Earable POCER: Development of a Point-of-Care Ear Sensor for Respiratory Rate Measurement
Abstract
:1. Introduction
2. Materials and Methods
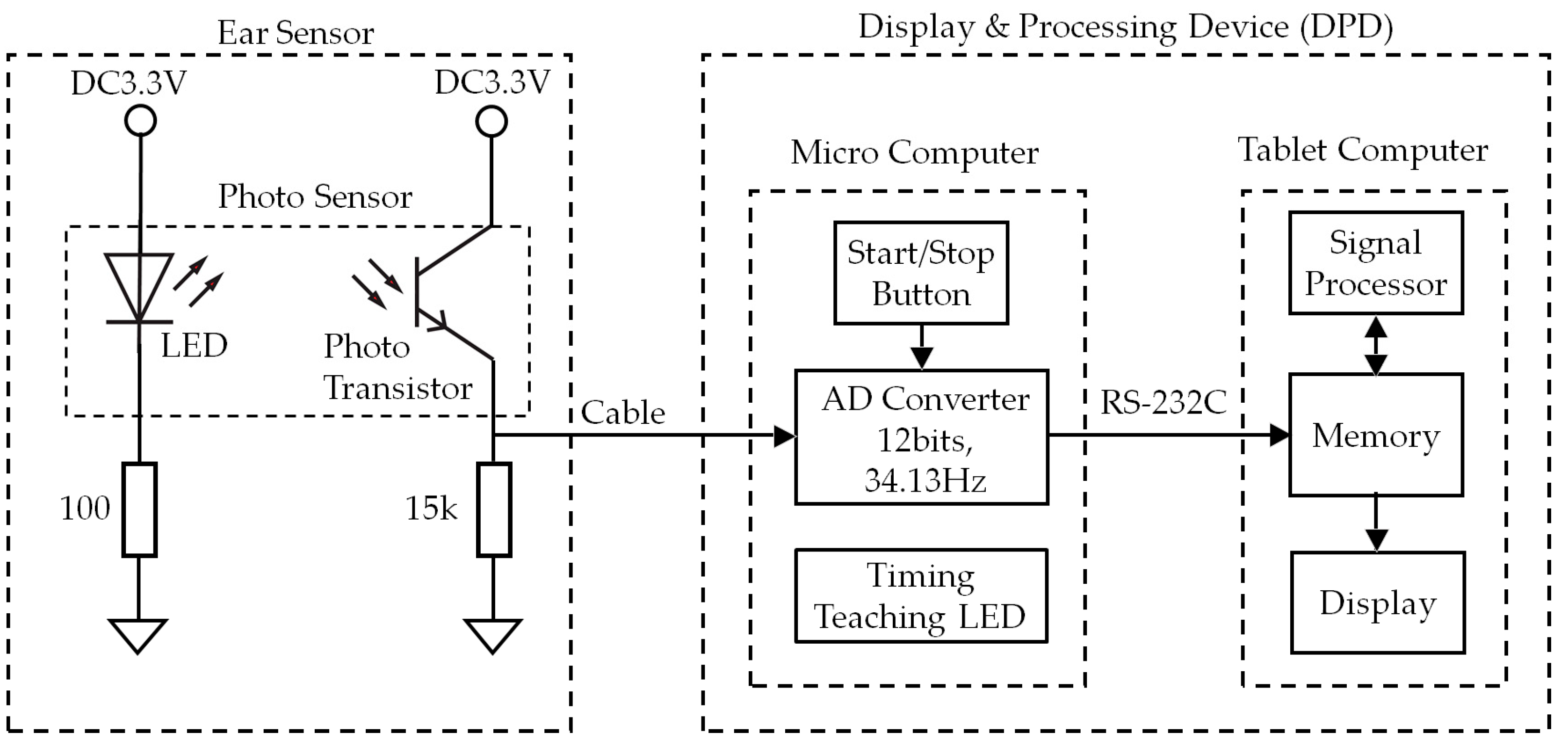
2.1. Hardware
2.2. Algorithm
3. Evaluation Experiments
3.1. Subjects
3.2. Earable Point-of-Care Ear Sensor for Respiratory Rate Measurement (POCER) Evaluation Experiments
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barrett, K.E.; Barman, S.M.; Boitano, S.; Brooks, H. Ganong’s Review of Medical Physiology, 24th ed.; McGraw-Hill Medical: New York, NY, USA, 2015; p. 619. ISBN 0071780033. [Google Scholar]
- Yuasa, Y.; Takahashi, K.; Suzuki, K. Wearable flexible device for respiratory phase measurement based on sound and chest movement. In Proceedings of the 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Banff, AB, Canada, 5–8 October 2017; pp. 2378–2383. [Google Scholar]
- Bestbier, A.; Fourie, P.R. Development of a vital signs monitoring wireless ear probe. In Proceedings of the 2018 3rd Biennial South African Biomedical Engineering Conference (SAIBMEC), Stellenbosch, South Africa, 4–6 April 2018; pp. 1–5. [Google Scholar]
- Tang, H.; Chen, H.; Li, T. Quantitative measurement of respiratory split in the second heart sound. In Proceedings of the 2017 Computing in Cardiology (CinC), Rennes, France, 24–27 September 2017; pp. 1–4. [Google Scholar]
- Yu, M.; Liou, J.; Kuo, S.; Lee, M.; Hung, Y. Noncontact respiratory measurement of volume change using depth camera. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August 2012; pp. 2371–2374. [Google Scholar]
- Alemaryeen, A.; Noghanian, S.; Fazel-Rezai, R. Antenna Effects on Respiratory Rate Measurement Using a UWB Radar System. IEEE J. Electromagn. RF Microw. Med. Biol. 2018, 2, 87–93. [Google Scholar] [CrossRef]
- Ansari, S.; Najarian, K.; Ward, K.; Tiba, M.H. Extraction of Respiratory Rate from Impedance Signal Measured on Arm: A Portable Respiratory Rate Measurement Device. In Proceedings of the 2009 IEEE International Conference on Bioinformatics and Biomedicine, Washington, DC, USA, 20 July 2009; pp. 197–202. [Google Scholar]
- Hasegawa, Y.; Kawaoka, H.; Mitsunari, Y.; Matsushima, M.; Kawabe, T.; Shikida, M. Catheter type thermal flow sensor with small footprint for measuring breathing function. Microsyst. Technol. 2018, 1–11. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kawaoka, H.; Yamada, T.; Matsushima, M.; Kawabe, T.; Shikida, M. Respiration and Heartbeat Signal Detection from Airflow at Airway in Rat by Catheter Flow Sensor with Temparature Compensation Function. J. Micromech. Microeng. 2017, 27. [Google Scholar] [CrossRef]
- Shikida, M.; Yoshikawa, K.; Matsuyama, T.; Yamazaki, Y.; Matsushima, M.; Kawabe, T. Catheter flow sensor with temperature compensation for tracheal intubation tube system. Sens. Actuators A 2014, 155–160. [Google Scholar] [CrossRef]
- Shaikh, J.G.; Daimiwal, N.M. Respiratory parameter measurement and analysis using differential pressure sensor. In Proceedings of the 2017 International Conference on Communication and Signal Processing (ICCSP), Tamilnadu, India, 4–6 April 2017; pp. 845–848. [Google Scholar]
- Maes, H.; Zivanovic, M.; Schoukens, J.; Vandersteen, G. Estimating Respiratory Impedance at Breathing Frequencies Using Regularized Least Squares on Forced Oscillation Technique Measurements. IEEE Trans. Instrum. Meas. 2017, 66, 479–491. [Google Scholar] [CrossRef]
- Hughes, M. Market trends and point of care testing. Point Care 2002, 1, 84–94. [Google Scholar] [CrossRef]
- Taniguchi, K.; Kondo, H.; Kurosawa, M.; Nishikawa, A. Earable TEMPO: A Novel, Hands-Free Input Device that Uses the Movement of the Tongue Measured with a Wearable Ear Sensor. Sensors 2018, 18, 733. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Kondo, H.; Tanaka, T.; Nishikawa, A. Earable RCC: Development of an Earphone-Type Reliable Chewing-Count Measurement Device. J. Healthc. Eng. 2018. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Chiaki, H.; Kurosawa, M.; Nishikawa, A. A Novel Earphone Type Sensor for Measuring Mealtime: Consideration of the Method to Distinguish between Running and Meals. Sensors 2017, 17, 252. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Kurosawa, M.; Nishikawa, A. earable: Wearable ear computer. In Proceedings of the 2017 International Conference for Top and Emerging Computer Scientists (IC-TECS 2017), Taipei, Taiwan, 21–24 December 2017. [Google Scholar]
- Kurosawa, M.; Taniguchi, K.; Nishikawa, A. A Basic Study of Occlusal Force Measured Using a Wearable Ear Sensor. In Proceedings of the 14th International Conference on Ubiquitous Healthcare (uHealthcare 2017), Seoul, Korea, 5–7 December 2017. [Google Scholar]
- Taniguchi, K.; Horise, Y.; Nishikawa, A.; Iwaki, S. A novel wearable input device using movement of ear-canals. In Proceedings of the TBIS2012, Textile Bioengineering and Informatics Symposium 2012, Ueda, Japan, 9–11 August 2012. [Google Scholar]
- Taniguchi, K.; Nishikawa, A.; Miyazaki, F.; Kokubo, A. Input Device, Wearable Computer, and Input Method. US Patent No. US8994647, 31 March 2015. [Google Scholar]
- Goverdovsky, V.; Rosenberg, W.; Nakamura, T.; Looney, D.; Sharp, D.J.; Papavassiliou, C.; Morrell, M.J.; Mandic, D.P. Hearables: Multimodal physiological in-ear sensing. Sci. Rep. 2017, 7, 6948. [Google Scholar] [CrossRef] [PubMed]
- Budidha, K.; Kyriacou, P.A. The human ear canal: Investigation of its suitability for monitoring photoplethysmographs and arterial oxygen saturation. Physiol. Meas. 2014, 35, 111. [Google Scholar] [CrossRef] [PubMed]
- Budidha, K.; Kyriacou, P.A. In vivo investigation of ear canal pulse oximetry during hypothermia. J. Clin. Monit. Comput. 2018, 32, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Dieffenderfer, J.P.; Goodell, H.; Bent, B.; Beppler, E.; Jayakumar, R.; Yokus, M.; Jur, J.S.; Bozkurt, A.; Peden, D. Wearable Wireless Sensors for Chronic Respiratory Disease Monitoring. In Proceedings of the 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Cambridge, MA, USA, 9–12 June 2015. [Google Scholar]
- Hung, P.D. Estimating respiration rate using an accelerometer sensor. In Proceedings of the 8th International Conference on Computational Systems-Biology and Bioinformatics, Nha Trang City, Vietnam, 7–8 December 2017; pp. 11–14. [Google Scholar]
- Preejith, S.P.; Dhinesh, R.; Joseph, J. Wearable ECG platform for continuous cardiac monitoring. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016. [Google Scholar]
- Preejith, S.P.; Ahamed, J.; Paresh, M. Accelerometer based system for continuous respiratory rate monitoring. In Proceedings of the 2017 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rochester, MN, USA, 7–10 May 2017. [Google Scholar]





| Subject | Item | FFT (mHz) | Total Number of Peaks (rpm) | ||
|---|---|---|---|---|---|
| First | Second | First | Second | ||
| A | EX200mHz12rpm | 200 | 200 | 12 | 12 |
| EX267mHz16rpm | 267 | 267 | 16 | 16 | |
| EX333mHz20rpm | 333 | 333 | 19 | 20 | |
| B | EX200mHz12rpm | 200 | 200 | 12 | 12 |
| EX267mHz16rpm | 17 | 267 | 10 | 15 | |
| EX333mHz20rpm | 333 | 333 | 19 | 17 | |
| C | EX200mHz12rpm | 200 | 200 | 12 | 13 |
| EX267mHz16rpm | 267 | 267 | 16 | 16 | |
| EX333mHz20rpm | 333 | 333 | 19 | 20 | |
| D | EX200mHz12rpm | 200 | 200 | 12 | 12 |
| EX267mHz16rpm | 267 | 267 | 16 | 16 | |
| EX333mHz20rpm | 333 | 333 | 19 | 19 | |
| E | EX200mHz12rpm | 200 | 200 | 12 | 12 |
| EX267mHz16rpm | 267 | 267 | 16 | 15 | |
| EX333mHz20rpm | 333 | 333 | 20 | 17 | |
| F | EX200mHz12rpm | 200 | 200 | 12 | 12 |
| EX267mHz16rpm | 267 | 267 | 16 | 16 | |
| EX333mHz20rpm | 333 | 333 | 20 | 20 | |
| G | EX200mHz12rpm | 200 | 200 | 12 | 12 |
| EX267mHz16rpm | 267 | 267 | 16 | 16 | |
| EX333mHz20rpm | 333 | 333 | 20 | 20 | |
| H | EX200mHz12rpm | 200 | 200 | 12 | 12 |
| EX267mHz16rpm | 267 | 267 | 14 | 16 | |
| EX333mHz20rpm | 333 | 17 | 20 | 18 | |
| Average | EX200mHz12rpm | 200.0 | 12.1 | ||
| EX267mHz16rpm | 251.4 | 15.4 | |||
| EX333mHz20rpm | 313.3 | 19.2 | |||
| Accuracy | EX200mHz12rpm | 1.000 | 1.000 | ||
| EX267mHz16rpm | 0.938 | 0.750 | |||
| EX333mHz20rpm | 0.938 | 0.500 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taniguchi, K.; Nishikawa, A. Earable POCER: Development of a Point-of-Care Ear Sensor for Respiratory Rate Measurement. Sensors 2018, 18, 3020. https://doi.org/10.3390/s18093020
Taniguchi K, Nishikawa A. Earable POCER: Development of a Point-of-Care Ear Sensor for Respiratory Rate Measurement. Sensors. 2018; 18(9):3020. https://doi.org/10.3390/s18093020
Chicago/Turabian StyleTaniguchi, Kazuhiro, and Atsushi Nishikawa. 2018. "Earable POCER: Development of a Point-of-Care Ear Sensor for Respiratory Rate Measurement" Sensors 18, no. 9: 3020. https://doi.org/10.3390/s18093020





