Estimation of Human Body Vital Signs Based on 60 GHz Doppler Radar Using a Bound-Constrained Optimization Algorithm
Abstract
:1. Introduction
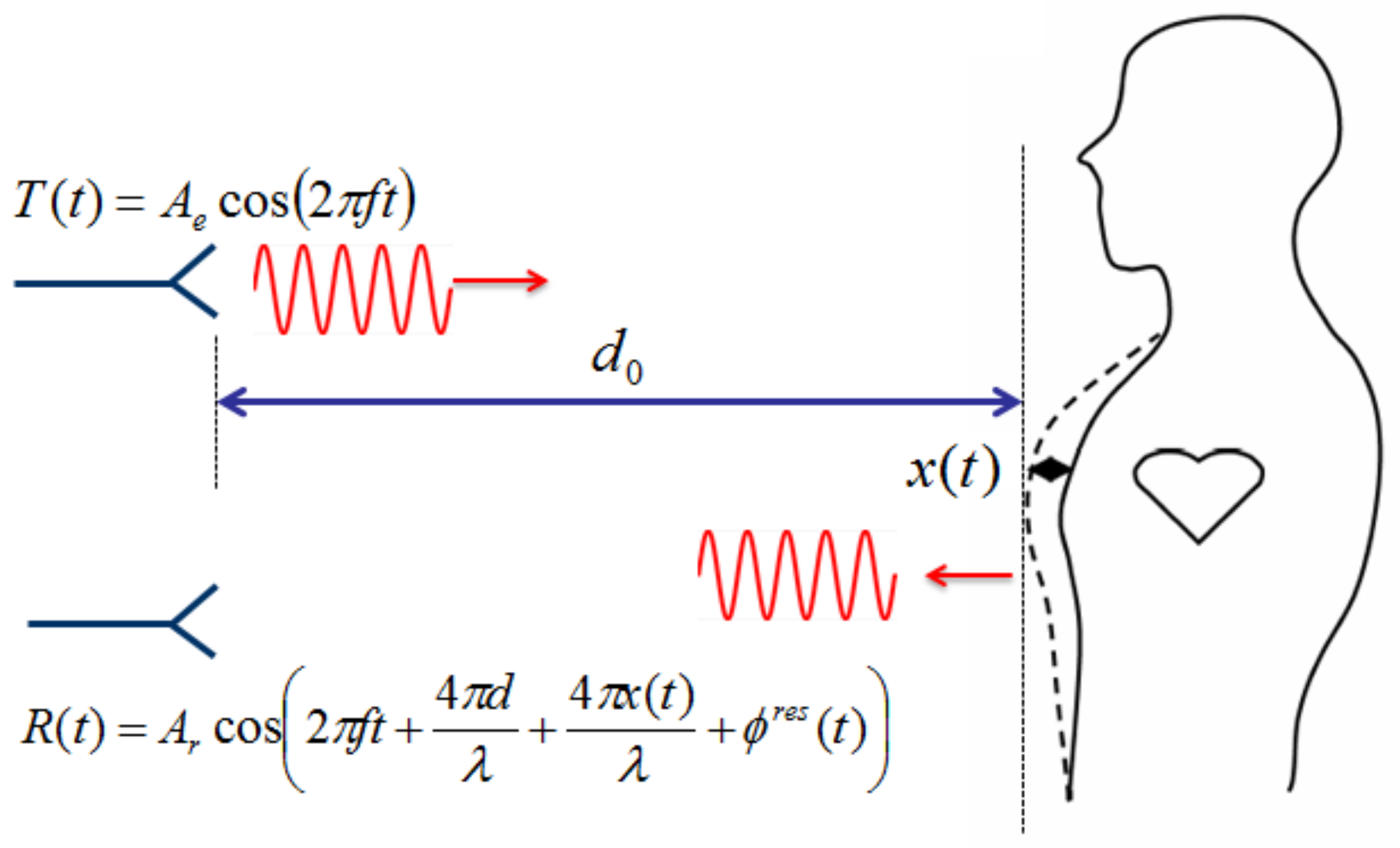
2. Nonlinearity in Doppler Radar Vital-Signal Detection
2.1. Arctangent Demodulation
2.2. Complex Demodulation
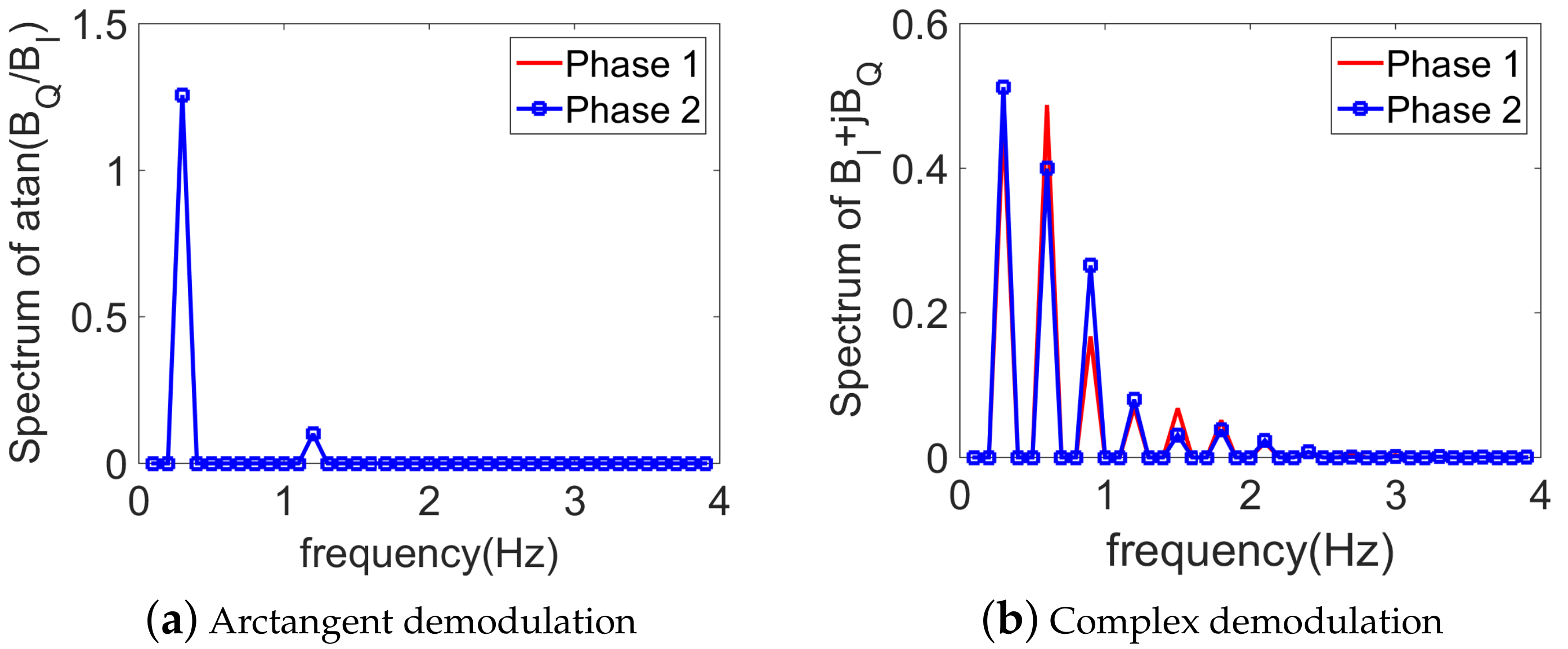
3. Numerical Spectrum Analysis
3.1. Without Noise
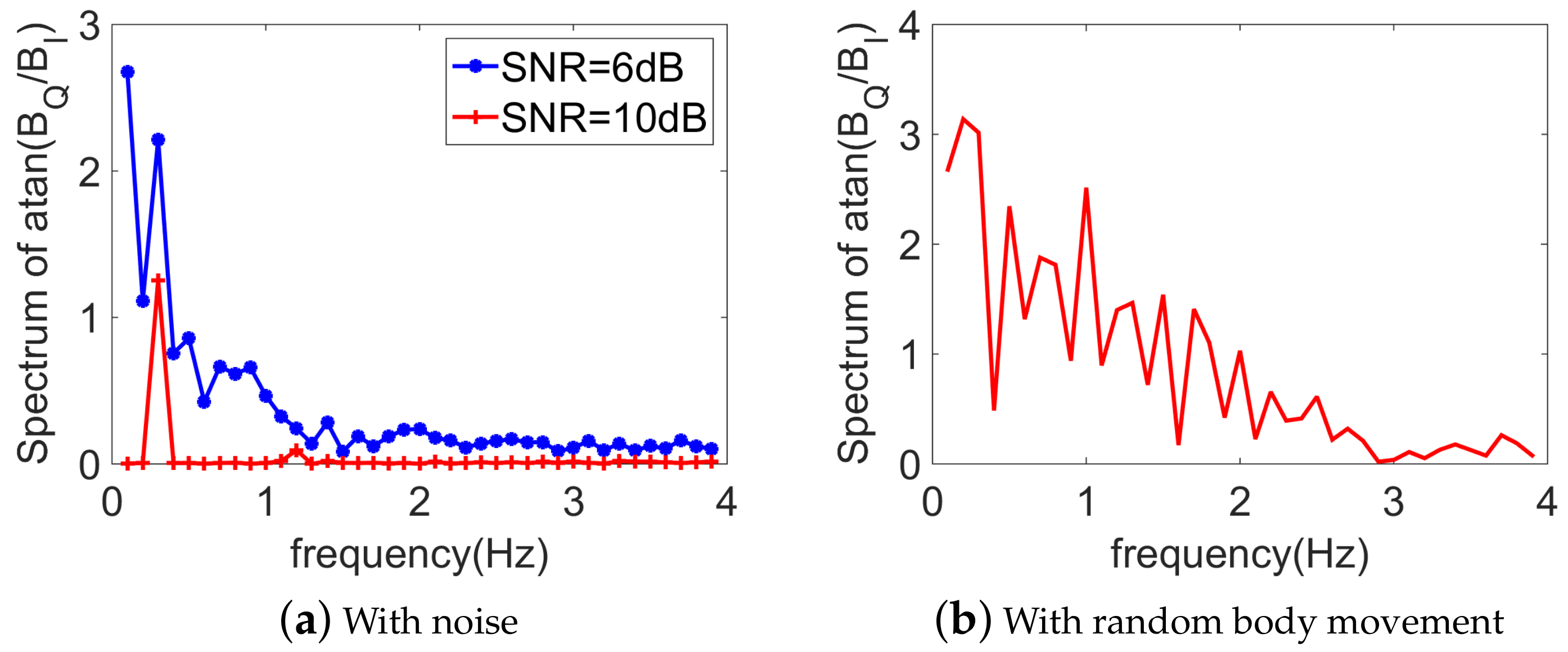
3.2. With Noise
3.3. Choice of the Demodulation Technique
4. Vital-Sign Detection Using Optimization Algorithms
4.1. Description of the Problem
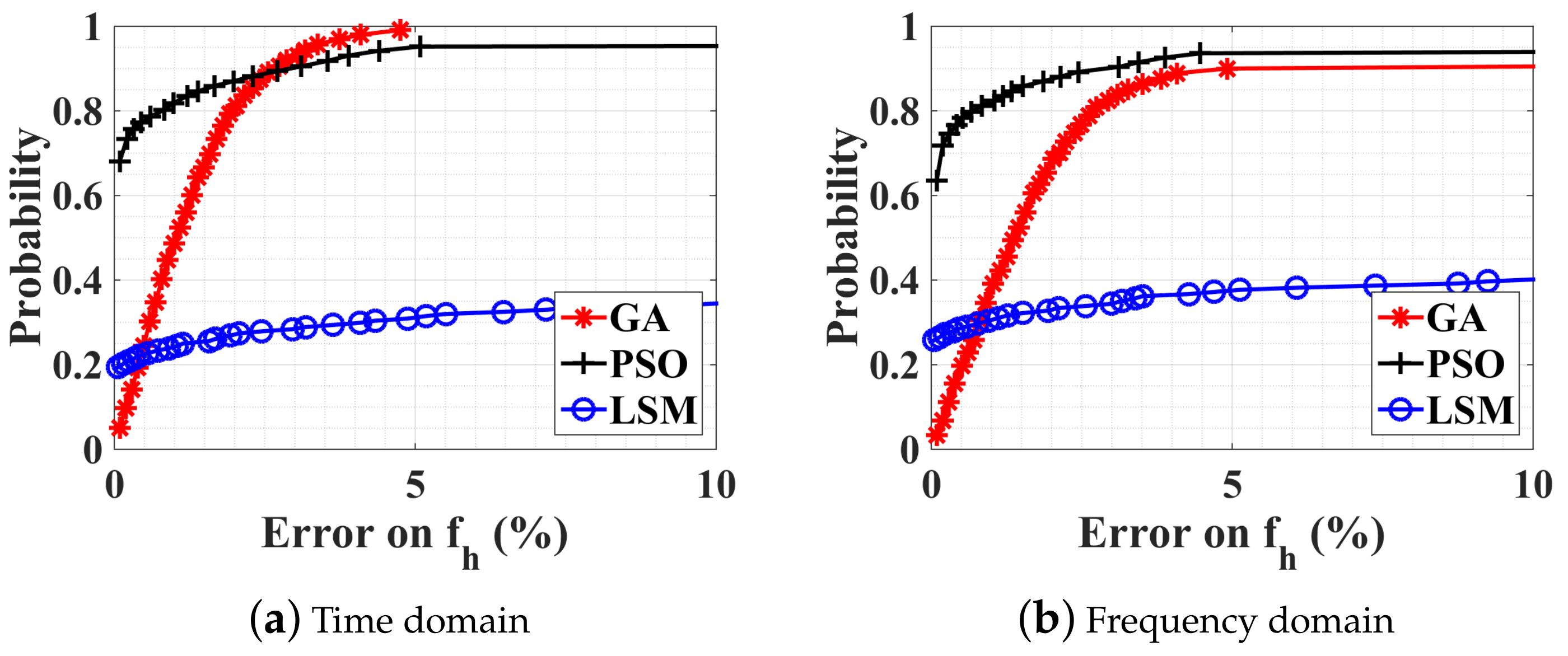
4.2. Numerical Results
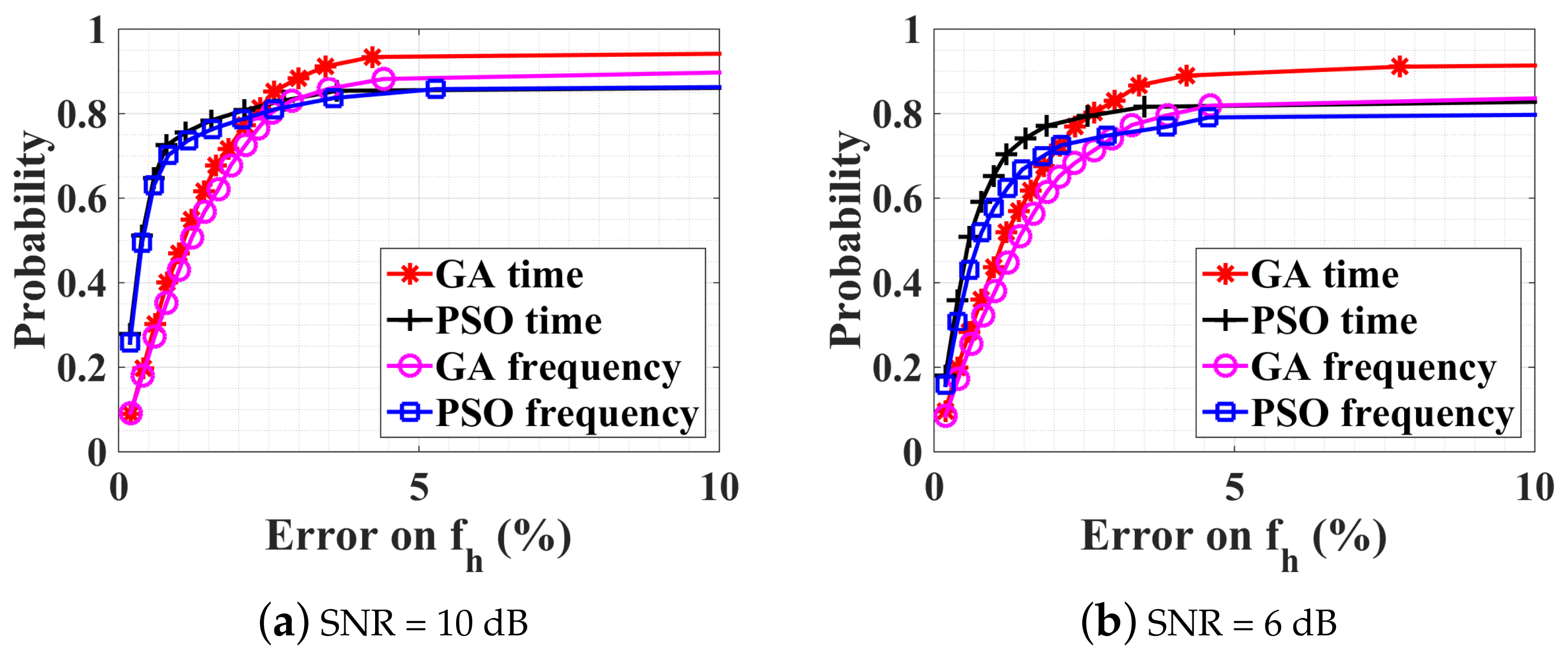
4.2.1. Without Noise, with Ambiguity
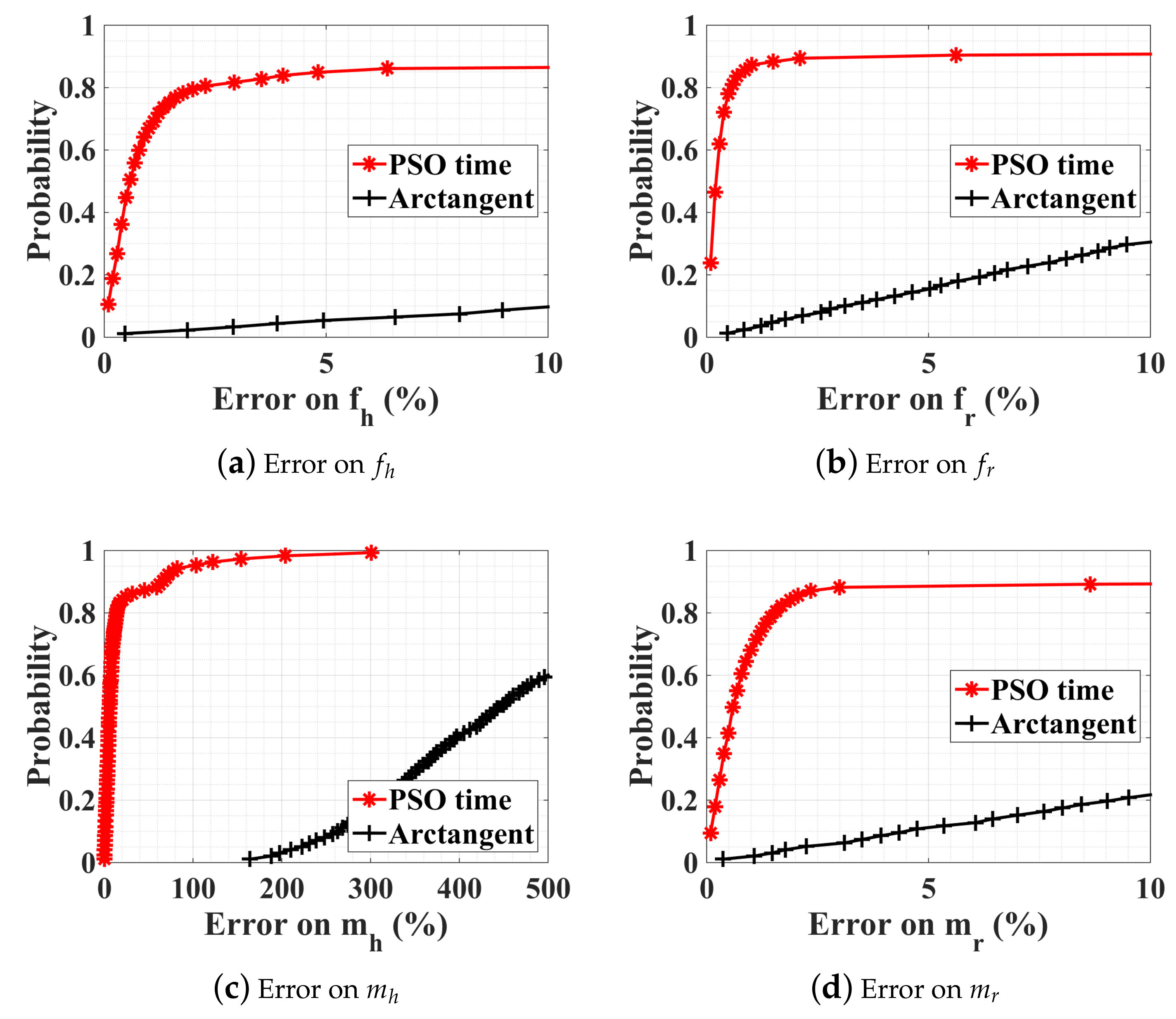
4.2.2. Noise Influence on the Optimization
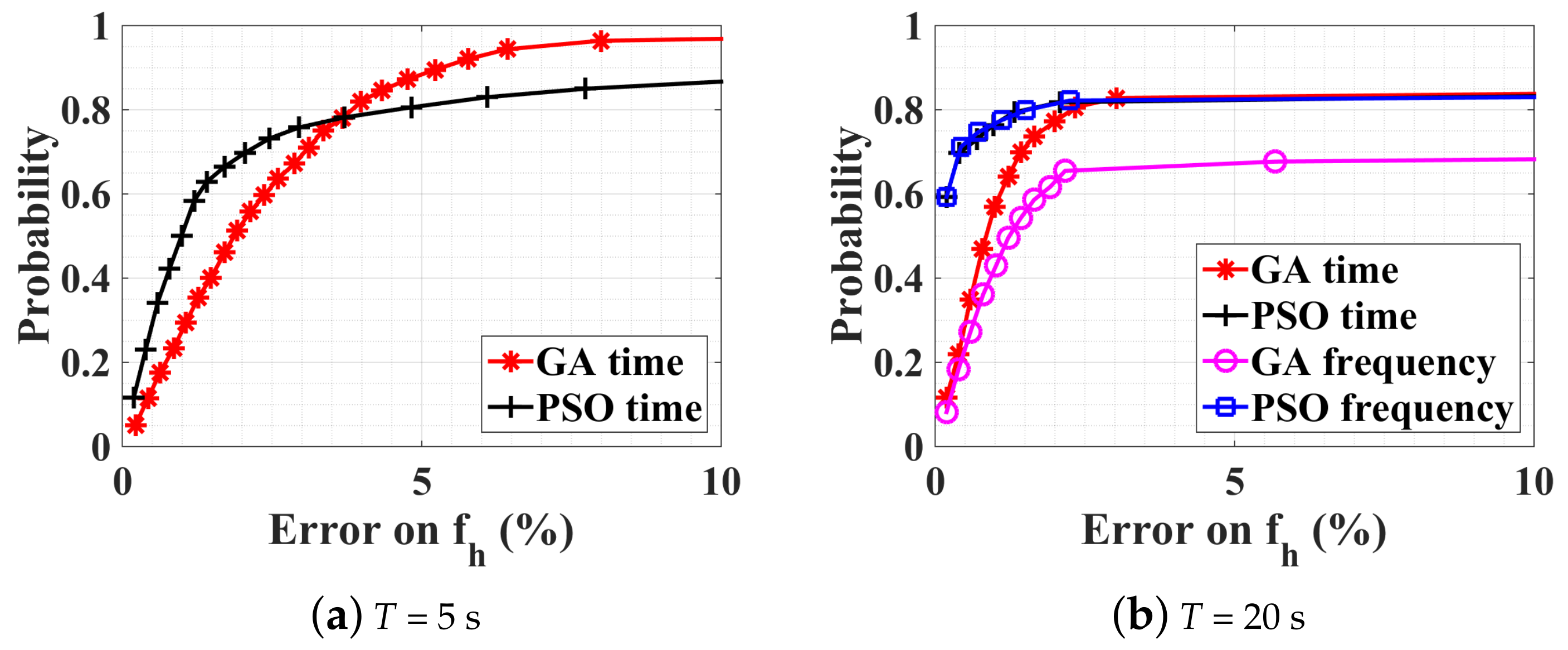
4.2.3. Observation-Time Influence on the Optimization
5. Large-Scale Constrained Bound: PSO Parallel Optimization
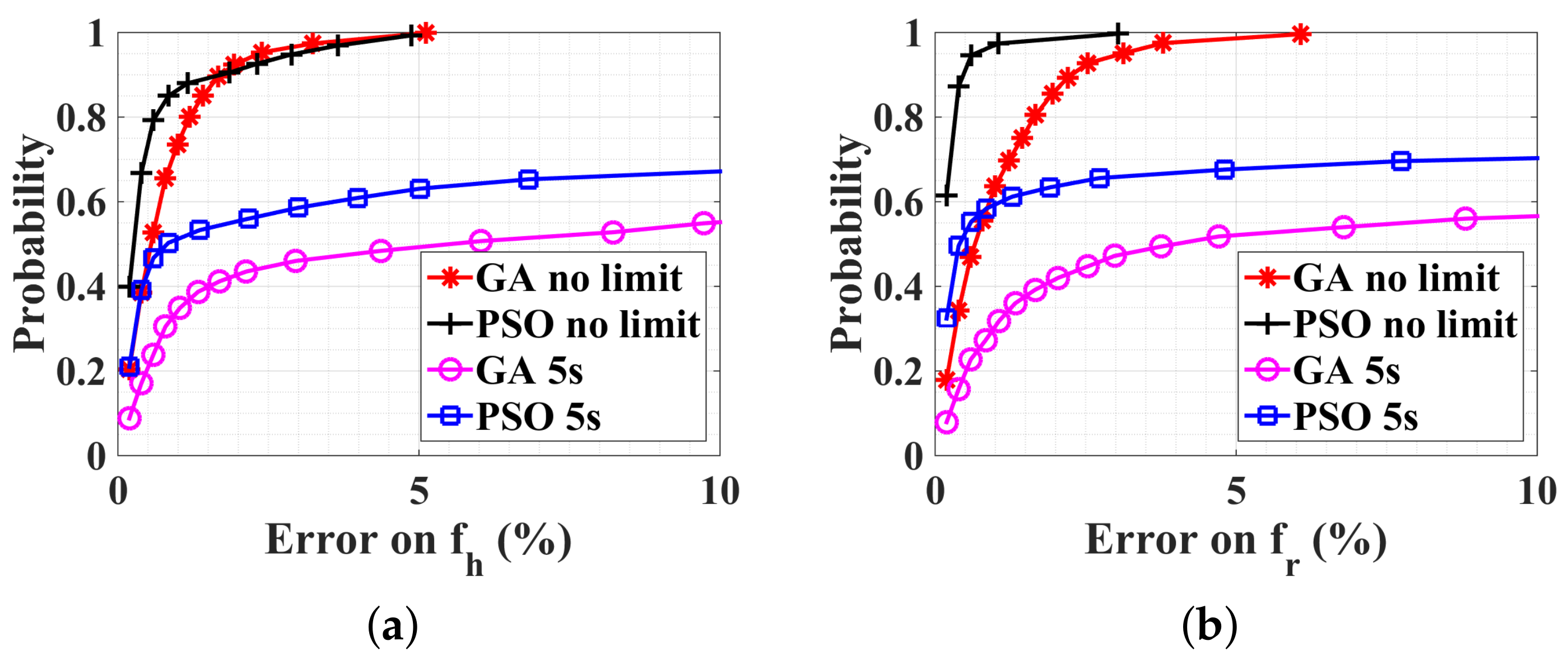
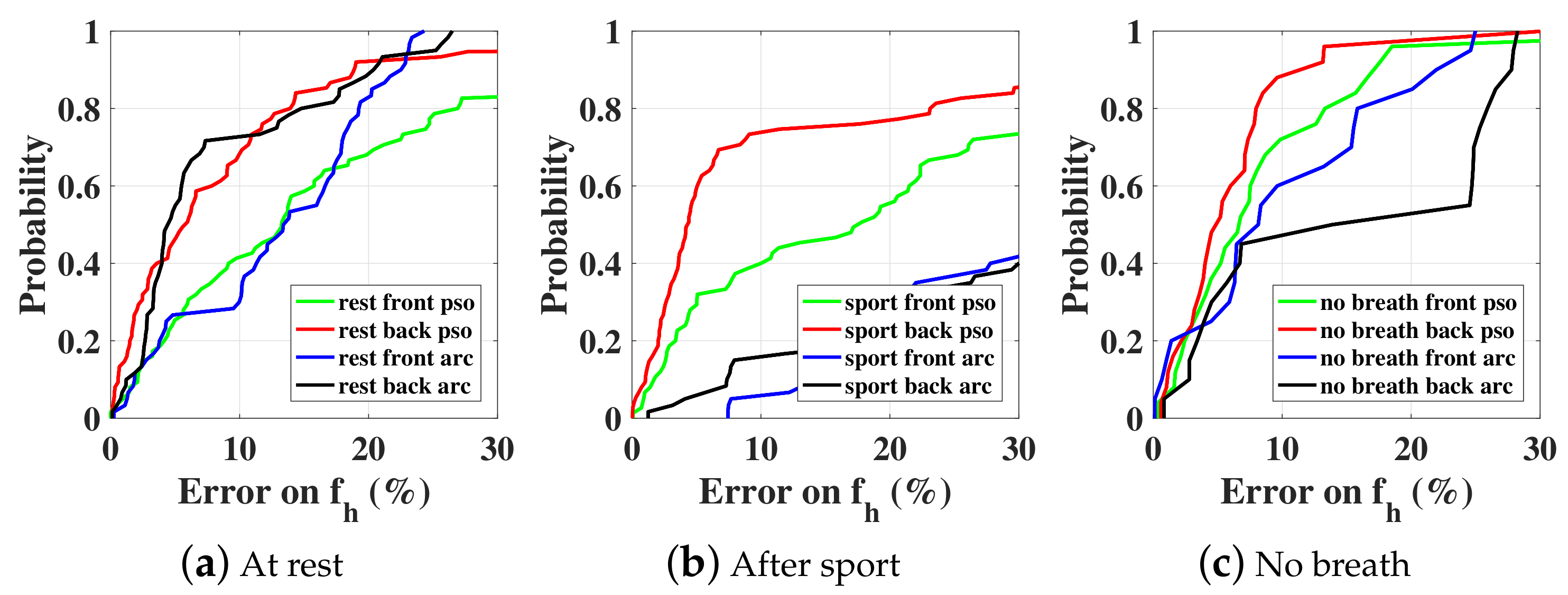
5.1. Normal Case
5.2. No-Breath Case
5.3. With a Random Body Motion
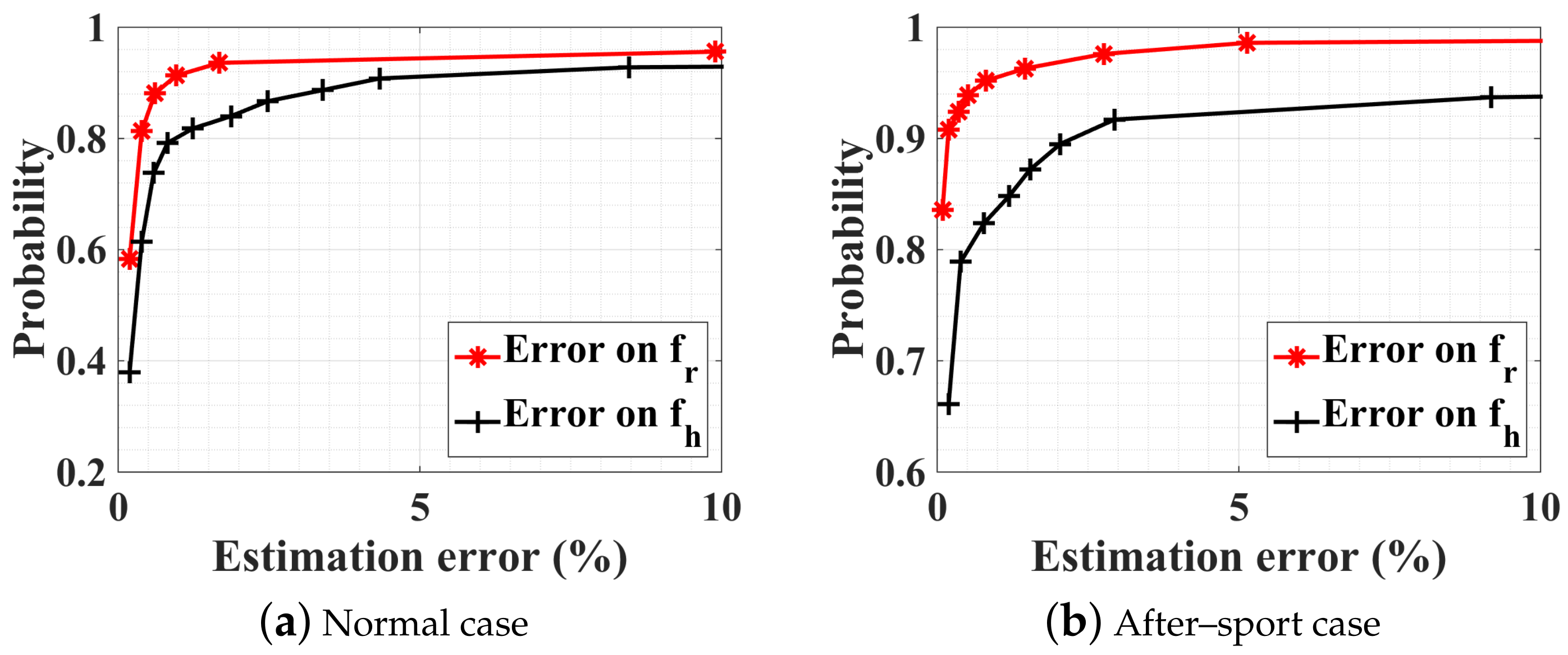
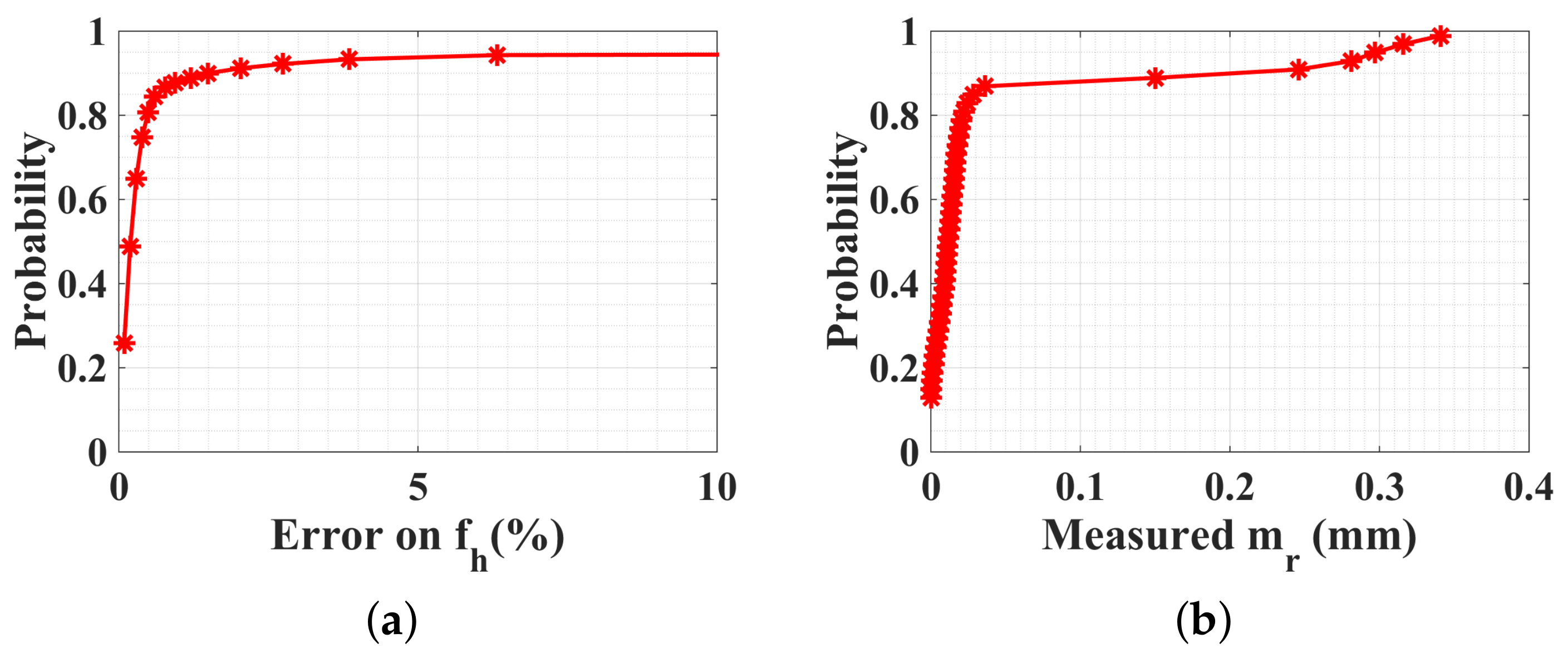
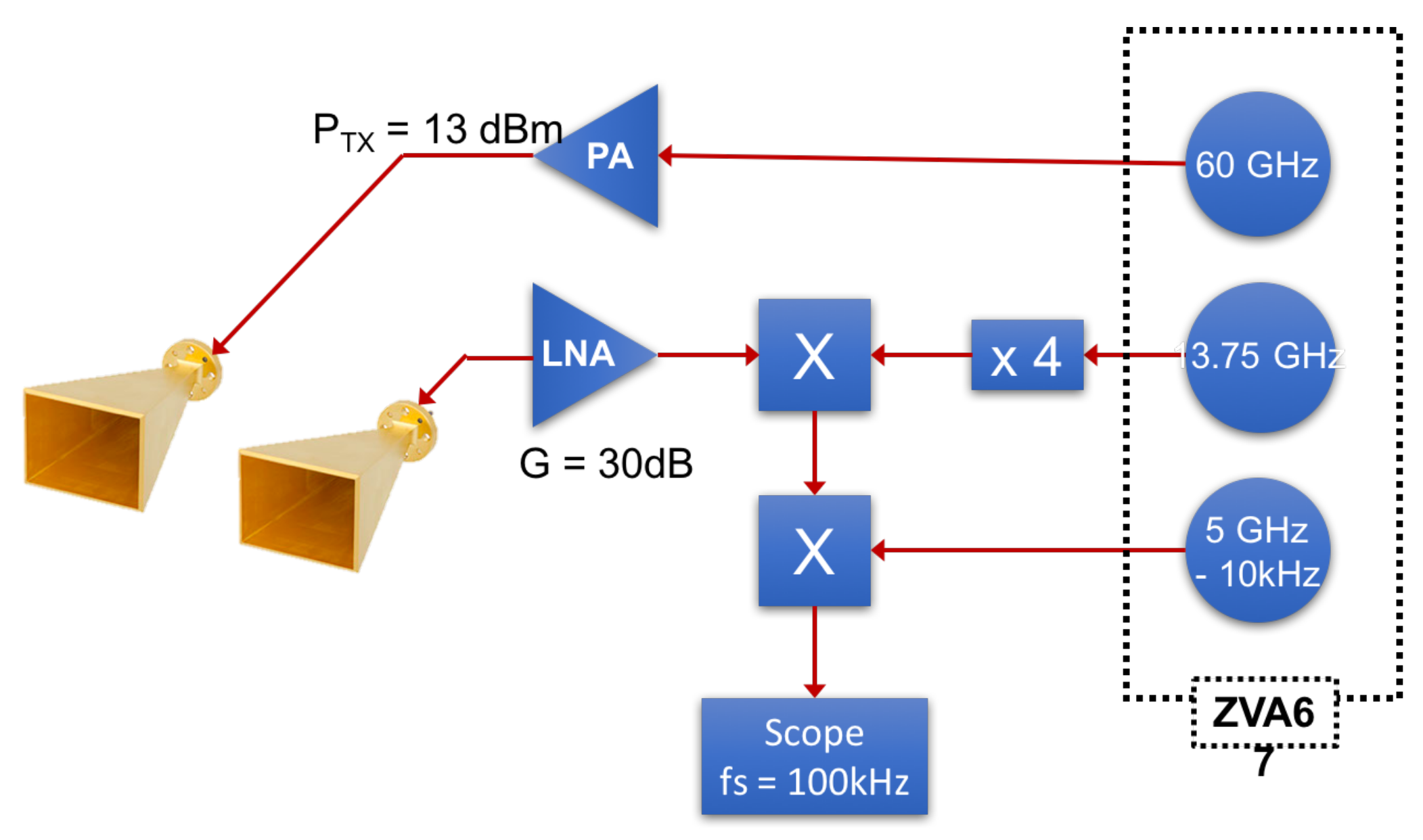
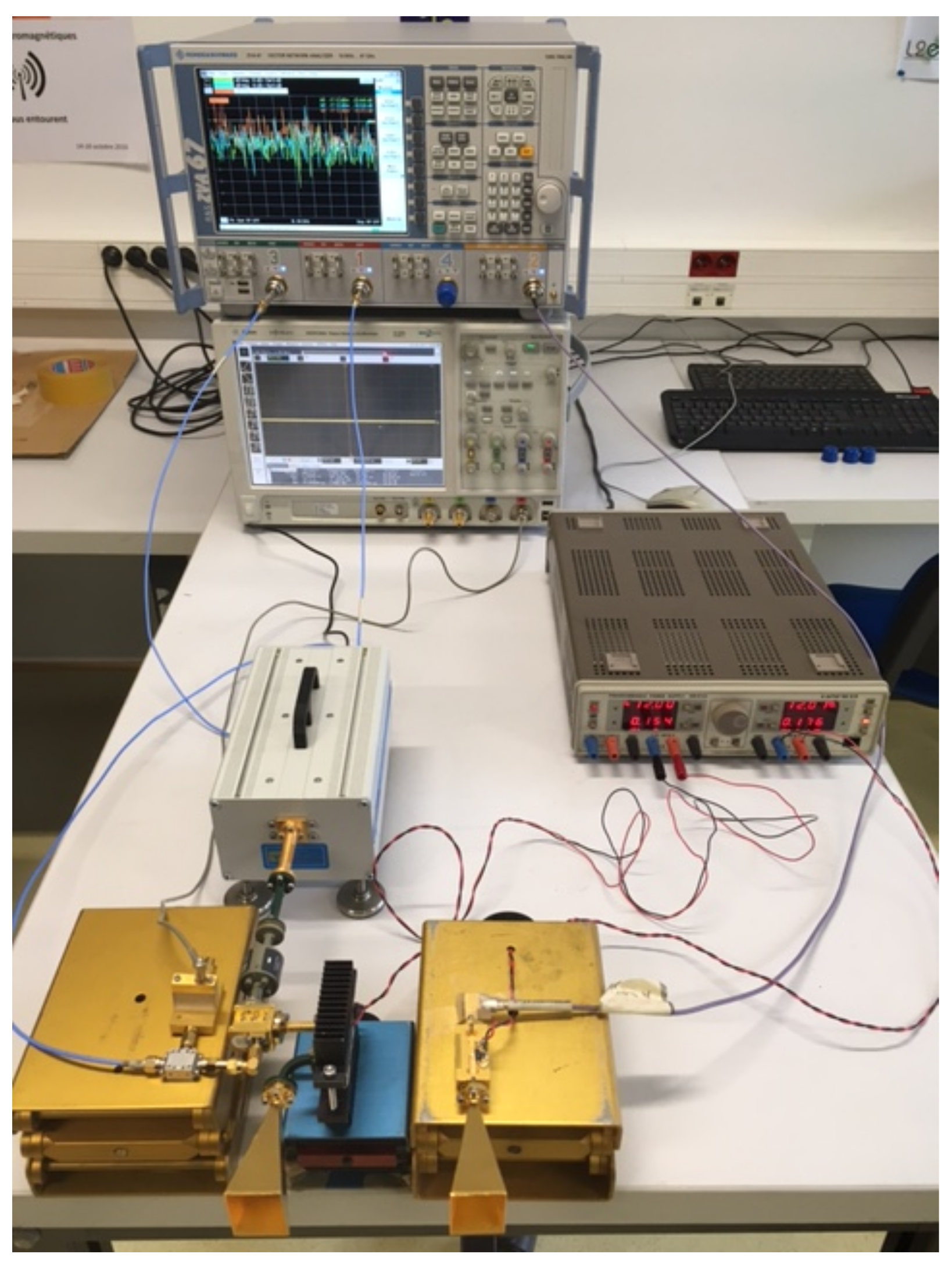
5.4. Experimental Measurements
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| WSN | Wireless sensor network |
| IQ | In-phase quadrature |
| LO | Local oscillator |
| EEMD | Ensemble empirical mode decomposition |
| CW | Continuous wave |
| CDF | Cumulative distribution function |
| LSM | Least-square minimization |
| GA | Genetic algorithm |
| PSO | Particle swarm optimization |
References
- Aguilar, P.A.C.; Boudy, J.; Istrate, D.; Dorizzi, B.; Mota, J.C.M. A Dynamic Evidential Network for Fall Detection. IEEE J. Biomed. Health Inform. 2014, 18, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chen, X.; Shen, M. A Framework for Daily Activity Monitoring and Fall Detection Based on Surface Electromyography and Accelerometer Signals. IEEE J. Biomed. Health Inform. 2013, 17, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ci, S.; Katsaggelos, A.K.; Liu, Y. A Multi-Camera Motion Capture System for Remote Health Care Monitoring. In Proceedings of the 2013 IEEE International Conference on Multimedia and Expo (ICME 2013), San Jose, CA, USA, 15–19 July 2013. [Google Scholar]
- Istrate, D.; Vacher, M.; Serignat, J.F. Generic implementation of a distress sound extraction system for elder care. In Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 854–857. [Google Scholar]
- Aguiar, B.; Rocha, T.; Silva, J.; Sousa, I. Accelerometer-Based Fall Detection for Smartphones. In Proceedings of the 2014 IEEE International Symposium on Medical Measurements and Applications (MEMEA), Lisboa, Portugal, 11–12 June 2014; pp. 480–485. [Google Scholar]
- Fischer, M.; Lim, Y.Y.; Lawrence, E.; Ganguli, L.K. ReMoteCare: Health Monitoring with Streaming Video. In Proceedings of the 7th International Conference on Mobile Business (ICMB ’08), Barcelona, Spain, 7–8 July 2008; pp. 280–286. [Google Scholar]
- LeBellego, G.; Noury, N.; Virone, G.; Mousseau, M.; Demongeot, J. A model for the measurement of patient activity in a hospital suite. IEEE Trans. Inf. Technol. Biomed. 2006, 10, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Noury, N.; Hadidi, T. Computer simulation of the activity of the elderly person living independently in a Health Smart Home. Comput. Methods Progr. Biomed. 2012, 108, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Kudo, M.; Pei, B.N.; Nonaka, H.; Toyama, J. Multiperson Locating and Their Soft Tracking in a Binary Infrared Sensor Network. IEEE Trans. Hum.-Mach. Syst. 2015, 45, 550–561. [Google Scholar] [CrossRef]
- Li, C.; Lubecke, V.M.; Boric-Lubecke, O.; Lin, J. A Review on Recent Advances in Doppler Radar Sensors for Noncontact Healthcare Monitoring. IEEE Trans. Microw. Theory Tech. 2013, 61, 2046–2060. [Google Scholar] [CrossRef]
- Kuutti, J.; Paukkunen, M.; Aalto, M.; Eskelinen, P.; Sepponen, R.E. Evaluation of a Doppler radar sensor system for vital signs detection and activity monitoring in a radio-frequency shielded room. Measurement 2015, 68, 135–142. [Google Scholar] [CrossRef]
- Li, C.; Xiao, Y.; Lin, J. Experiment and Spectral Analysis of a Low-power Ka-band Heartbeat Detector Measuring from Four Sides of a Human Body. IEEE Trans. Microw. Theory Tech. 2006, 54, 4464–4471. [Google Scholar] [CrossRef]
- Droitcour, A.D.; Boric-Lubecke, O.; Lubecke, V.M.; Lin, J.; Kovacs, G.T. Range Correlation and I/Q Performance Benefits in Single-chip Silicon Doppler Radars for Noncontact Cardiopulmonary Monitoring. IEEE Trans. Microw. Theory Tech. 2004, 52, 838–848. [Google Scholar] [CrossRef]
- Li, C.; Lin, J. Random Body Movement Cancellation in Doppler Radar Vital Sign Detection. IEEE Trans. Microw. Theory Tech. 2008, 56, 3143–3152. [Google Scholar]
- Li, C.; Lin, J. Complex Signal Demodulation and Random Body Movement Cancellation Techniques for Non-contact Vital Sign Detection. In Proceedings of the IEEE MTT-S International Microwave Symposium Digest, Atlanta, GA, USA, 15–20 June 2008; pp. 566–569. [Google Scholar]
- Hu, W.; Zhao, Z.; Wang, Y.; Zhang, H.; Lin, F. Noncontact Accurate Measurement of Cardiopulmonary Activity Using a Compact Quadrature Doppler Radar Sensor. IEEE Trans. Biomed. Eng. 2014, 61, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Boric-Lubecke, O.; Lubecke, V.M. Arctangent Demodulation With DC Offset Compensation in Quadrature Doppler Radar Receiver Systems. IEEE Trans. Microw. Theory Tech. 2007, 55, 1073–1079. [Google Scholar] [CrossRef]
- Li, C.; Lin, J. Recent Advances in Doppler Radar Sensors for Pervasive Healthcare Monitoring. In Proceedings of the Asia-Pacific Microwave Conference, Yokohama, Japan, 7–10 December 2010; pp. 283–290. [Google Scholar]
- Li, C.; Lin, J. Optimal Carrier Frequency of Non-contact Vital Sign Detectors. In Proceedings of the IEEE Radio and Wireless Symposium, Long Beach, CA, USA, 9–11 January 2007; pp. 490–493. [Google Scholar]
- Chioukh, L.; Boutayeb, H.; Li, L.; Yahia, L.H.; Wu, K. Integrated Radar Systems for Precision Monitoring of Heartbeat and Respiratory Status. In Proceedings of the APMC: Asia Pacific Microwave Conference, Singapore, 7–10 December 2009; pp. 405–408. [Google Scholar]
- Chuang, H.R.; Kuo, H.C.; Lin, F.L.; Huang, T.H.; Kuo, C.S.; Ou, Y.W. 60-GHz Millimeter-Wave Life Detection System (MLDS) for Noncontact Human Vital-Signal Monitoring. IEEE Sens. J. 2012, 12, 602–609. [Google Scholar] [CrossRef]
- Nguyen, V.; Javaid, A.Q.; Weitnauer, M.A. Harmonic Path (HAPA) Algorithm for Non-contact Vital Signs Monitoring with IR-UWB Radar. In Proceedings of the Biomedical Circuits and Systems Conference, Rotterdam, The Netherlands, 31 October–2 November 2013; pp. 146–149. [Google Scholar]
- Nguyen, V.; Javaid, A.Q.; Weitnauer, M.A. Spectrum-Averaged Harmonic Path (SHAPA) Algorithm for Non-Contact Vital Sign Monitoring with Ultra-wideband (UWB) Radar. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 2241–2244. [Google Scholar]
- Li, C.; Ling, J.; Li, J.; Lin, J. Accurate Doppler Radar Noncontact Vital Sign Detection Using the RELAX Algorithm. IEEE Trans. Instrum. Meas. 2010, 59, 687–695. [Google Scholar]
- Mostafanezhad, I.; Boric-Lubecke, O.; Lubecke, V.; Mandic, D.P. Application of Empirical Mode Decomposition in Removing Fidgeting Interference in Doppler Radar Life Signs Monitoring Devices. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 340–343. [Google Scholar]
- Oum, J.H.; Kim, D.W.; Hong, S. Two frequency Radar Sensor for Non-contact Vital Signal Monitor. In Proceedings of the IEEE MTT-S International Microwave Symposium Digest, Atlanta, GA, USA, 15–20 June 2008; pp. 1181–1184. [Google Scholar]
- Wiesner, A. A Multifrequency Interferometric CW Radar for Vital Signs Detection. In Proceedings of the IEEE Radar Conference, Pasadena, CA, USA, 4–8 May 2009; pp. 291–294. [Google Scholar]
- Gu, C.; Wang, G.; Li, Y.; Inoue, T.; Li, C. A Hybrid Radar-Camera Sensing System With Phase Compensation for Random Body Movement Cancellation in Doppler Vital Sign Detection. IEEE Trans. Microw. Theory Tech. 2013, 61, 4678–4688. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, D.; Zhang, Z. Doppler Radar Vital Signs Detection Method Based on Higher Order Cyclostationary. Sensors 2018, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Park, Y. Accurate Heartbeat Frequency Extraction Method using UWB Impulse Radar. IEIE Trans. Smart Process. Comput. 2017, 6, 246–252. [Google Scholar] [CrossRef]
- Kim, J.G.; Sim, S.H.; Cheon, S.; Hong, S. 24 GHz circularly polarized Doppler radar with a single antenna. In Proceedings of the 2005 European Microwave Conference, Paris, France, 4–6 October 2005; p. 4. [Google Scholar]
- Suzuki, S.; Matsui, T.; Asao, T.; Kotani, K. An investigation using high-precision CCD laser displacement sensor to measure body surface motion induced by heartbeat. J. Biomed. Sci. Eng. 2012, 5, 672–677. [Google Scholar] [CrossRef]
- Droitcour, A. Non-Contact Measurement of Heart and Respiration Rates with a Single-Chip Microwave Doppler Radar. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 2006. [Google Scholar]
- MacGill, M. Heart Rate: What Is a Normal Heart Rate? 2015. Available online: http://www.medicalnewstoday.com/articles/235710.php (accessed on 11 July 2018).
- McFadden, J.P.; Price, R.C.; Eastwood, H.D.; Briggs, R.S. Raised Respiratory Rate in ELdely Patients—A Valuable Physical Sign. Br. Med. J. 1982, 284, 626–627. [Google Scholar] [CrossRef]
- Respiratory Responses to Exercise. Available online: http://www.ptdirect.com/training-design/anatomy-and-physiology/acute-respiratory-responses (accessed on 11 July 2018).
- Zhang, T.; Valerio, G.; Sarrazin, J.; Istrate, D. Non-Contact Estimation at 60 GHz for Human Vital Signs Monitoring Using a Robust Optimization Algorithm. In Proceedings of the 2016 IEEE International Symposium on Antennas and Propagation, Fajardo, Puerto Rico, 26 June–1 July 2016. [Google Scholar]
- Hu, X. PSO Tutorial. Available online: http://www.swarmintelligence.org/tutorials.php (accessed on 11 July 2018).
- Kao, T.Y.J.; Yan, Y.; Shen, T.M.; Chen, A.Y.K.; Lin, J. Design and Analysis of a 60-GHz CMOS Doppler Micro-Radar System-in-Package for Vital-Sign and Vibration Detection. IEEE Trans. Microw. Theory Tech. 2013, 61, 1649–1659. [Google Scholar] [CrossRef]

| (bpm) | (bpm) | (mm) | (mm) | ||
|---|---|---|---|---|---|
| At rest | lb | 12 | 48 | 0 | 0.05 |
| ub | 30 | 90 | 6.0 | 1.0 | |
| After sport | lb | 30 | 90 | 0 | 0.05 |
| ub | 60 | 180 | 6.0 | 1.0 |
| Working Domain | Methods | Advantages | Disadvantages | |
|---|---|---|---|---|
| Frequency domain | Peak detection | Arctangent demodulation | Fast, No ambiguity | Sensitive to noise and to random body movements, Needs accurate DC offset compensation |
| Complex demodulation | Fast, Robust to noise | Intermodulation, ambiguity | ||
| Optimization | LSM, GA, and PSO | Handle ambiguity | At least 10 s time window, Not adaptable to nonstationary signal | |
| Time domain | Optimization | LSM | Converge quickly | Sensitive to initial estimates, Easy to fall into local minima |
| GA | Robustness, Stable | Computationally expensive if applied to large bounds | ||
| PSO | Converges more quickly than GA | |||
| PSO in parallel | Robust, Less optimization time | Multiple processors required |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Sarrazin, J.; Valerio, G.; Istrate, D. Estimation of Human Body Vital Signs Based on 60 GHz Doppler Radar Using a Bound-Constrained Optimization Algorithm. Sensors 2018, 18, 2254. https://doi.org/10.3390/s18072254
Zhang T, Sarrazin J, Valerio G, Istrate D. Estimation of Human Body Vital Signs Based on 60 GHz Doppler Radar Using a Bound-Constrained Optimization Algorithm. Sensors. 2018; 18(7):2254. https://doi.org/10.3390/s18072254
Chicago/Turabian StyleZhang, Ting, Julien Sarrazin, Guido Valerio, and Dan Istrate. 2018. "Estimation of Human Body Vital Signs Based on 60 GHz Doppler Radar Using a Bound-Constrained Optimization Algorithm" Sensors 18, no. 7: 2254. https://doi.org/10.3390/s18072254





