A Review on Bacteriorhodopsin-Based Bioelectronic Devices
Abstract
:1. Introduction
2. Working Principle and Properties of Bacteriorhodopsin
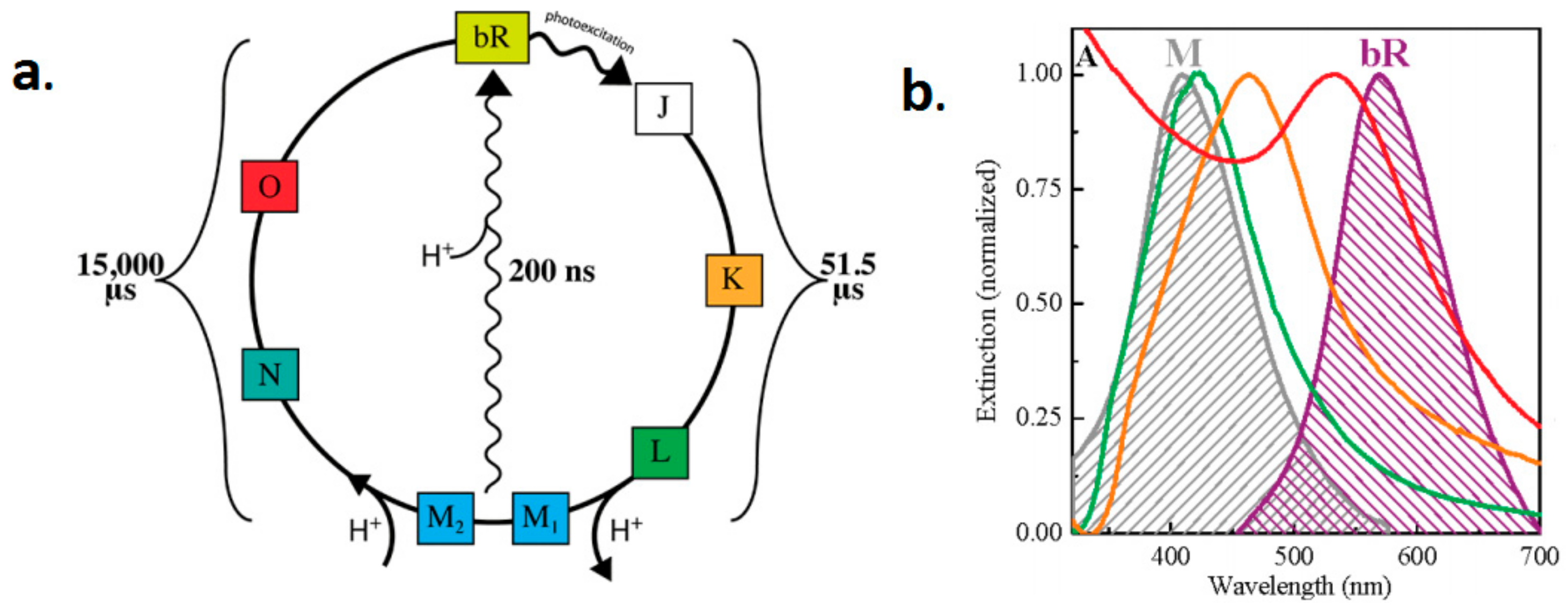
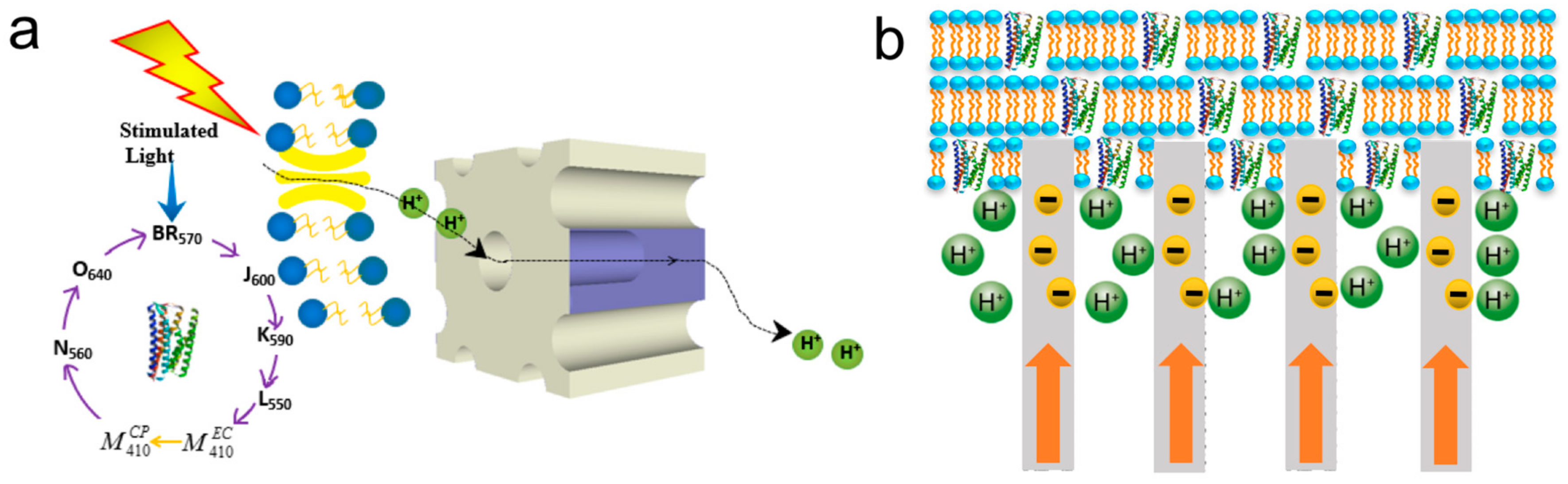
2.1. General Structure and Operation Principles of bR
2.2. Methods of bR Layer Preparation
3. Photochemical Application of Bacteriorhodopsin
3.1. Optical Volumetric Memories
3.2. Holographic Associative Processors
4. Photoelectric Application of Bacteriorhodopsin
4.1. Motion Biosensors
4.2. X-ray Sensor
4.3. Photovoltaic Cells
4.4. bR Related Immunosensor
4.5. bR Related Artificial Retinal Prostheses
5. Conclusions/Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berggren, M.; Richter-Dahlfors, A. Organic bioelectronics. Adv. Mater. 2007, 19, 3201–3213. [Google Scholar] [CrossRef]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Szent-Györgyi, A. Bioelectronics. Science 1968, 161, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Baron, R.; Willner, B. Integrated nanoparticle–biomolecule systems for biosensing and bioelectronics. Biosens. Bioelectron. 2007, 22, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Katz, E. Bioelectronics: From Theory to Applications; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Willner, I.; Willner, B. Biomaterials integrated with electronic elements: En route to bioelectronics. Trends Biotechnol. 2001, 19, 222–230. [Google Scholar] [CrossRef]
- Zhang, A.; Lieber, C.M. Nano-bioelectronics. Chem. Rev. 2015, 116, 215–257. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.-Q.; Zhang, K.-N.; Tian, H.; Liu, Y.; Wang, D.-Y.; Chen, Y.-Q.; Yang, Y.; Ren, T.-L. Graphene-Paper Pressure Sensor for Detecting Human Motions. ACS Nano 2017, 11, 8790–8795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Béja, O.; Aravind, L.; Koonin, E.V.; Suzuki, M.T.; Hadd, A.; Nguyen, L.P.; Jovanovich, S.B.; Gates, C.M.; Feldman, R.A.; Spudich, J.L. Bacterial rhodopsin: Evidence for a new type of phototrophy in the sea. Science 2000, 289, 1902–1906. [Google Scholar] [CrossRef] [PubMed]
- Béjà, O.; Spudich, E.N.; Spudich, J.L.; Leclerc, M.; DeLong, E.F. Proteorhodopsin phototrophy in the ocean. Nature 2001, 411, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.T. Molecular sensors based on the photoelectric effect of bacteriorhodopsin: origin of differential responsivity. Mater. Sci. Eng. C 1997, 4, 267–285. [Google Scholar]
- Knoblauch, C.; Griep, M.; Friedrich, C. Recent advances in the field of bionanotechnology: An insight into optoelectric bacteriorhodopsin, quantum dots, and noble metal nanoclusters. Sensors 2014, 14, 19731–19766. [Google Scholar] [CrossRef] [PubMed]
- Lozier, R.H.; Bogomolni, R.A.; Stoeckenius, W. Bacteriorhodopsin: A light-driven proton pump in Halobacterium Halobium. Biophys. J. 1975, 15, 955–962. [Google Scholar] [CrossRef]
- Peck, R.F.; Echavarri-Erasun, C.; Johnson, E.A.; Ng, W.V.; Kennedy, S.P.; Hood, L.; DasSarma, S.; Krebs, M.P. brp and blh are required for synthesis of the retinal cofactor of bacteriorhodopsin in Halobacterium salinarum. J. Biol. Chem. 2001, 276, 5739–5744. [Google Scholar] [CrossRef] [PubMed]
- Stoeckenius, W.; Kunau, W.H. Further characterization of particulate fractions from lysed cell envelopes of Halobacterium halobium and isolation of gas vacuole membranes. J. Cell Biol. 1968, 38, 337–357. [Google Scholar] [CrossRef]
- Oesterhelt, D. Bacteriorhodopsin as a light-drive ion exchanger? FEBS Lett. 1976, 64, 20–22. [Google Scholar] [CrossRef]
- Frydrych, M.; Silfsten, P.; Parkkinen, S.; Parkkinen, J.; Jaaskelainen, T. Color sensitive retina based on bacteriorhodopsin. Biosystems 2000, 54, 131–140. [Google Scholar] [CrossRef]
- Fischer, T.; Neebe, M.; Juchem, T.; Hampp, N.A. Biomolecular optical data storage and data encryption. IEEE Trans. Nanobiosci. 2003, 2, 1–5. [Google Scholar] [CrossRef]
- Imhof, M.; Rhinow, D.; Hampp, N. Two-photon polarization data storage in bacteriorhodopsin films and its potential use in security applications. Appl. Phys. Lett. 2014, 104, 081921. [Google Scholar] [CrossRef]
- Yu, X.; Yao, B.; Lei, M.; Gao, P.; Ma, B. Femtosecond laser-induced permanent anisotropy in bacteriorhodopsin films and applications in optical data storage. J. Mod. Opt. 2013, 60, 309–314. [Google Scholar] [CrossRef]
- Greco, J.A.; Wagner, N.L.; Birge, R.R. Fourier Transform holographic associative processors based on bacteriorhodopsin. Int. J. Unconv. Comput. 2012, 8, 433–457. [Google Scholar]
- Hillebrecht, J.R.; Koscielecki, J.F.; Wise, K.J.; Marcy, D.L.; Tetley, W.; Rangarajan, R.; Sullivan, J.; Brideau, M.; Krebs, M.P.; Stuart, J.A. Optimization of protein-based volumetric optical memories and associative processors by using directed evolution. NanoBiotechnology 2005, 1, 141–151. [Google Scholar] [CrossRef]
- Kasai, K.; Haruyama, Y.; Yamada, T.; Akiba, M.; Kaji, T.; Tominari, Y.; Tanaka, S.; Okada-Shudo, Y.; Otomo, A. Optical-Flow Sensing Using a Bacteriorhodopsin-Based Bipolar Photosensor Array; Optical Sensors, 2016; Optical Society of America: Washington, DC, USA, 2016; p. SeW2F.2. [Google Scholar]
- Okada-Shudo, Y.; Tanabe, T.; Mukai, T.; Kasai, K.; Zhang, Y.; Watanabe, M. Directionally selective motion detection with bacteriorhodopsin patterned sensor. Synth. Met. 2016, 222, 249–254. [Google Scholar] [CrossRef]
- Wang, W.W.; Knopf, G.K.; Bassi, A.S. Protein-based photocell for high-speed motion detection. In Proceedings of the 2005 IEEE Conference on Control Applications CCA 2005, Toronto, ON, Canada, 28–31 August 2005; IEEE: Piscataway, NJ, USA, 2005; pp. 731–736. [Google Scholar]
- Allam, N.K.; Yen, C.-W.; Near, R.D.; El-Sayed, M.A. Bacteriorhodopsin/TiO2 nanotube arrays hybrid system for enhanced photoelectrochemical water splitting. Energy Environ. Sci. 2011, 4, 2909–2914. [Google Scholar] [CrossRef]
- Guo, Z.; Liang, D.; Rao, S.; Xiang, Y. Heterogeneous bacteriorhodopsin/gold nanoparticle stacks as a photovoltaic system. Nano Energy 2015, 11, 654–661. [Google Scholar] [CrossRef]
- Lu, S.; Guo, Z.; Xiang, Y.; Jiang, L. Photoelectric frequency response in a bioinspired bacteriorhodopsin/alumina nanochannel hybrid nanosystem. Adv. Mater. 2016, 28, 9851–9856. [Google Scholar] [CrossRef] [PubMed]
- Rakovich, A.; Nabiev, I.; Sukhanova, A.; Lesnyak, V.; Gaponik, N.; Rakovich, Y.P.; Donegan, J.F. Large enhancement of nonlinear optical response in a hybrid nanobiomaterial consisting of bacteriorhodopsin and cadmium telluride quantum dots. ACS Nano 2013, 7, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Guo, Z.; Liang, D.; Chen, D.; Li, Y.; Xiang, Y. 3D Proton Transfer Augments Bio-Photocurrent Generation. Adv. Mater. 2015, 27, 2668–2673. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-W.; Chu, L.-K.; El-Sayed, M.A. Plasmonic field enhancement of the bacteriorhodopsin photocurrent during its proton pump photocycle. J. Am. Chem. Soc. 2010, 132, 7250–7251. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-W.; Hayden, S.C.; Dreaden, E.C.; Szymanski, P.; El-Sayed, M.A. Tailoring plasmonic and electrostatic field effects to maximize solar energy conversion by bacteriorhodopsin, the other natural photosynthetic system. Nano Lett. 2011, 11, 3821–3826. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Yeow, J.T. Fabrication and characterization of a radiation sensor based on bacteriorhodopsin. Biosens. Bioelectron. 2011, 26, 2171–2176. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Roy, S. Optoelectronic logic gates based on photovoltaic response of bacteriorhodopsin polymer composite thin films. IEEE Trans. Nanobiosci. 2012, 11, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Jheng, K.-R.; Yu, A.-D. Direct, label-free, selective, and sensitive microbial detection using a bacteriorhodopsin-based photoelectric immunosensor. Biosens. Bioelectron. 2017, 91, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Padros, E.; Sanz, C.; Lazarova, T.; Marquez, M.; Sepulcre, F.; Trapote, X.; Munoz, F.-X.; Gonzalez-Moreno, R.; Bourdelande, J.-L.; Querol, E. Extracellular mutants of bacteriorhodopsin as possible materials for bioelectronic applications. In NATO Science Series Sub Series I Life and Behavioural Sciences; IOS Press: Amsterdam, The Netherlands, 2001; Volume 335, pp. 120–136. [Google Scholar]
- Wagner, N.L.; Greco, J.A.; Ranaghan, M.J.; Birge, R.R. Directed evolution of bacteriorhodopsin for applications in bioelectronics. J. R. Soc. Interface 2013, 10, 20130197. [Google Scholar] [CrossRef] [PubMed]
- Wise, K.J.; Gillespie, N.B.; Stuart, J.A.; Krebs, M.P.; Birge, R.R. Optimization of bacteriorhodopsin for bioelectronic devices. Trends Biotechnol. 2002, 20, 387–394. [Google Scholar] [CrossRef]
- Engelman, D.; Goldman, A.; Steitz, T. [11] The identification of helical segments in the polypeptide chain of bacteriorhodopsin. In Methods in Enzymology; Elsevier: New York, NY, USA, 1982; Volume 88, pp. 81–88. [Google Scholar]
- Luecke, H.; Schobert, B.; Richter, H.-T.; Cartailler, J.-P.; Lanyi, J.K. Structure of bacteriorhodopsin at 1.55 Å resolution 1. J. Mol. Biol. 1999, 291, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.J.; Kessler, M.; Oesterhelt, F.; Möller, C.; Oesterhelt, D.; Gaub, H. Stability of bacteriorhodopsin α-helices and loops analyzed by single-molecule force spectroscopy. Biophys. J. 2002, 83, 3578–3588. [Google Scholar] [CrossRef]
- Pebay-Peyroula, E.; Rummel, G.; Rosenbusch, J.P.; Landau, E.M. X-ray structure of bacteriorhodopsin at 2.5 angstroms from microcrystals grown in lipidic cubic phases. Science 1997, 277, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Von Heijne, G. Proline kinks in transmembrane α-helices. J. Mol. Biol. 1991, 218, 499–503. [Google Scholar] [CrossRef]
- Bayley, H.; Huang, K.-S.; Radhakrishnan, R.; Ross, A.H.; Takagaki, Y.; Khorana, H.G. Site of attachment of retinal in bacteriorhodopsin. Proc. Natl. Acad. Sci. USA 1981, 78, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Heyn, M.; Westerhausen, J.; Wallat, I.; Seiff, F. High-sensitivity neutron diffraction of membranes: Location of the Schiff base end of the chromophore of bacteriorhodopsin. Proc. Natl. Acad. Sci. USA 1988, 85, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Lemke, H.-D.; Oesterhelt, D. Lysine 216 is a binding site of the retinyl moiety in bacteriorhodopsin. FEBS Lett. 1981, 128, 255–260. [Google Scholar] [CrossRef]
- Henderson, R.; Baldwin, J.M.; Ceska, T.; Zemlin, F.; Beckmann, E.; Downing, K. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J. Mol. Biol. 1990, 213, 899–929. [Google Scholar] [CrossRef]
- Ovchinnikov, Y.A. Rhodopsin and bacteriorhodopsin: Structure-function relationships. FEBS Lett. 1982, 148, 179–191. [Google Scholar] [CrossRef]
- Rakovich, A.; Donegan, J.F.; Oleinikov, V.; Molinari, M.; Sukhanova, A.; Nabiev, I.; Rakovich, Y.P. Linear and nonlinear optical effects induced by energy transfer from semiconductor nanoparticles to photosynthetic biological systems. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 17–32. [Google Scholar] [CrossRef]
- Kato, Y. Function and Mechanism Study of Light-Driven Ion Pumps. Biochem. Biophys. Res. Commun. 1977, 78, 237–243. [Google Scholar]
- Lanyi, J.K.; Schobert, B. Effects of chloride and pH on the chromophore and photochemical cycling of halorhodopsin. Biochemistry 1983, 22, 2763–2769. [Google Scholar] [CrossRef]
- Spudich, J.L.; Bogomolni, R.A. Sensory rhodopsins of halobacteria. Annu. Rev. Biophys. Biophys. Chem. 1988, 17, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Iwasa, T.; Yoshizawa, T. Isomeric composition of retinal chromophore in dark-adapted bacteriorhodopsin. J. Biochem. 1977, 82, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Birge, R.R. Photophysics and molecular electronic applications of the rhodopsins. Annu. Rev. Phys. Chem. 1990, 41, 683–733. [Google Scholar] [CrossRef] [PubMed]
- Trissl, H.-W.; Montal, M. Electrical demonstration of rapid light-induced conformational changes in bacteriorhodopsin. Nature 1977, 266, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Birge, R.R. A nonlinear proton pump. Nature 1994, 371, 659–660. [Google Scholar] [CrossRef]
- Drachev, L.; Kaulen, A.; Skulachev, V. Correlation of photochemical cycle, H+ release and uptake, and electric events in bacteriorhodopsin. FEBS Lett. 1984, 178, 331–335. [Google Scholar] [CrossRef]
- Edman, K.; Nollert, P.; Royant, A.; Belrhali, H.; Pebay-Peyroula, E.; Hajdu, J.; Neutze, R.; Landau, E.M. High-resolution X-ray structure of an early intermediate in the bacteriorhodopsin photocycle. Nature 1999, 401, 822–826. [Google Scholar] [PubMed]
- Han, B.-G.; Vonck, J.; Glaeser, R.M. The bacteriorhodopsin photocycle: Direct structural study of two substrates of the M-intermediate. Biophys. J. 1994, 67, 1179–1186. [Google Scholar] [CrossRef]
- Luecke, H.; Richter, H.-T.; Lanyi, J.K. Proton transfer pathways in bacteriorhodopsin at 2.3 angstrom resolution. Science 1998, 280, 1934–1937. [Google Scholar] [CrossRef] [PubMed]
- Oesterhelt, D.; Stoeckenius, W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat. New Biol. 1971, 233, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Polland, H.-J.; Franz, M.; Zinth, W.; Kaiser, W.; Kölling, E.; Oesterhelt, D. Early picosecond events in the photocycle of bacteriorhodopsin. Biophys. J. 1986, 49, 651–662. [Google Scholar] [CrossRef]
- Shichida, Y.; Matuoka, S.; Hidaka, Y.; Yoshizawa, T. Absorption spectra of intermediates of bacteriorhodopsin measured by laser photolysis at room temperatures. Biochim. Biophys. Acta (BBA)-Bioenerg. 1983, 723, 240–246. [Google Scholar] [CrossRef]
- Kobayashi, T.; Saito, T.; Ohtani, H. Real-time spectroscopy of transition states in bacteriorhodopsin during retinal isomerization. Nature 2001, 414, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.A.; Liu, E.; Burkard, F.; Berry, E.A.; Glaeser, R.M. Characterization of the conformational change in the M1 and M2 substates of bacteriorhodopsin by the combined use of visible and infrared spectroscopy. J. Struct. Biol. 1992, 109, 142–151. [Google Scholar] [CrossRef]
- Varo, G.; Lanyi, J.K. Thermodynamics and energy coupling in the bacteriorhodopsin photocycle. Biochemistry 1991, 30, 5016–5022. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, N.B.; Wise, K.J.; Ren, L.; Stuart, J.A.; Marcy, D.L.; Hillebrecht, J.; Li, Q.; Ramos, L.; Jordan, K.; Fyvie, S. Characterization of the branched-photocycle intermediates P and Q of bacteriorhodopsin. J. Phys. Chem. B 2002, 106, 13352–13361. [Google Scholar] [CrossRef]
- Wang, J.-P.; Yoo, S.-K.; Song, L.; El-Sayed, M.A. Molecular mechanism of the differential photoelectric response of bacteriorhodopsin. J. Phys. Chem. B 1997, 101, 3420–3423. [Google Scholar] [CrossRef]
- Popp, A.; Wolperdinger, M.; Hampp, N.; Brüchle, C.; Oesterhelt, D. Photochemical conversion of the O-intermediate to 9-cis-retinal-containing products in bacteriorhodopsin films. Biophys. J. 1993, 65, 1449–1459. [Google Scholar] [CrossRef]
- Chang, C.-H.; Liu, S.-Y.; Jonas, R.; Govindjee, R. The pink membrane: The stable photoproduct of deionized purple membrane. Biophys. J. 1987, 52, 617–623. [Google Scholar] [CrossRef]
- Birge, R.R.; Gillespie, N.B.; Izaguirre, E.W.; Kusnetzow, A.; Lawrence, A.F.; Singh, D.; Song, Q.W.; Schmidt, E.; Stuart, J.A.; Seetharaman, S. Biomolecular Electronics: Protein-Based Associative Processors and Volumetric Memories; ACS Publications: Washington, DC, USA, 1999. [Google Scholar]
- Stuart, J.A.; Marcy, D.L.; Wise, K.J.; Birge, R.R. Volumetric optical memory based on bacteriorhodopsin. Synth. Met. 2002, 127, 3–15. [Google Scholar] [CrossRef]
- Váró, G.; Keszthelyi, L. Photoelectric signals from dried oriented purple membranes of Halobacterium halobium. Biophys. J. 1983, 43, 47–51. [Google Scholar] [CrossRef]
- Nagy, K. Photoelectric activity of dried, oriented layers of purple membrane from Halobacterium halobium. Biochem. Biophys. Res. Commun. 1978, 85, 383–390. [Google Scholar] [CrossRef]
- Choi, H.-G.; Min, J.; Choi, J.-W.; Lee, W.H. Molecular photoreceptor consisting of bacteriorhodopsin/flavin complex Langmuir–Blodgett films1. Biosens. Bioelectron. 1998, 13, 1069–1075. [Google Scholar] [CrossRef]
- Choi, H.-G.; Oh, B.-K.; Lee, W.H.; Choi, J.-W. Deposition behavior and photoelectrochemical characteristics of chlorophylla Langmuir-Blodgett films. Biotechnol. Bioprocess Eng. 2001, 6, 183–188. [Google Scholar] [CrossRef]
- DeRose, J.; Leblanc, R. Scanning tunneling and atomic force microscopy studies of Langmuir-Blodgett films. Surf. Sci. Rep. 1995, 22, 73–126. [Google Scholar] [CrossRef]
- Flanagan, M. The deposition of Langmuir-Blodgett films containing purple membrane on lipid-and paraffin-impregnated filters. Thin Solid Films 1983, 99, 133–138. [Google Scholar] [CrossRef]
- Ikonen, M.; Peltonen, J.; Vuorimaa, E.; Lemmetyinen, H. Study of photocycle and spectral properties of bacteriorhodopsin in Langmuir-Blodgett films. Thin Solid Films 1992, 213, 277–284. [Google Scholar] [CrossRef]
- Ikonen, M.; Sharonov, A.; Tkachenko, N.; Lemmetyinen, H. The photovoltage signals of bacteriorhodopsin in Langmuir–Blodgett films with different molecular orientations. Adv. Funct. Mater. 1993, 2, 115–122. [Google Scholar] [CrossRef]
- Ikonen, M.; Sharonov, A.Y.; Tkachenko, N.V.; Lemmetyinen, H. The kinetics of charges in dry bacteriorhodopsin Langmuir–Blodgett films—An analysis and comparison of electrical and optical signals. Adv. Funct. Mater. 1993, 2, 211–220. [Google Scholar] [CrossRef]
- Korenbrot, J.I.; Hwang, S. Proton transport by bacteriorhodopsin in planar membranes assembled from air-water interface films. J. Gen. Physiol. 1980, 76, 649–682. [Google Scholar] [CrossRef] [PubMed]
- Maximychev, A.; Kholmansky, A.; Levin, E.; Rambidi, N.; Chamorovsky, S.; Kononenko, A.; Erokhin, V.; Checulaeva, L. Oriented purple membrane multilayers of halobacteria fabricated by langmuir–blodgett and electrophoretic sedimentation techniques. Adv. Funct. Mater. 1992, 1, 105–115. [Google Scholar] [CrossRef]
- Niemi, H.; Ikonen, M.; Levlin, J.; Lemmetyinen, H. Bacteriorhodopsin in Langmuir-Blodgett films imaged with a scanning tunneling microscope. Langmuir 1993, 9, 2436–2447. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Inoue, T.; Ikematsu, M.; Iseki, M.; Sekiguchi, T. Controlling the orientation of purple membrane fragments on an air/water interface by a new method of direct electric field application during purple membrane spreading. Jpn. J. Appl. Phys. 1997, 36, 5674–5679. [Google Scholar] [CrossRef]
- Wang, J.P.; Li, J.R.; De Tao, P.; Li, X.C.; Jiang, L. Photoswitch based on bacteriorhodopsin Langmuir–Blodgett films. Adv. Funct. Mater. 1994, 4, 219–224. [Google Scholar] [CrossRef]
- Min, J.; Choi, H.-G.; Choi, J.-W.; Lee, W.H.; Kim, R. Optimal fabrication condition of bacteriorhodopsin films by electrophoretic sedimentation technique. Supramol. Sci. 1998, 5, 687–690. [Google Scholar] [CrossRef]
- Min, J.; Choi, H.-G.; Choi, J.-W.; Lee, W.H.; Kim, U.R. Photocurrent of bacteriorhodopsin films deposited by electrophoretic method. Thin Solid Films 1998, 327, 698–702. [Google Scholar] [CrossRef]
- Uehara, K.; Kawai, K.; Kouyama, T. Photoelectric response of oriented purple membrane electrodeposited onto poly(vinyl alcohol) film. Thin Solid Films 1993, 232, 271–277. [Google Scholar] [CrossRef]
- Al-Aribe, K.M.; Knopf, G.K.; Bassi, A.S. Photoelectric monolayers based on self-assembled and oriented purple membrane patches. J. Microelectromech. Syst. 2011, 20, 800–810. [Google Scholar] [CrossRef]
- Chen, D.-L.; Lu, Y.-J.; Sui, S.-F.; Xu, B.; Hu, K.-S. Oriented assembly of purple membrane on solid support, mediated by molecular recognition. J. Phys. Chem. B 2003, 107, 3598–3605. [Google Scholar] [CrossRef]
- Koyama, K.; Yamaguchi, N.; Miyasaka, T. Antibody-mediated bacteriorhodopsin orientation for molecular device architectures. Science 1994, 265, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, T. Design of intelligent optical sensors with organized bacteriorhodopsin films. Japn. J. Appl. Phys. 1995, 34, 3920–3924. [Google Scholar] [CrossRef]
- Fisher, K.A.; Yanagimoto, K.; Stoeckenius, W. Oriented adsorption of purple membrane to cationic surfaces. J. Cell Biol. 1978, 77, 611–621. [Google Scholar] [CrossRef] [PubMed]
- He, J.-A.; Samuelson, L.; Li, L.; Kumar, J.; Tripathy, S.K. Oriented bacteriorhodopsin/polycation multilayers by electrostatic layer-by-layer assembly. Langmuir 1998, 14, 1674–1679. [Google Scholar] [CrossRef]
- He, J.-A.; Samuelson, L.; Li, L.; Kumar, J.; Tripathy, S.K. Photoelectric properties of oriented bacteriorhodopsin/polycation multilayers by electrostatic layer-by-layer assembly. J. Phys. Chem. B 1998, 102, 7067–7072. [Google Scholar] [CrossRef]
- Lvov, Y.; Ariga, K.; Ichinose, I.; Kunitake, T. Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J. Am. Chem. Soc. 1995, 117, 6117–6123. [Google Scholar] [CrossRef]
- Pei, R.; Cui, X.; Yang, X.; Wang, E. Assembly of alternating polycation and DNA multilayer films by electrostatic layer-by-layer adsorption. Biomacromolecules 2001, 2, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Knopf, G.K.; Bassi, A.S. Photoelectric properties of a detector based on dried bacteriorhodopsin film. Biosens. Bioelectron. 2006, 21, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Osei, E.K.; Yeow, J.T. Bacteriorhodopsin for superficial X-ray sensing. Sens. Actuators B Chem. 2012, 166, 177–183. [Google Scholar] [CrossRef]
- Li, Y.-T.; Tian, H.; Zhao, H.-M.; Jian, M.-Q.; Lv, Y.-J.; Tian, Y.; Wang, Q.; Yang, Y.; Xiang, Y.; Zhang, Y. A novel cell-scale bio-nanogenerator based on electron-ion interaction for fast light power conversion. Nanoscale 2018, 10, 526–532. [Google Scholar] [CrossRef] [PubMed]



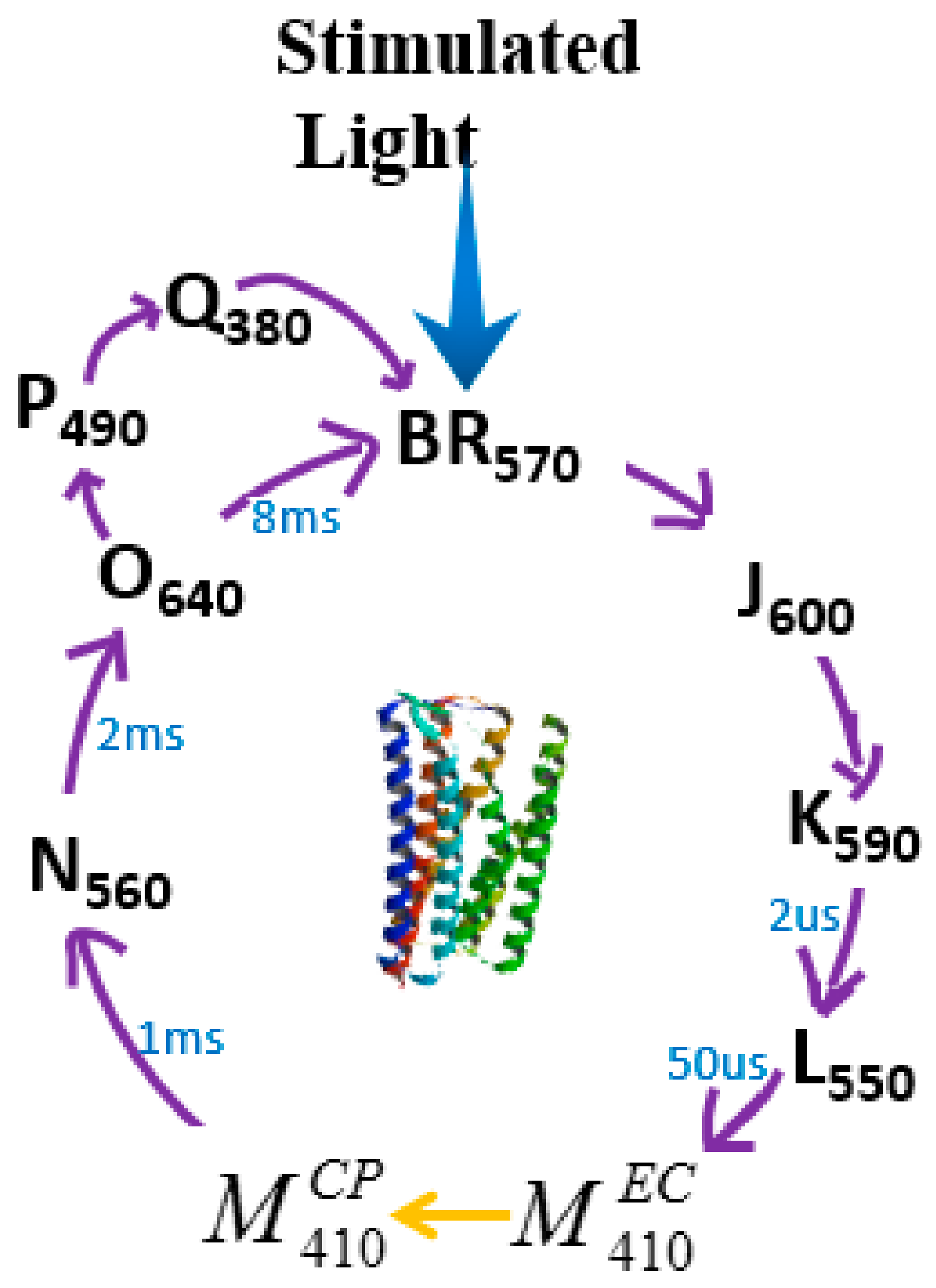



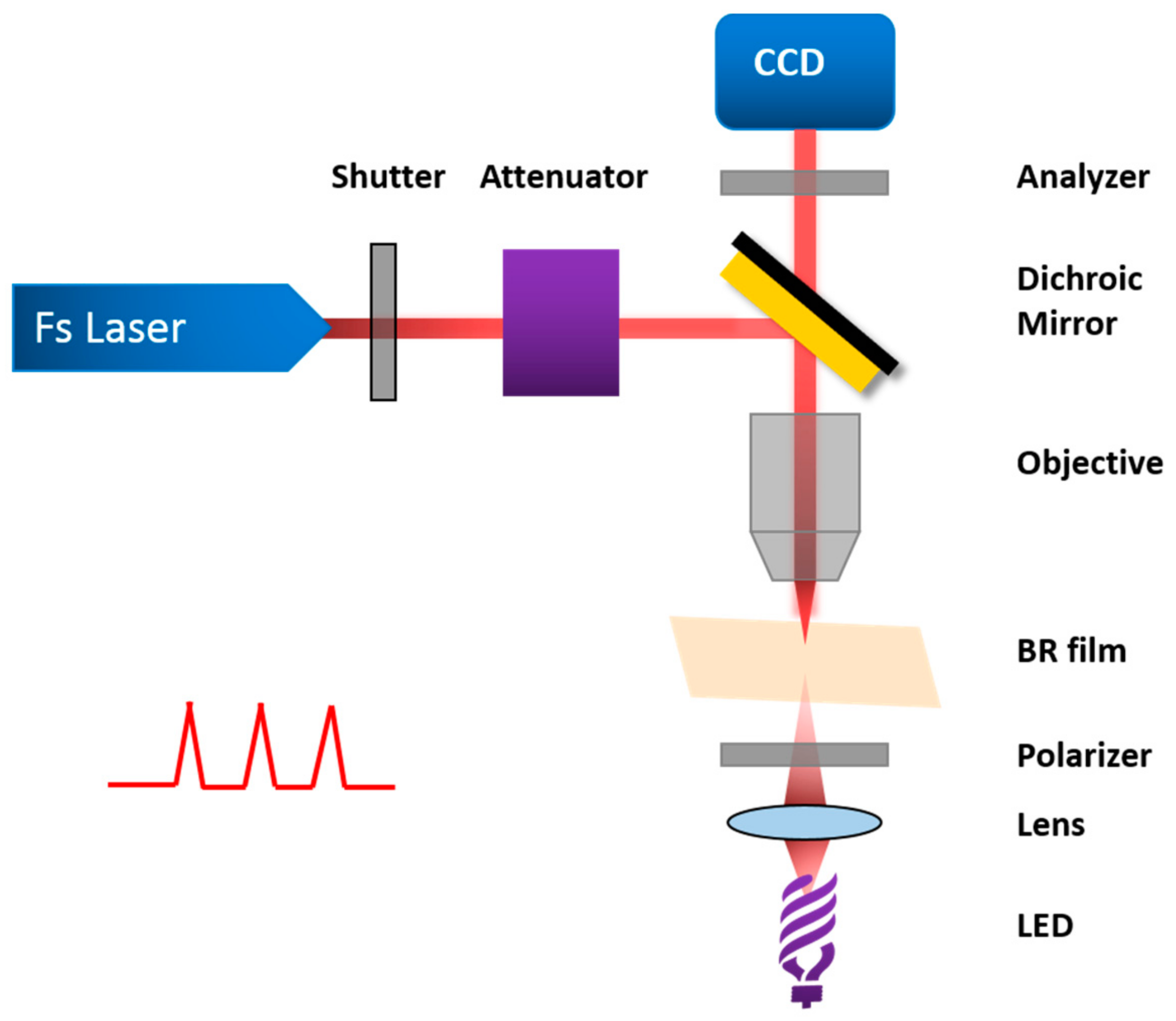


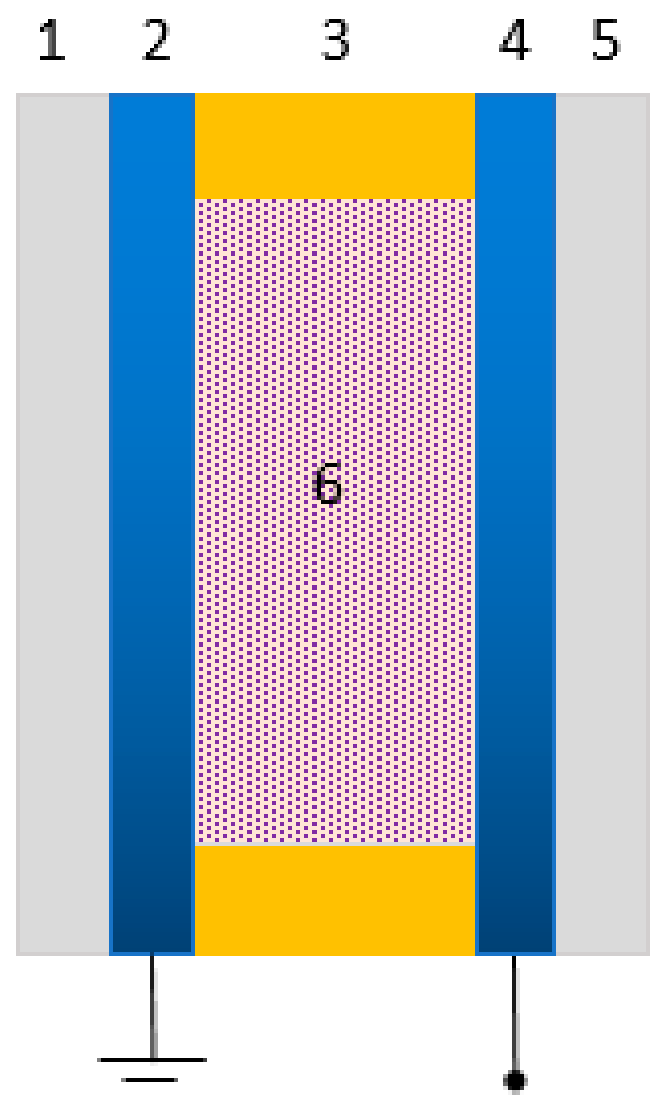
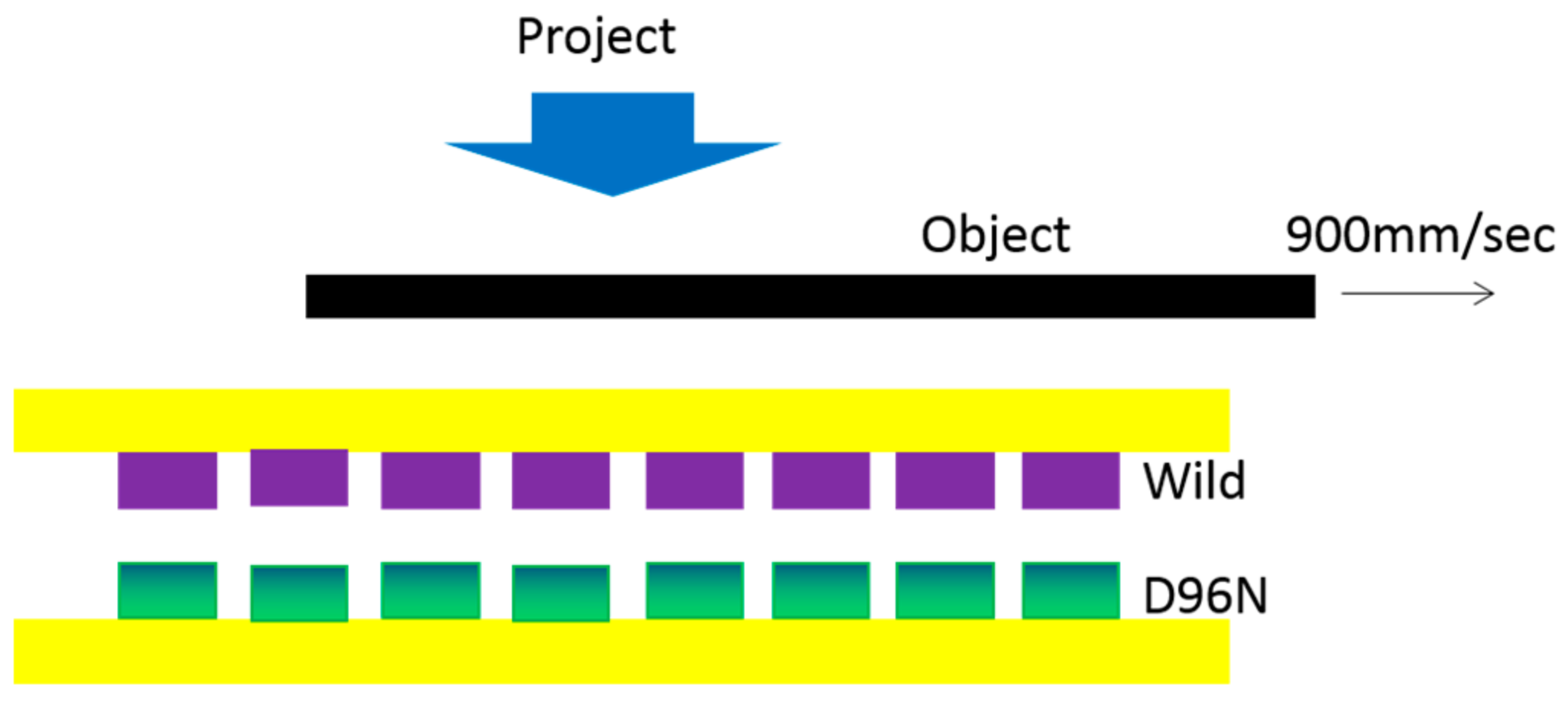
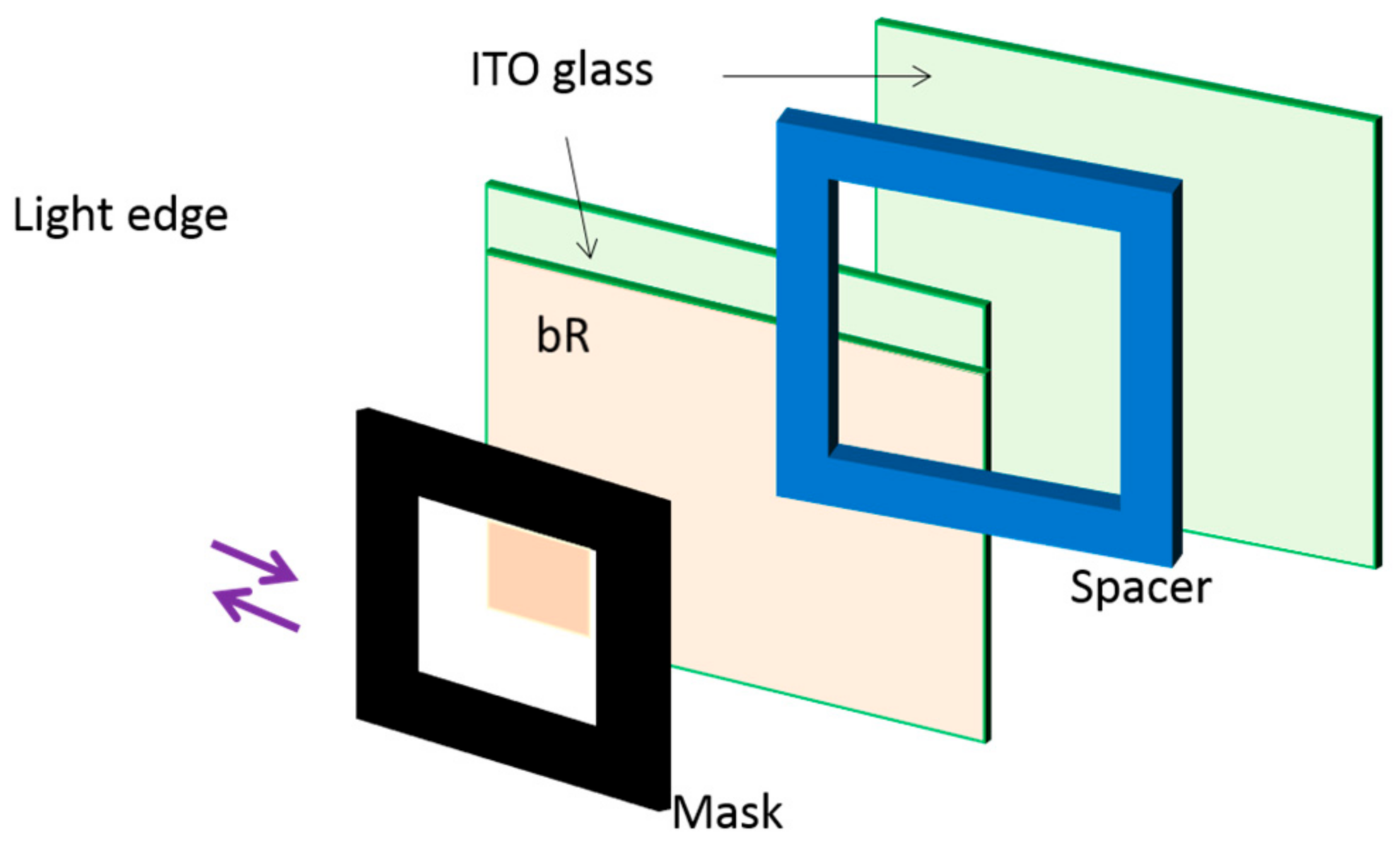
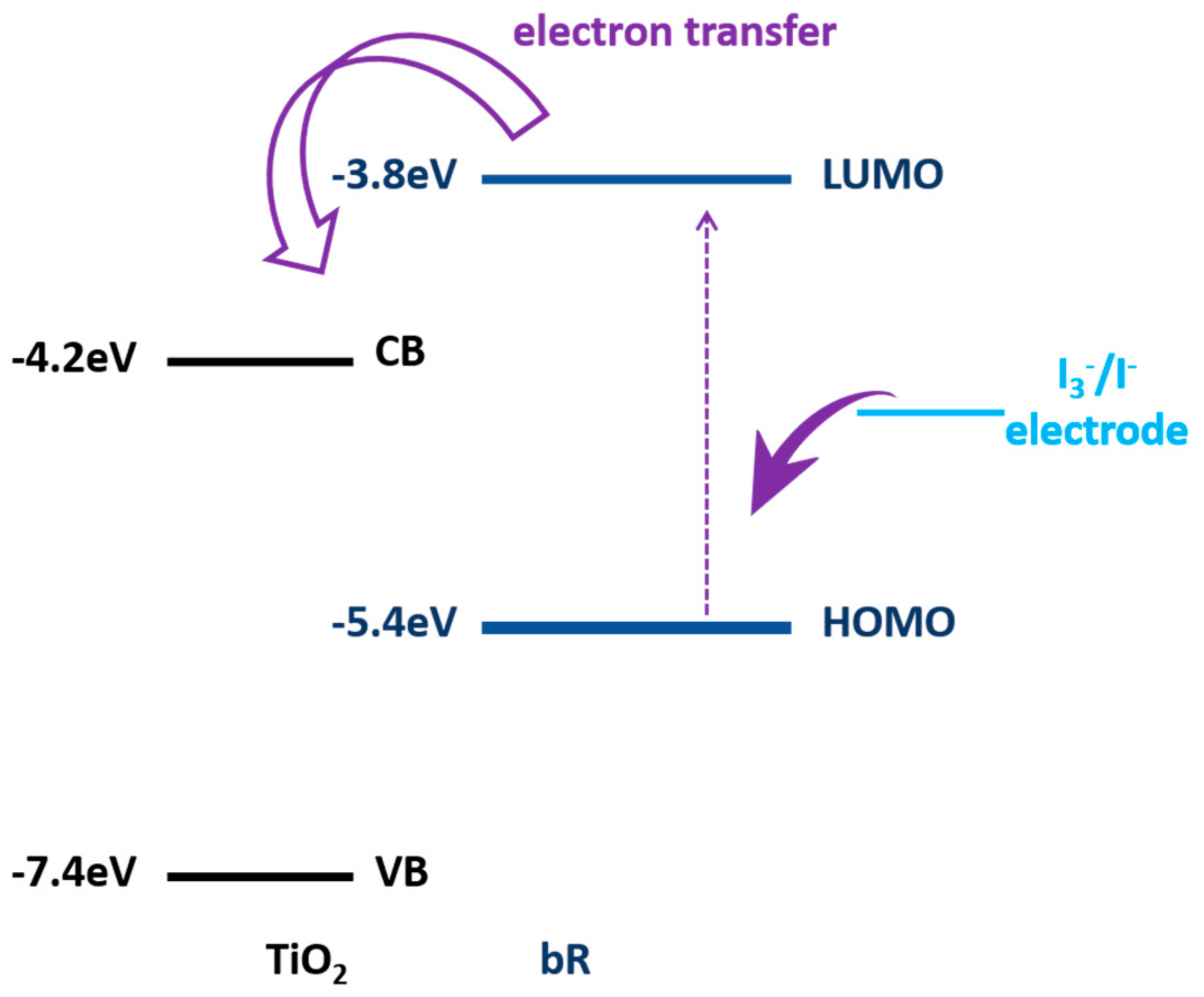
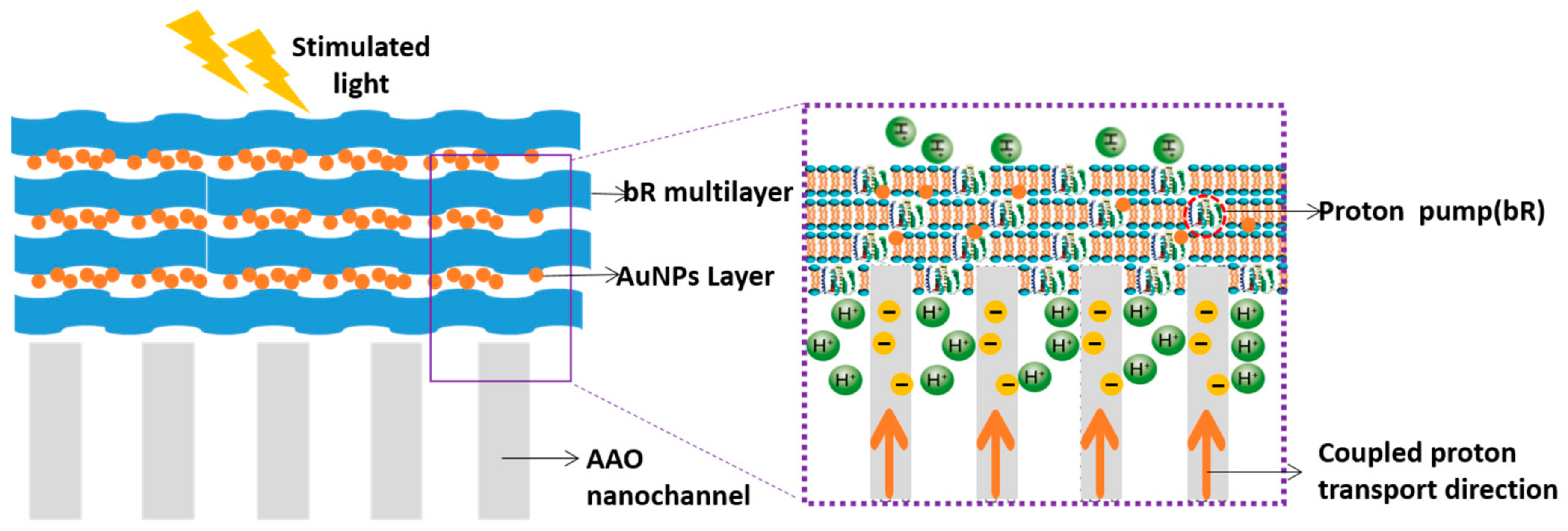
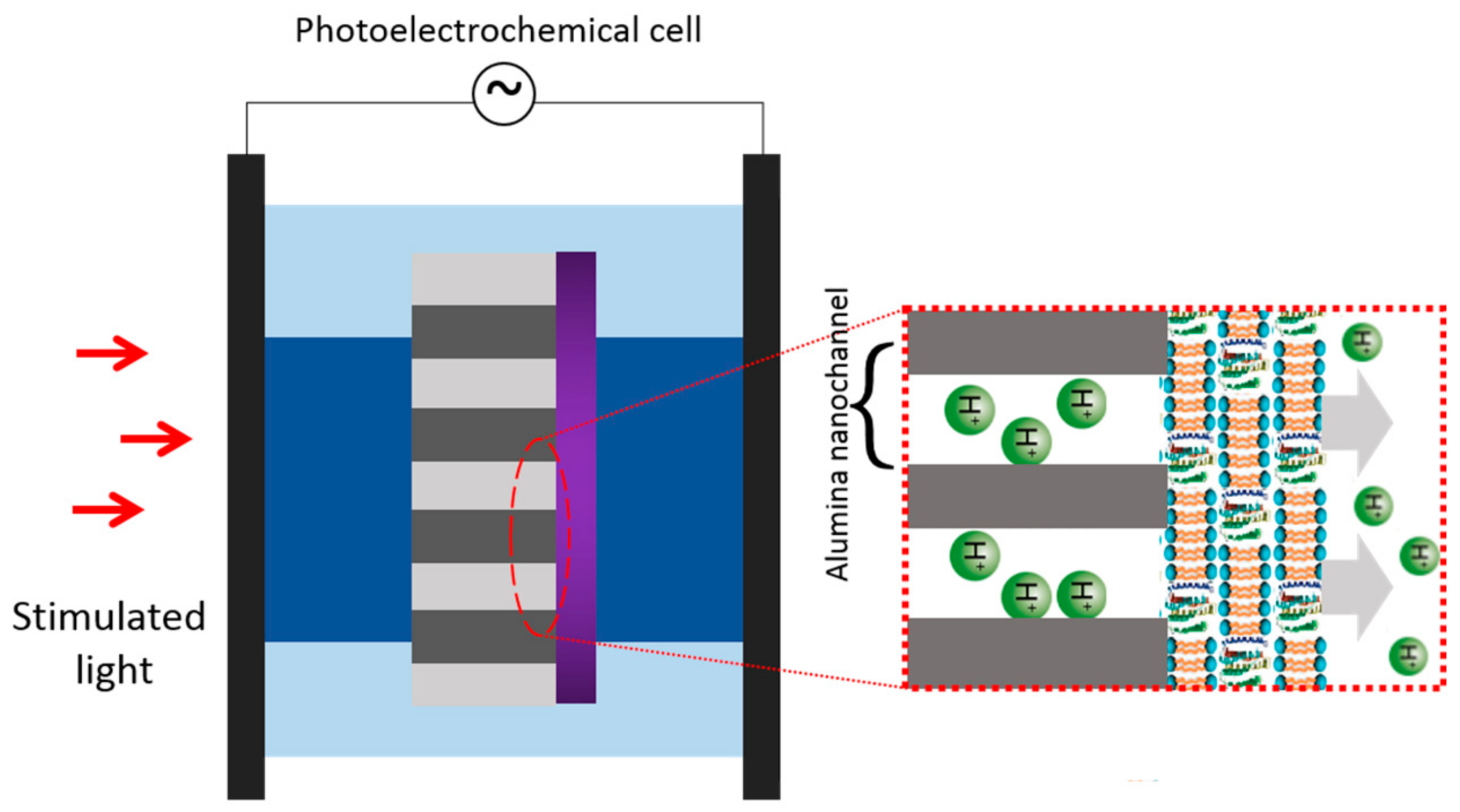

| Protein | Ion | Sensitive Light Color | Function | Contents |
|---|---|---|---|---|
| bR | H+ | Green | Energy conversion | 2,000,000/bacteria |
| hR | Cl− | Green | Maintain osmolarity | 20,000/bacteria |
| sR-I | H+ | Blue/Green | Avoid blue light | 5000/bacteria |
| sR-II | H+ | purple | Avoid purple light | 5000/bacteria |
| Methods | Orientation | Number of Layer | References |
|---|---|---|---|
| Langmuir–Blodgett deposition | High degree of control | Excellent control over layer numbers | [77,78,79,80,81,82,83,84,85,86,87,88] |
| Electrophoretic sedimentation | High degree of control | Multilayer | [85,89,90,91] |
| Biotin-streptavidin mediated | High degree of control | Monolayer | [92,93] |
| Antibody-mediated monolayer | High degree of control | Monolayer | [94,95] |
| Electrostatic adsorption | Difficult to confirm | <12 | [96,97,98,99] |
| Composite Materials | Operation Mechanisms | Literature |
|---|---|---|
| bR/TiO2 | Electron transfer effect | Energy Environ. Sci., 2011 [28] |
| bR/Au | Coupled proton transportation | Nano Energy, 2015 [29] |
| pR/kpw | 3D Proton transfer | Adv. Mater., 2015 [32] |
| bR/AAO | Protons transportation | Adv. Mater., 2016 [30] |
| bR/Nafion | Plasmonic enhancement | Nano letters, 2011 [34] |
| bR/QDs | Förster resonance energy transfer | ACS nano, 2013 [31] |
| bR/CNTs | Electron–ion interaction | Nanoscale, 2018 [103] |
| Application Direction | Application Category | Currently Being Researched | Reason for Stop |
|---|---|---|---|
| Photochemical application | Optical volumetric memories | Yes | — |
| Holographic associative processors | No | Poor compatibility and replaced by new optical information recording materials | |
| Photoelectric application | Motion biosensors | No | Expensive material and weaker electrical signal |
| X-ray sensor | Yes | — | |
| Photovoltaic cells | Yes | — | |
| Immunosensor | Yes | — | |
| Artificial retinal prostheses | No | Security and human biological compatibility |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-T.; Tian, Y.; Tian, H.; Tu, T.; Gou, G.-Y.; Wang, Q.; Qiao, Y.-C.; Yang, Y.; Ren, T.-L. A Review on Bacteriorhodopsin-Based Bioelectronic Devices. Sensors 2018, 18, 1368. https://doi.org/10.3390/s18051368
Li Y-T, Tian Y, Tian H, Tu T, Gou G-Y, Wang Q, Qiao Y-C, Yang Y, Ren T-L. A Review on Bacteriorhodopsin-Based Bioelectronic Devices. Sensors. 2018; 18(5):1368. https://doi.org/10.3390/s18051368
Chicago/Turabian StyleLi, Yu-Tao, Ye Tian, He Tian, Tao Tu, Guang-Yang Gou, Qian Wang, Yan-Cong Qiao, Yi Yang, and Tian-Ling Ren. 2018. "A Review on Bacteriorhodopsin-Based Bioelectronic Devices" Sensors 18, no. 5: 1368. https://doi.org/10.3390/s18051368





