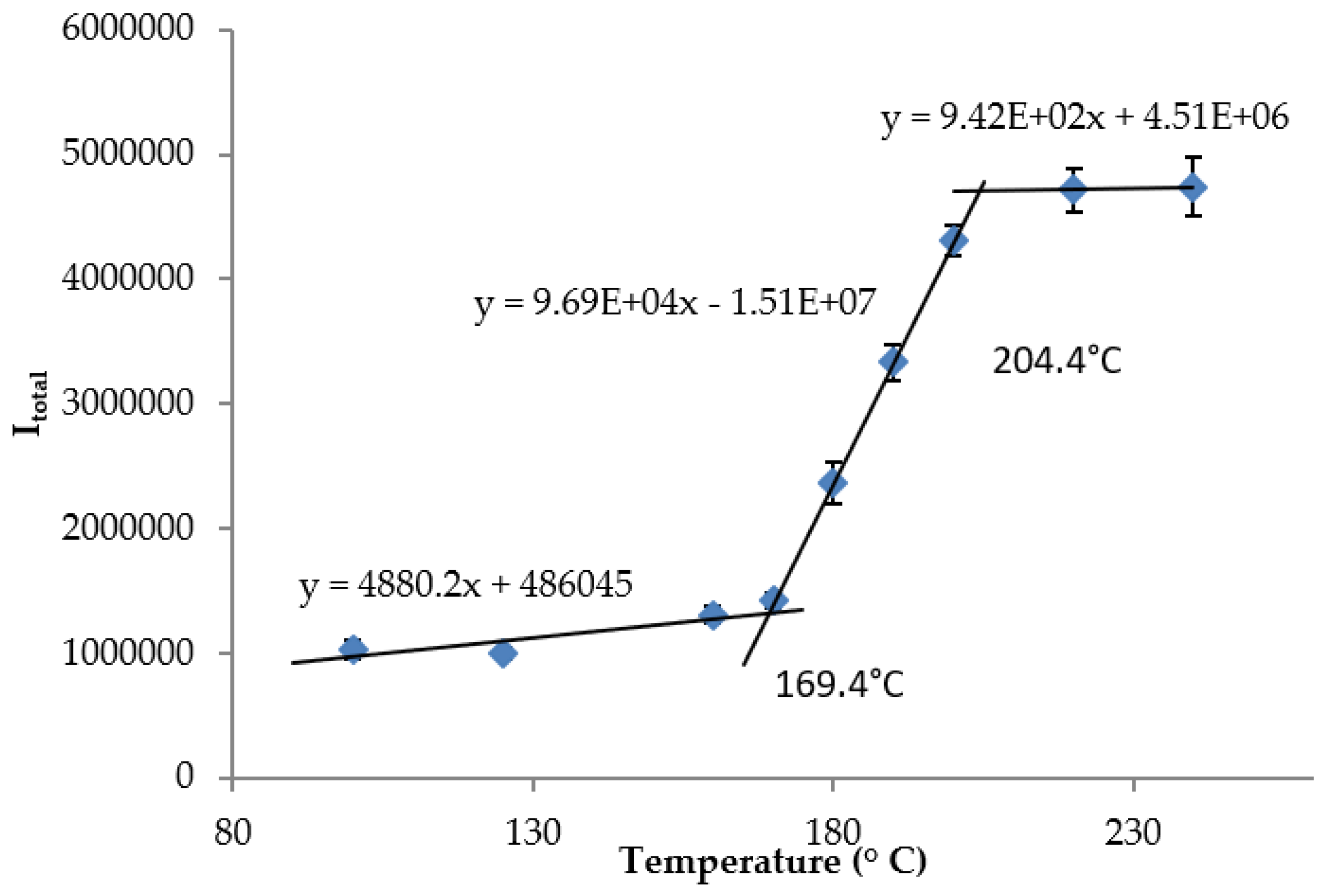
Appendix B. Tangent Line Fitting for Dynamic Temperature Range for M1-PDMS Sensor
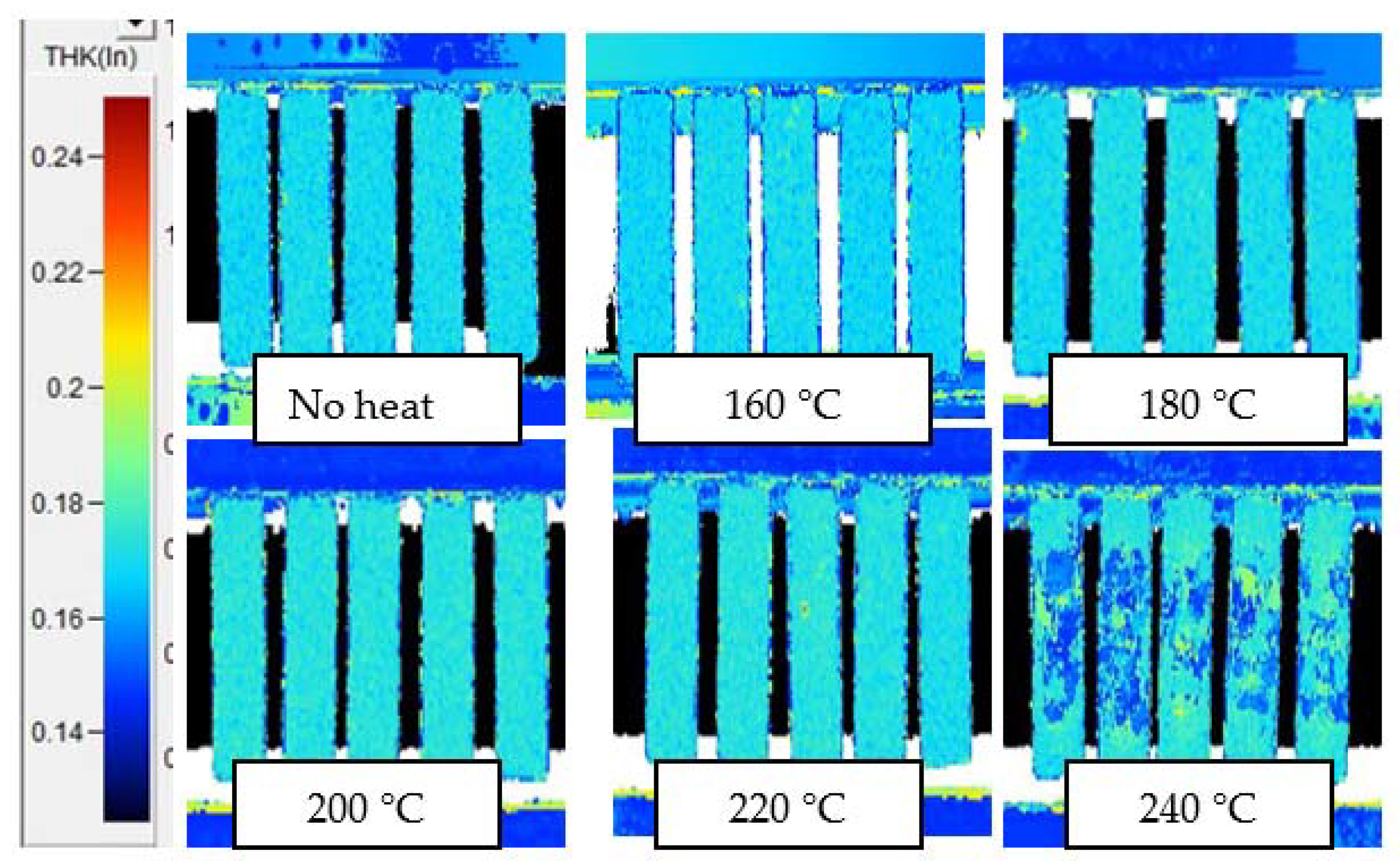
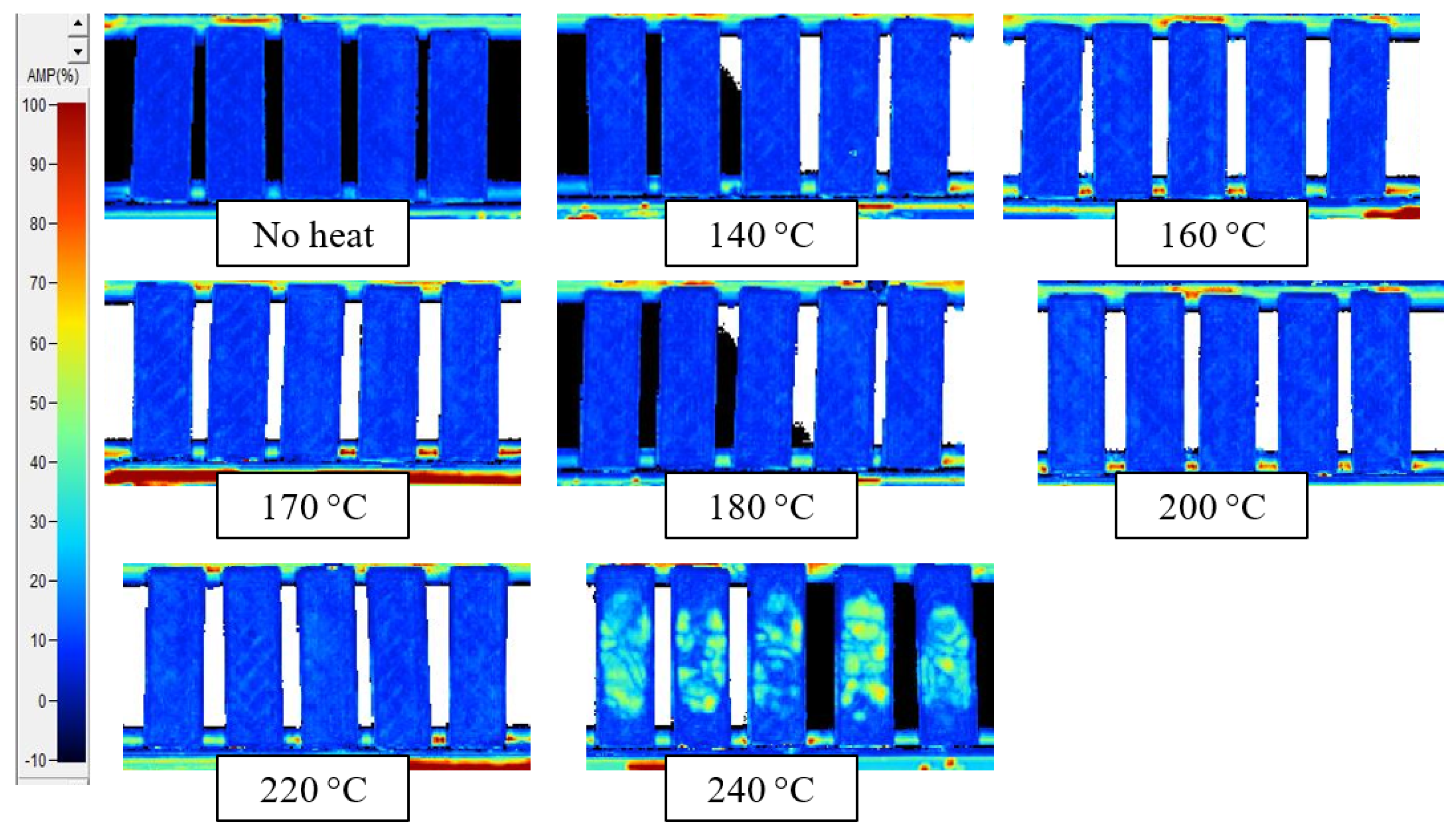
Appendix C. C-Scans of Samples after 1 h Thermal Exposure, Using Alternate Gate Signals

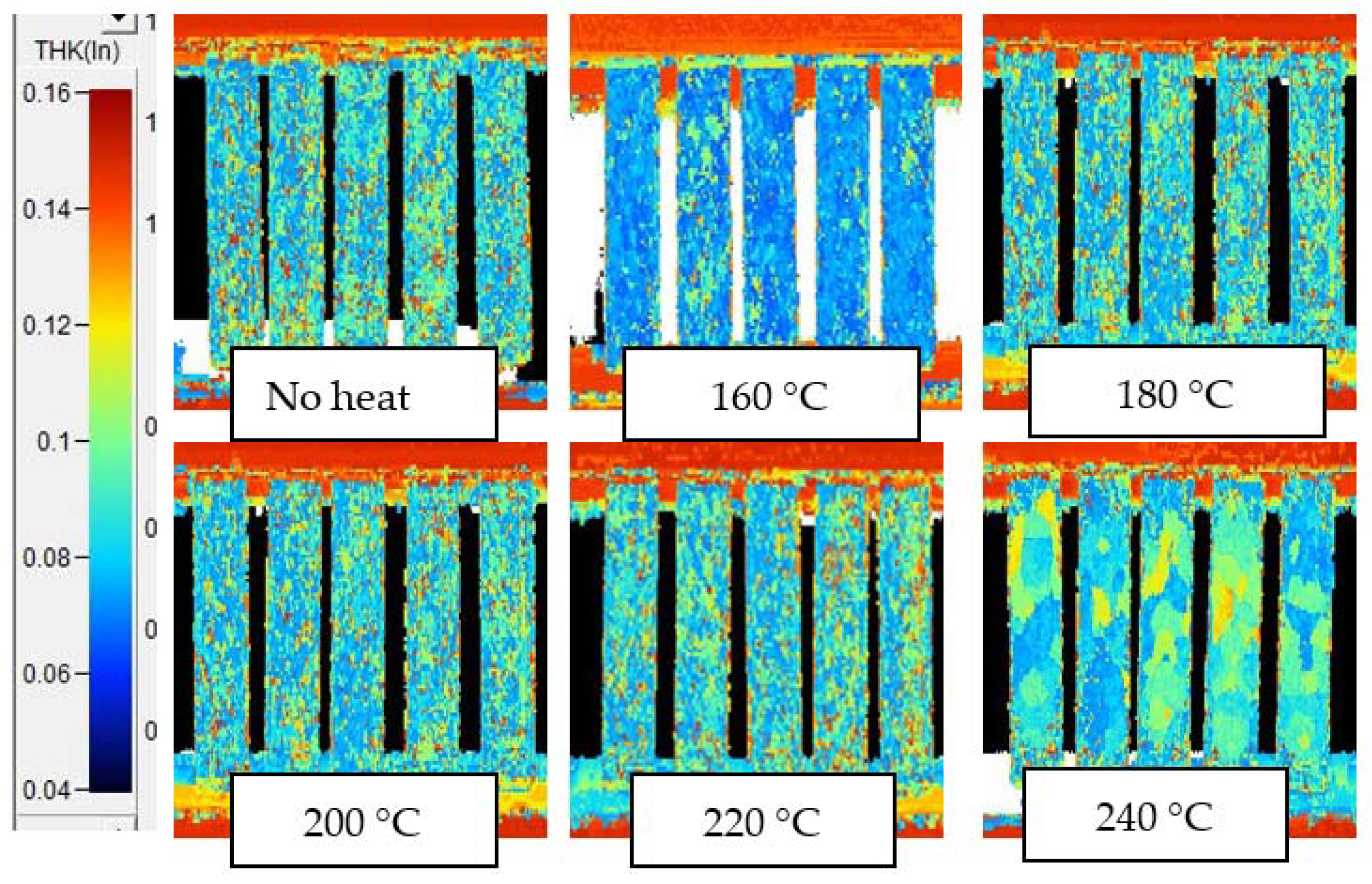
Appendix D. C-Scans Using Amplitude of Interior Echo, for Samples after 10 min and 6 h Thermal Exposures

Appendix E. p-Values Resulting from Comparison of ‘No Heat’ Samples’ ILSS with Thermally Exposed Samples’ ILSS Using Student’s t-Test.
| Temperature (°C) | 10 min | 1 h | 6 h |
| 140 | 0.506 | ||
| 160 | 0.020498 | 0.05466 | |
| 170 | 0.0277 | 0.0106 | |
| 180 | 0.00579 | 9.01 × 10−5 | 1.865 × 10−5 |
| 190 | 0.00856 | ||
| 200 | 0.00157 | 3.09 × 10−5 | 4.63 × 10−5 |
| 210 | 0.000342 | ||
| 220 | 0.000475 | 8.94 × 10−7 | 1.26 × 10−10 |
| 230 | 0.00101 | ||
| 240 | 9.27 × 10−5 | 9.75 × 10−7 | 4.01 × 10−6 |
| 250 | 0.000247 | ||
| 260 | 3.92 × 10−6 | ||
| 280 | 3.70 × 10−10 |
Appendix F. Comparison of High and Low Heating and Cooling Rates

References
- Krishnamurthy, S.; Badcock, R.; Machavaram, V.; Fernando, G. Monitoring Pre-Stressed Composites Using Optical Fibre Sensors. Sensors 2016, 16, 777. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.A.; Wang, L.; Curtis, P.T.; Fernando, G.F. Self-sensing composites: In-situ detection of fibre fracture. Sensors 2016, 16, 615. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.B.; Joyce, P.; Mechtel, D. Localized temperature variations in laser-irradiated composites with embedded fiber bragg grating sensors. Sensors 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, W.; Cui, H.-L.; Shi, C.; Han, X.; Ma, Y.; Chen, J.; Chang, T.; Wei, D.; Zhang, Y.; et al. Nondestructive Evaluation of Carbon Fiber Reinforced Polymer Composites Using Reflective Terahertz Imaging. Sensors 2016, 16, 875. [Google Scholar] [CrossRef] [PubMed]
- Hamerton, I.; Kratz, J. The use of thermosets in modern aerospace applications. In Thermosets: Structure, Properties, and Applications; Guo, Q., Ed.; Elsevier: Cambridge, MA, USA, 2018; p. 303. [Google Scholar]
- Kageyama, K.; Kimpara, I.; Oshawa, I.; Hojo, M.; Kabashima, S. Mode I amd Mode II Delamination Growth of Interlayer Toughened Carbon/Epoxy (T800/3900-2) Composite System. Compos. Mater. Fatigue Fract. 1995, 5, 19–37. [Google Scholar]
- Zhang, J.; Fox, B.L. Manufacturing influence on the delamination fracture behavior of the T800H/3900-2 carbon fiber reinforced polymer composites. Mater. Manuf. Process. 2007, 22, 768–772. [Google Scholar] [CrossRef]
- Swanson, S.R.; Qian, Y. Muitiaxial characterization of T800/3900-2 carbon/epoxy composites. Compos. Sci. Technol. 1992, 43, 197–203. [Google Scholar] [CrossRef]
- Griffiths, B. Boeing sets pace for composites usage in large civil aircraft. High Perform. Compos. 2005, 13, 68–71. [Google Scholar]
- Dara, I.H.; Ankara, A.; Akovali, G.; Suzer, S. Heat-damage assessment of carbon-fiber-reinforced polymer composites by diffuse reflectance infrared spectroscopy. J. Appl. Polym. Sci. 2005, 96, 1222–1230. [Google Scholar] [CrossRef]
- Farquharson, S.; Bassilakis, R.; Ditaranto, M.B.; Haigis, J.R.; Smith, W.W.; Solomon, P.R.; Hartford, E.; Elleithy, R.; Ebeling, T.; Wallace, J.F. Measurement of thermal degradation in epoxy composites by FT-Raman spectroscopy. In SPIE Vol 2072: Fiber Optic Physical Sensors in Manufacturing and Transportation; Berthold, J.W., III, Claus, R.O., Marcus, M.A., Rogowski, R.S., Eds.; SPIE: Bellingham, WA, USA, 1994; Volume 2072, pp. 319–331. [Google Scholar]
- Brady, S.K.; Conradi, M.S.; Vaccaro, C.M. NMR detection of thermal damage in carbon fiber reinforced epoxy resins. J. Magn. Reson. 2005, 172, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Fisher, W.G.; Storey, J.M.E.; Sharp, S.L.; Janke, C.J.; Wachter, E.A. Nondestructive Inspection of Graphite-Epoxy Composites for Heat Damage Using Laser-Induced Fluorescence. Appl. Spectrosc. 1995, 49, 1225–1231. [Google Scholar] [CrossRef]
- Howie, T.; Tracey, A.; Flinn, B. Composite Thermal Damage Measurement with Handheld Fourier Transform Infrared Spectroscopy; FAA Final Report DOT/FAA/TC-15/51; Federal Aviation Administration: Atlantic City, NJ, USA, 2017.
- Rein, A. Advanced Fourier Transform Infrared Spectroscopy for Analyzing Damage in Aircraft; FAA Final Report DOT/FAA/TC-14/56; Federal Aviation Administration: Atlantic City, NJ, USA, 2014.
- Heckner, S.; Geistbeck, M.; Grosse, C.U.; Eibl, S.; Helwig, A. FTIR Spectroscopy As a Nondestructive Testing Method for CFRP Surfaces in Aerospace. In 7th International Symposium on NDT in Aerospace; German Society for Non-Destructive Testing: Bremen, Germany, 2015. [Google Scholar]
- Eibl, S. Comparison of surface and bulk analytical techniques for the distinct quantification of a moderate thermal pre-load on a carbon fibre reinforced plastic material. Polym. Degrad. Stab. 2017, 135, 31–42. [Google Scholar] [CrossRef]
- Musto, P.; Ragosta, G.; Russo, P.; Mascia, L. Thermal-oxidative degradation of epoxy and epoxy-bismaleimide networks: Kinetics and mechanism. Macromol. Chem. Phys. 2001, 202, 3445–3458. [Google Scholar] [CrossRef]
- Morgan, R.J.; Mones, E.T. The cure reactions, network structure, and mechanical response of diaminodiphenylsulfone-cured tetraglycidyl 4,4′diaminodiphenyl methane epoxies. J. Appl. Polym. Sci. 1987, 33, 999–1020. [Google Scholar] [CrossRef]
- Bondzic, S.; Hodgkin, J.; Krstina, J.; Mardel, J. Chemistry of thermal ageing in aerospace epoxy composites. J. Appl. Polym. Sci. 2006, 100, 2210–2219. [Google Scholar] [CrossRef]
- Wang, X.; Wolfbeis, O.S.; Meier, R.J. Luminescent probes and sensors for temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef] [PubMed]
- How It Works: Irreversible Temperature Indicator Labels. Available online: http://temperature-indicators.co.uk/articles/education/irreversible-temerature-indicators-educatio.htm (accessed on 1 February 2017).
- Taoukis, P.S.; Labuza, T.P. Applicability of time temperature indicators as shelf life monitors of food products. J. Food Sci. 1989, 54, 783–788. [Google Scholar] [CrossRef]
- Nuin, M.; Alfaro, B.; Cruz, Z.; Argarate, N.; George, S.; Le Marc, Y.; Olley, J.; Pin, C. Modelling spoilage of fresh turbot and evaluation of a time-temperature integrator (TTI) label under fluctuating temperature. Int. J. Food Microbiol. 2008, 127, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Wanihsuksombat, C.; Hongtrakul, V.; Suppakul, P. Development and characterization of a prototype of a lactic acid-based time-temperature indicator for monitoring food product quality. J. Food Eng. 2010, 100, 427–434. [Google Scholar] [CrossRef]
- Fortin, C.; Goodwin, H.L., Jr. Valuation of Temp-Time’s Fresh-Check® Indicator on Perishable Food Products in Belgium. In Proceedings of the Southern Agricultural Economics Association Annual Meeting, Dallas, TX, USA, 2–6 February 2008. [Google Scholar]
- Wu, D.; Wang, Y.; Chen, J.; Ye, X.; Wu, Q.; Liu, D.; Ding, T. Preliminary study on time-temperature indicator (TTI) system based on urease. Food Control 2013, 34, 230–234. [Google Scholar] [CrossRef]
- Toivola, R.; Howie, T.; Yang, J.; Lai, P.; Shi, Z.; Jang, S.-H.; Jen, A.K.-Y.; Flinn, B.D. Highly Sensitive Thermal Damage Sensors for Polymer Composites: Time Temperature Indicator Based on Thermochromic Fluorescence Turn-On Response. Smart Mater. Struct. 2017, 26, 85039. [Google Scholar] [CrossRef]
- Khan, M.R.R.; Kang, S.W. Highly sensitive temperature sensors based on fiber-optic PWM and capacitance variation using thermochromic sensing membrane. Sensors 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Rabhiou, A.; Feist, J.; Kempf, A.; Skinner, S.; Heyes, A. Phosphorescent thermal history sensors. Sens. Actuators A Phys. 2011, 169, 18–26. [Google Scholar] [CrossRef]
- Talghader, J.J.; Mah, M.L.; Yukihara, E.G.; Coleman, A.C. Thermoluminescent microparticle thermal history sensors. Microsyst. Nanoeng. 2016, 2, 16037. [Google Scholar] [CrossRef]
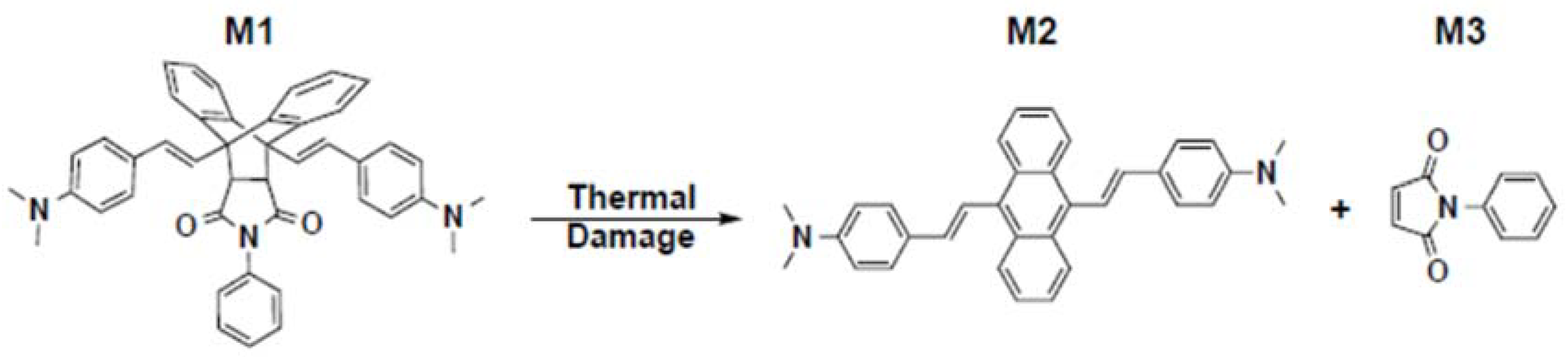
- Shi, Z.; Liang, W.; Luo, J.; Huang, S.; Polishak, B.M.; Li, X.; Younkin, T.R.; Block, B.A.; Jen, A.K.Y. Tuning the kinetics and energetics of Diels-Alder cycloaddition reactions to improve poling efficiency and thermal stability of high-temperature cross-linked electro-optic polymers. Chem. Mater. 2010, 22, 5601–5608. [Google Scholar] [CrossRef]
- ASTM International. ASTM E1356 Standard Test Method for Assignment of the Glass Transition Temperatures by Differential Scanning Calorimetry; ASTM International: West Conshohocken, PA, USA, 2008; Volume 8, pp. 1–4. [Google Scholar]
- ASTM International. ASTM E1640 Standard Test Method for Assignment of the Glass Transition Temperature by Dynamic Mechanical Analysis; ASTM International: West Conshohocken, PA, USA, 2013; Volume 1, pp. 1–6. [Google Scholar]
- ASTM International. ASTM D2344-16: Standard Test Method for Short-Beam Strength of Polymer Matrix Composite Materials; Annual B ASTM Standard; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Agarwal, B.D.; Broutman, L.J. Analysis and Performance of Fiber Composites, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Henneke, E.G. Ultrasonic Nondestructive Evaluation of Advanced Composites. In Non-Destructive Testing of Fibre-Reinforced Plastics Composites; Summerscales, J., Ed.; Elsevier Applied Science: New York, NY, USA, 1990; Volume 2, pp. 55–160. [Google Scholar]
- Mouritz, A.P.; Gallagher, J.; Goodwin, A.A. Flexural strength and interlaminar shear strength of stitched GRP laminates following repeated impacts. Compos. Sci. Technol. 1997, 57, 509–522. [Google Scholar] [CrossRef]
- Tsenoglou, C.J.; Pavlidou, S.; Papaspyrides, C.D. Evaluation of interfacial relaxation due to water absorption in fiber-polymer composites. Compos. Sci. Technol. 2006, 66, 2855–2864. [Google Scholar] [CrossRef]
- Shin, K.B.; Kim, C.G.; Hong, C.S.; Lee, H.H. Prediction of failure thermal cycles in graphite/epoxy composite materials under simulated low earth orbit environments. Compos. Part B Eng. 2000, 31, 223–235. [Google Scholar] [CrossRef]
- Raju, T.N. William Sealy Gosset and William A. Silverman: Two “students” of science. Pediatrics 2005, 116, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Hancox, N.L. Thermal effects on polymer matrix composites: Part 1. Thermal cycling. Mater. Des. 1998, 19, 85–91. [Google Scholar] [CrossRef]

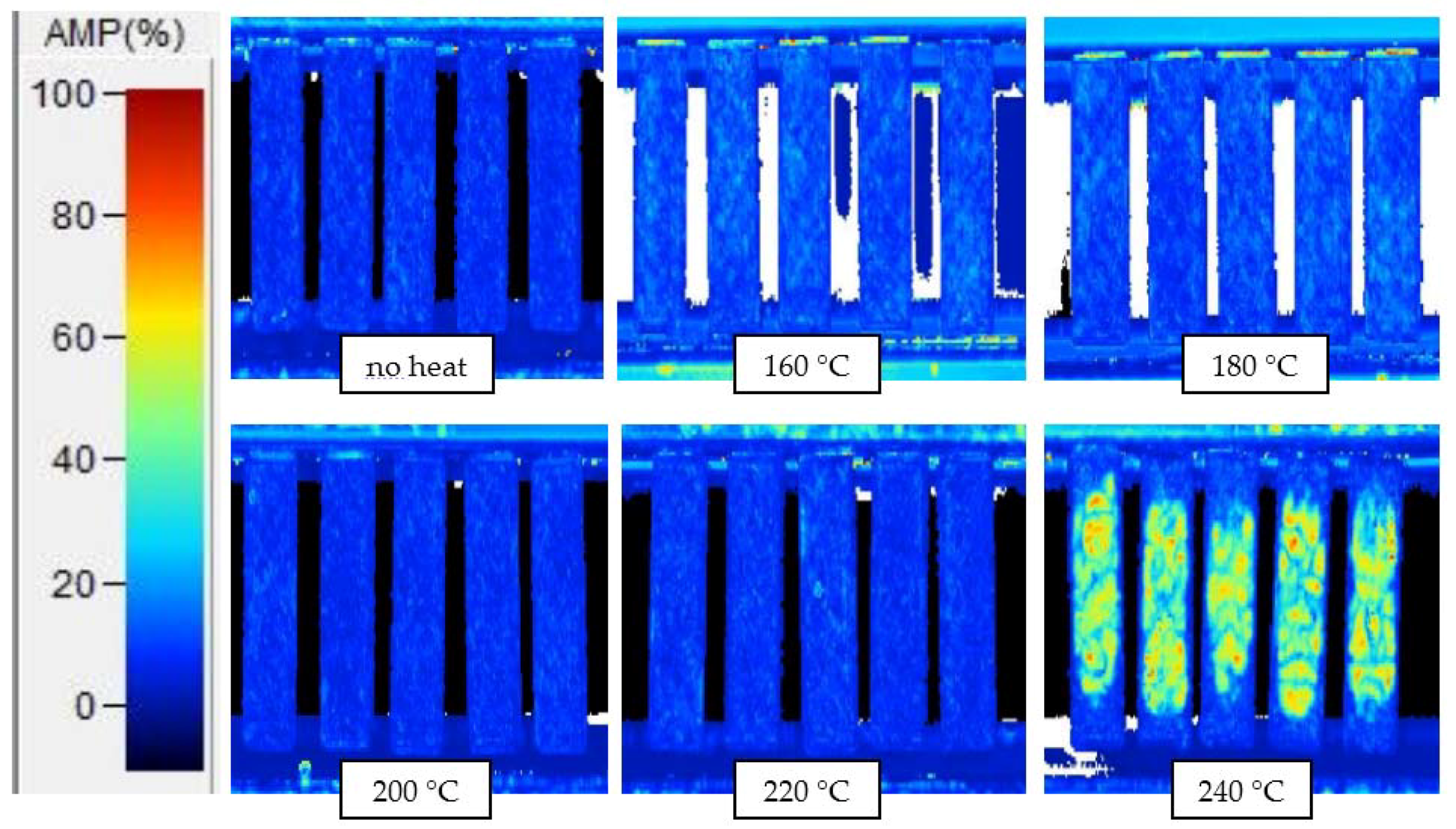



| Exposure Time | ITD Region (°C) | Sensor Dynamic Range (°C) |
|---|---|---|
| 10 min | 180–260 | 181–218 |
| 1 h | 180–240 | 169–204 |
| 6 h | 170–240 | 141–180 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toivola, R.; Jang, S.-H.; Baker, S.; Jen, A.K.-Y.; Flinn, B.D. Thermochromic Polymer Film Sensors for Detection of Incipient Thermal Damage in Carbon Fiber–Epoxy Composites. Sensors 2018, 18, 1362. https://doi.org/10.3390/s18051362
Toivola R, Jang S-H, Baker S, Jen AK-Y, Flinn BD. Thermochromic Polymer Film Sensors for Detection of Incipient Thermal Damage in Carbon Fiber–Epoxy Composites. Sensors. 2018; 18(5):1362. https://doi.org/10.3390/s18051362
Chicago/Turabian StyleToivola, Ryan, Sei-Hum Jang, Shawn Baker, Alex K. -Y. Jen, and Brian D. Flinn. 2018. "Thermochromic Polymer Film Sensors for Detection of Incipient Thermal Damage in Carbon Fiber–Epoxy Composites" Sensors 18, no. 5: 1362. https://doi.org/10.3390/s18051362





