A Room Temperature VOCs Gas Sensor Based on a Layer by Layer Multi-Walled Carbon Nanotubes/Poly-ethylene Glycol Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
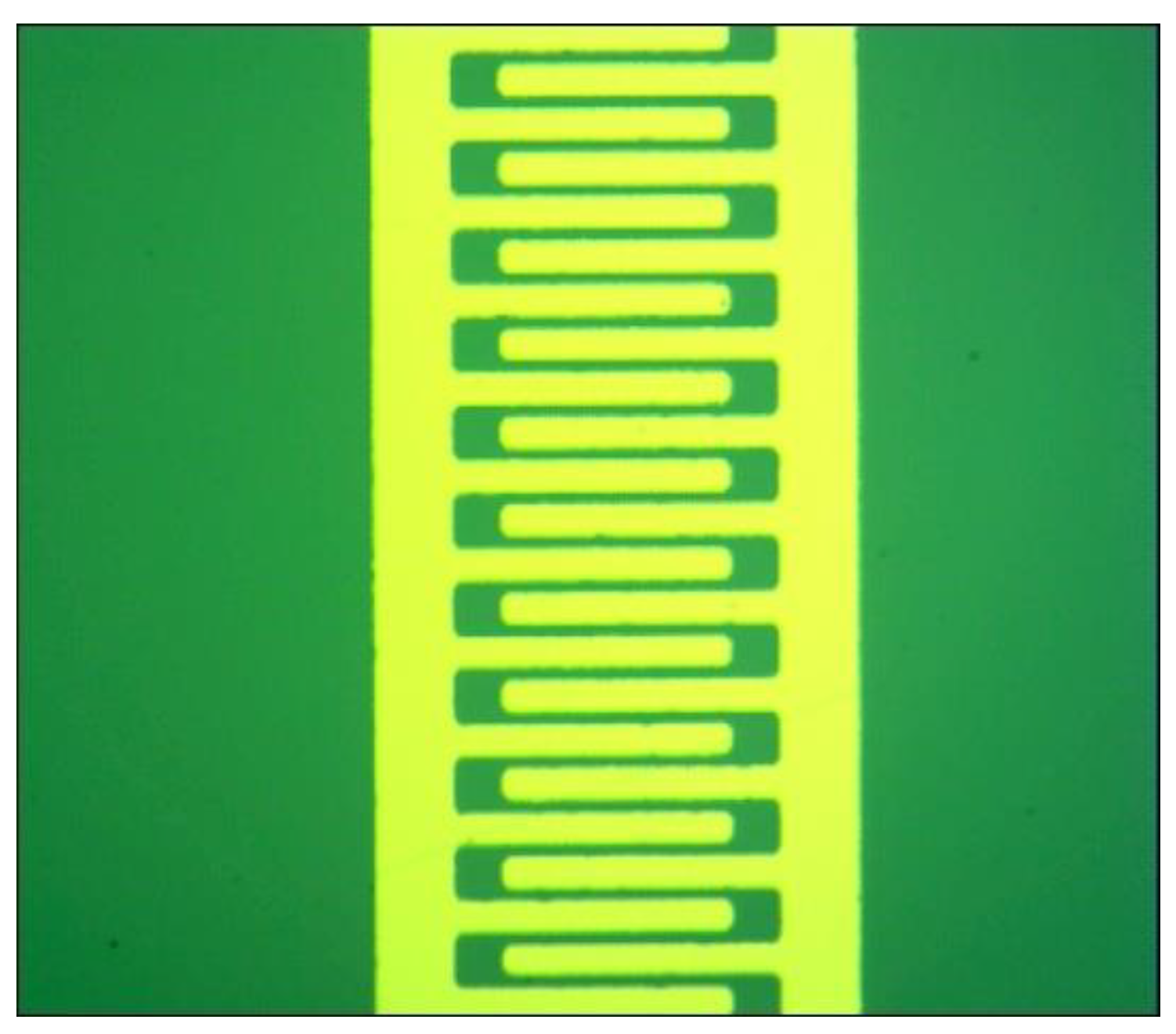
2.2. Gas Sensor Fabrication
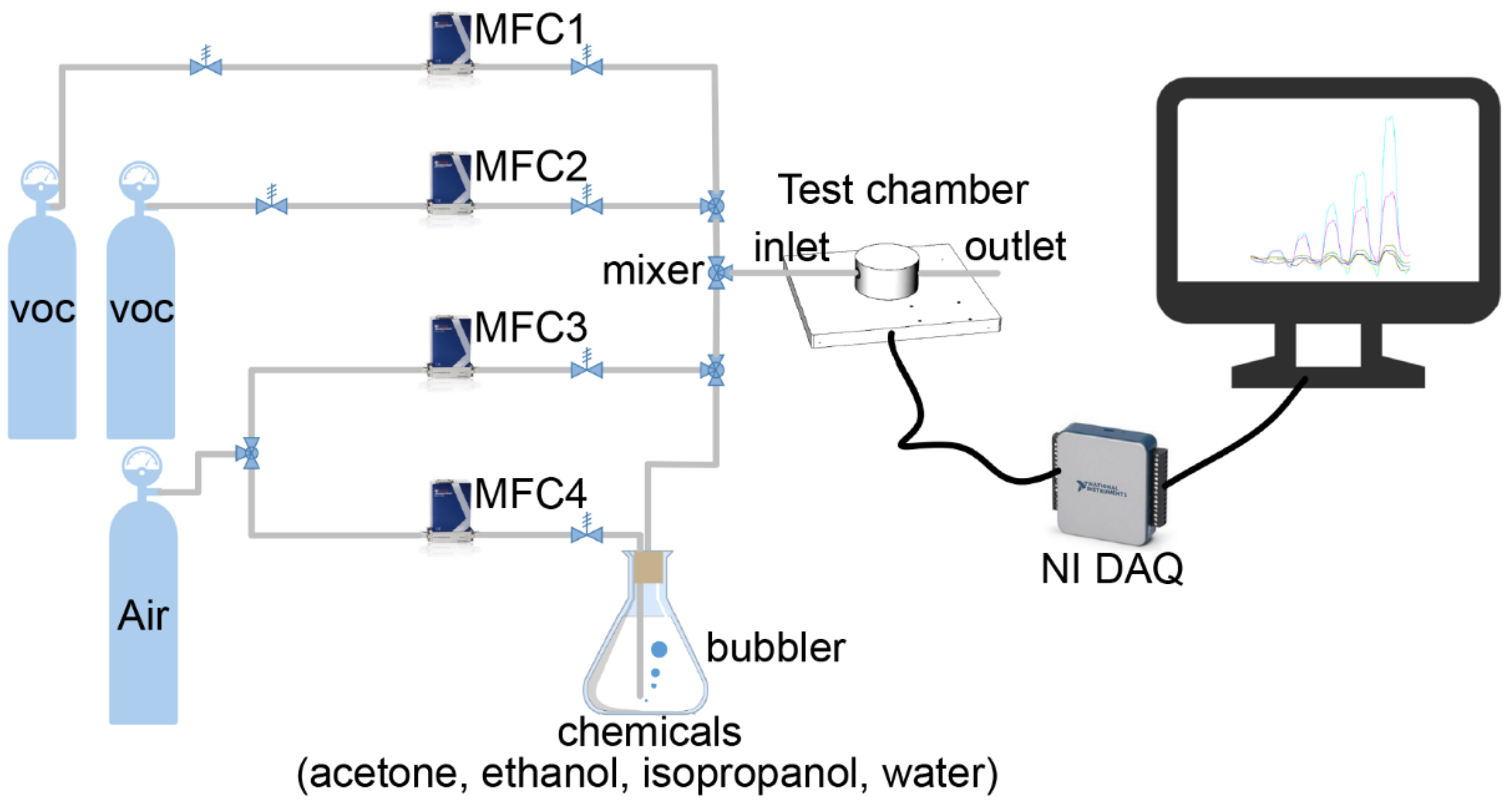
2.3. Gas Sensing Experiments
3. Results and Discussion
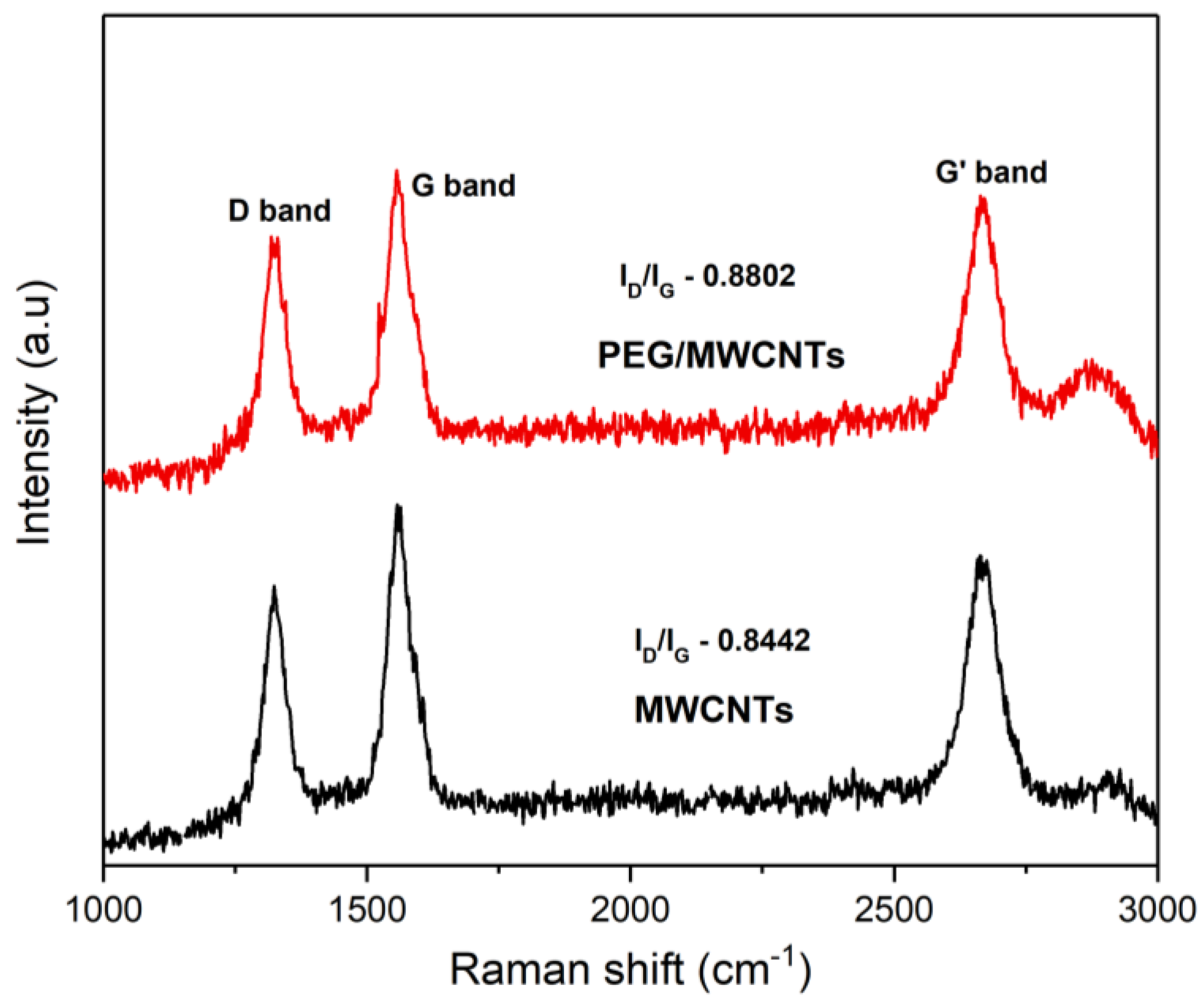
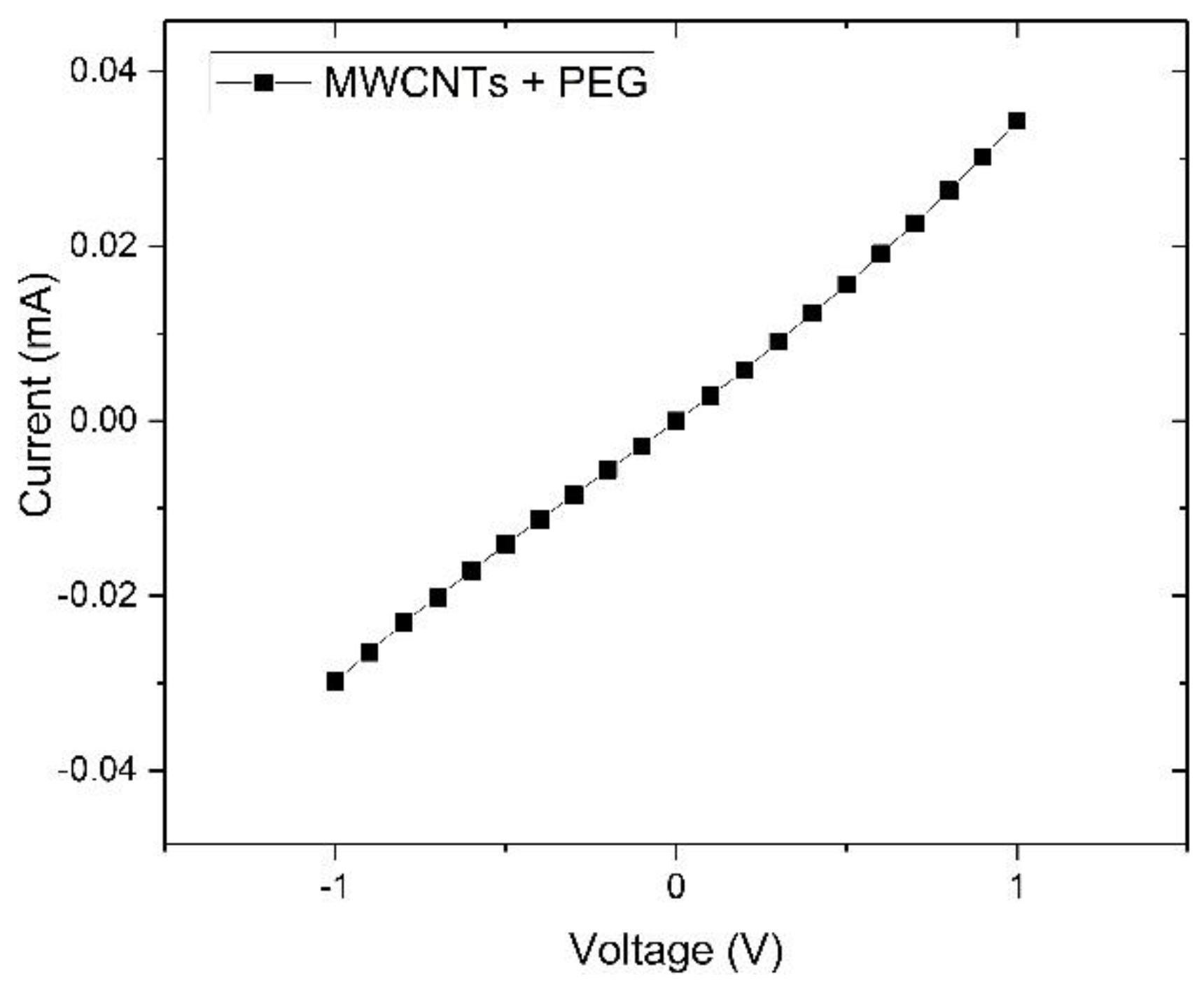
3.1. Device Fabrication
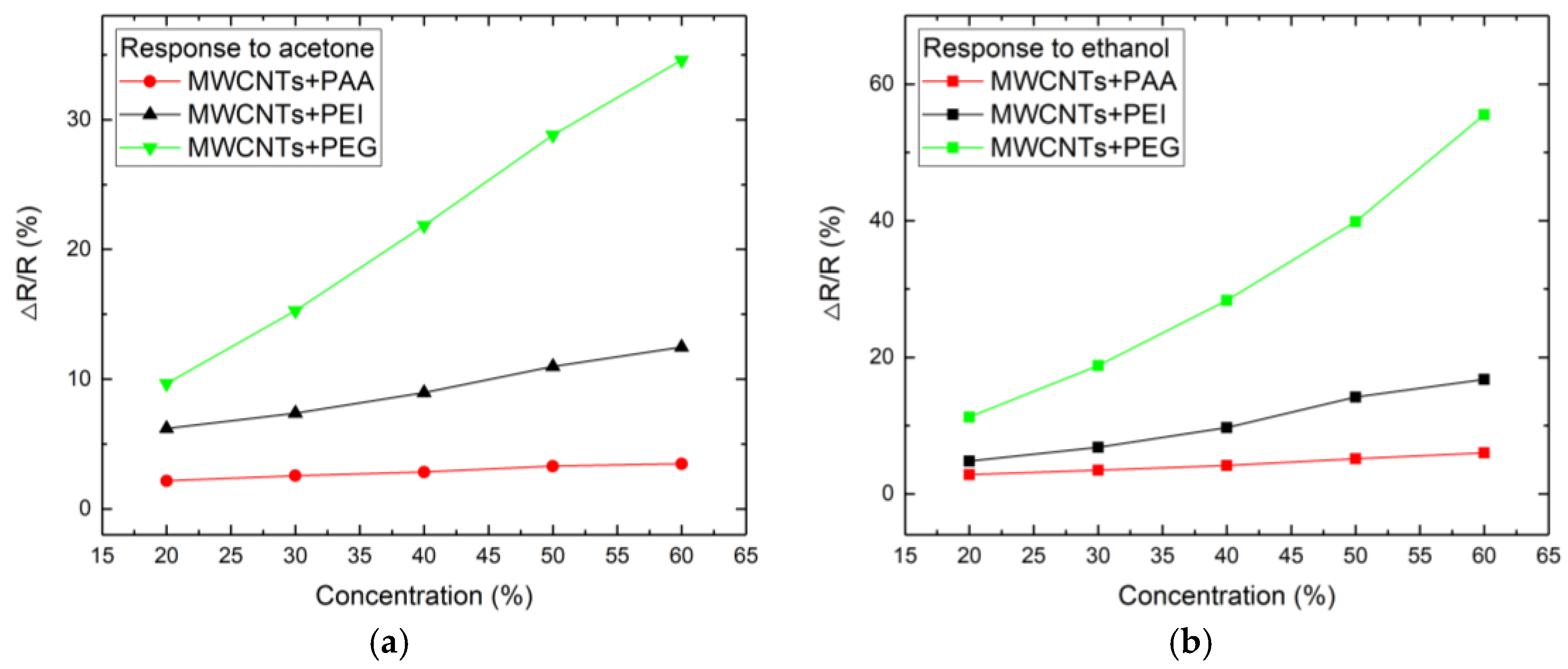
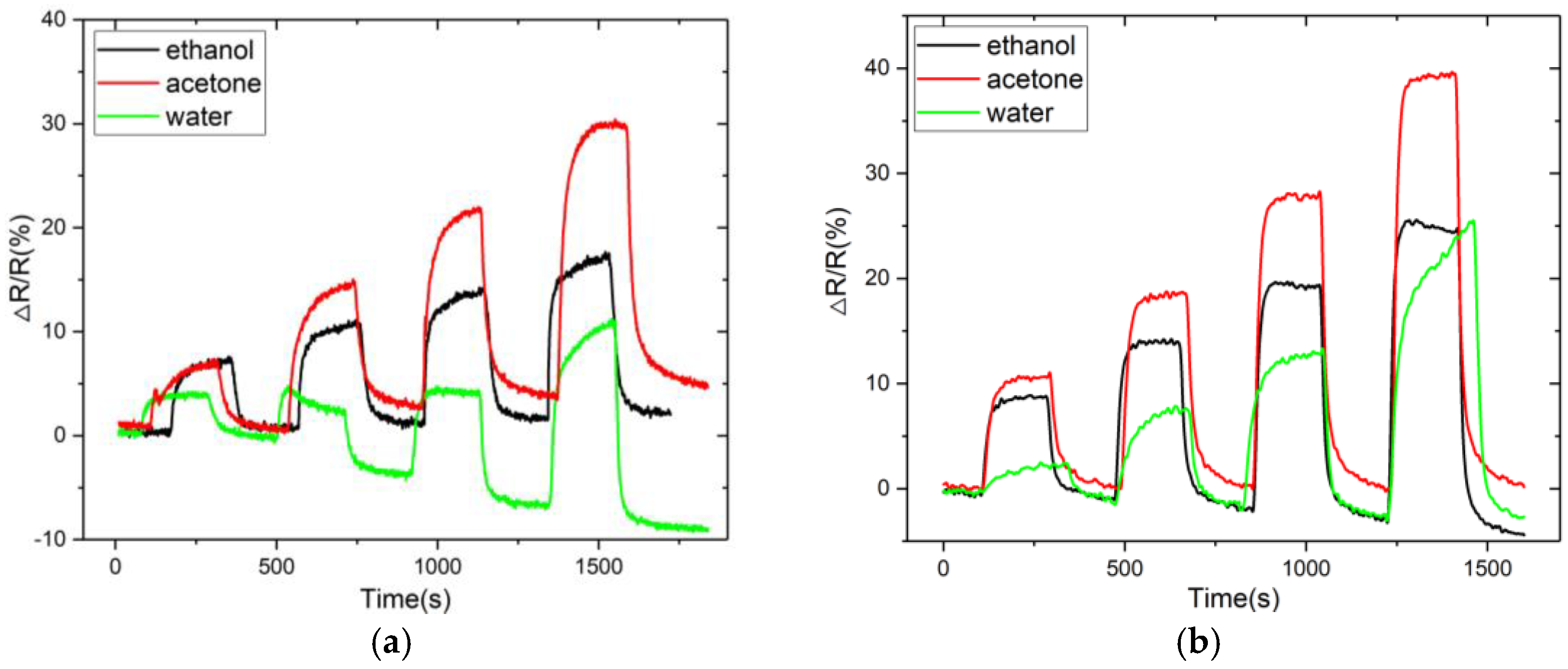
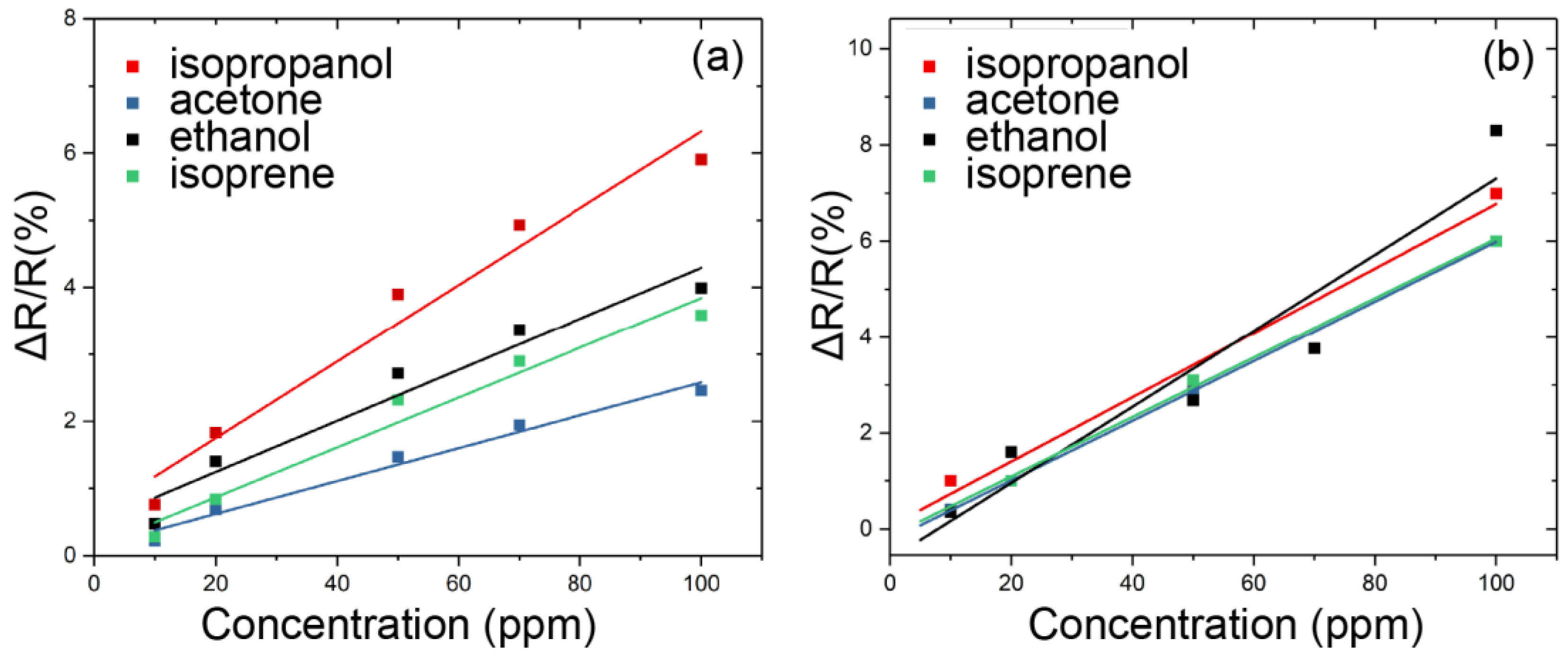
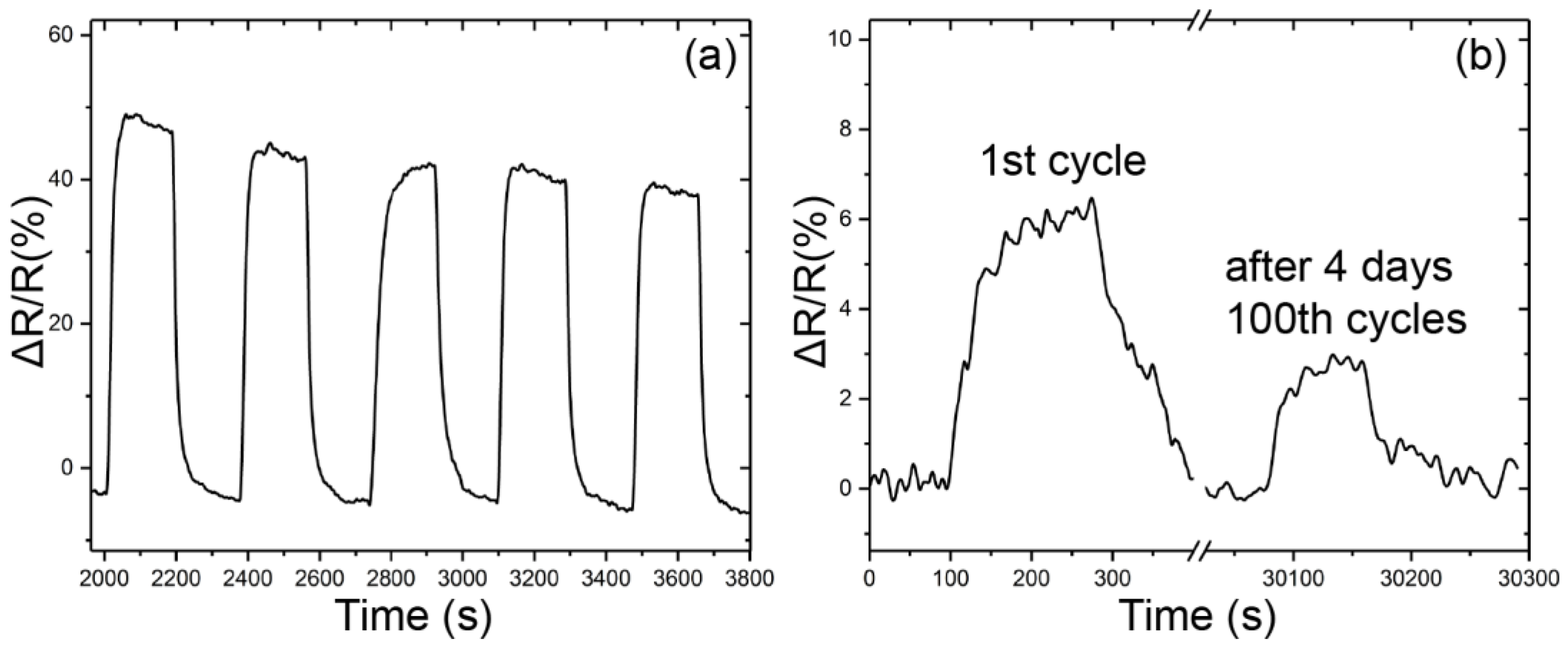
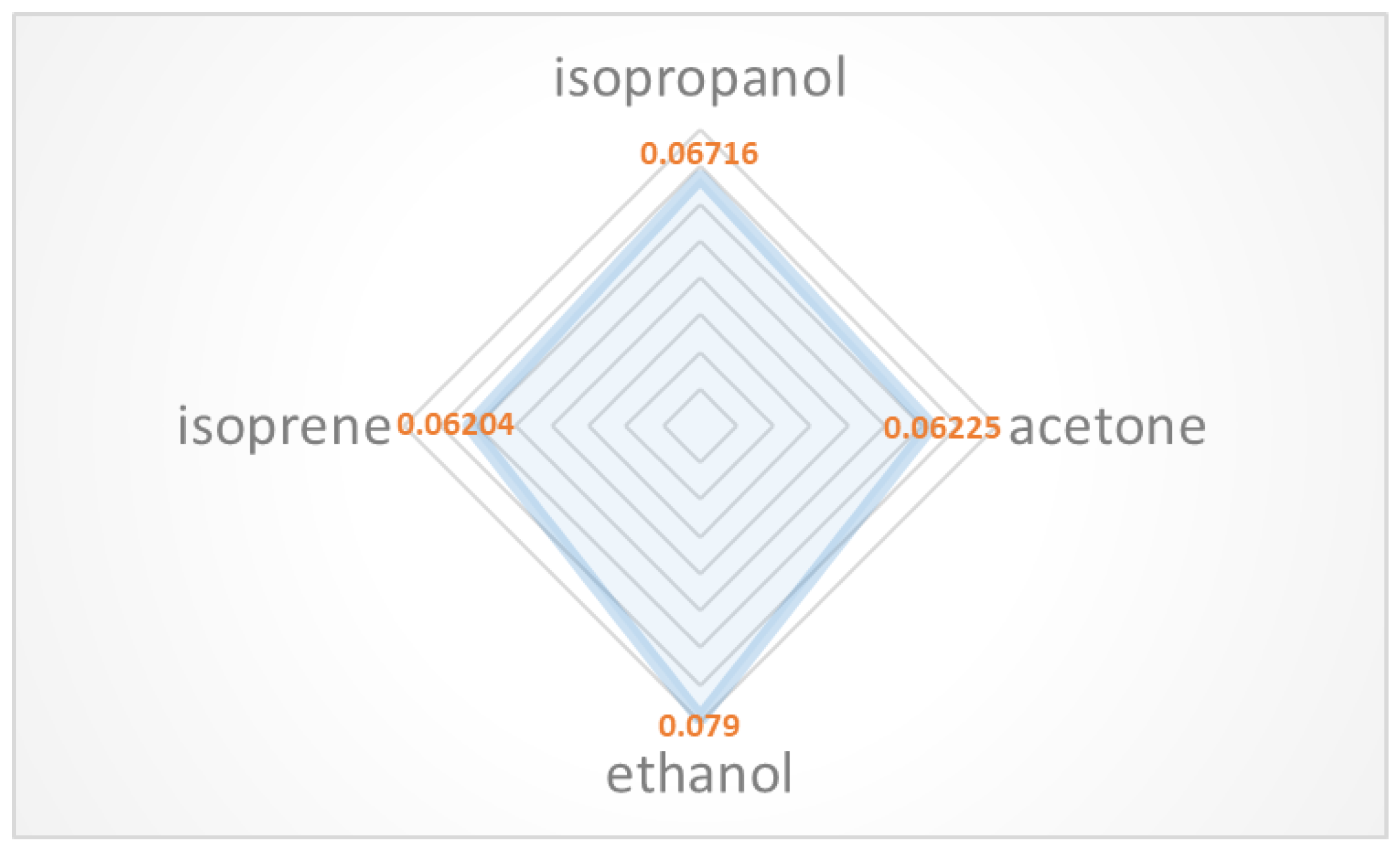
3.2. Sensor Response
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boots, A.W.; Bos, L.D.; van der Schee, M.P.; van Schooten, F.J.; Sterk, P.J. Exhaled molecular fingerprinting in diagnosis and monitoring: Validating volatile promises. Trends Mol. Med. 2015, 21, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.E.; Lee, D.S.; Ban, S.W.; Oh, J.; Jung, M.Y.; Kim, S.H.; Park, S.; Persaud, K.; Jheon, S. Analysis of volatile organic compounds in exhaled breath for lung cancer diagnosis using a sensor system. Sens. Actuators B Chem. 2018, 255, 800–807. [Google Scholar] [CrossRef]
- Gregis, G.; Sanchez, J.B.; Bezverkhyy, I.; Guy, W.; Berger, F.; Fierro, V.; Bellat, J.P.; Celzard, A. Detection and quantification of lung cancer biomarkers by a micro-analytical device using a single metal oxide-based gas sensor. Sens. Actuators B Chem. 2018, 255, 391–400. [Google Scholar] [CrossRef]
- Greiter, M.B.; Keck, L.; Siegmund, T.; Hoeschen, C.; Oeh, U.; Paretzke, H.G. Differences in exhaled gas profiles between patients with type 2 diabetes and healthy controls. Diabetes Technol. Ther. 2010, 12, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Tang, N.; Qu, H.; Liu, J.; Zhang, D.; Zhang, H.; Pang, W.; Duan, X.X. Detection of volatile organic compounds by self-assembled monolayer coated sensor array with concentration-independent fingerprints. Sci. Rep. 2016, 6, 23970. [Google Scholar] [CrossRef] [PubMed]
- Benammar, M.; Abdaoui, A.; Ahmad, S.H.; Touati, F.; Kadri, A. A Modular IoT Platform for Real-Time Indoor Air Quality Monitoring. Sensors 2018, 18, 581. [Google Scholar] [CrossRef] [PubMed]
- Szulczyński, B.; Gębicki, J. Currently commercially available chemical sensors employed for detection of volatile organic compounds in outdoor and indoor air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 082001. [Google Scholar] [CrossRef]
- Güntner, A.T.; Pineau, N.J.; Chie, D.; Krumeich, F.; Pratsinis, S.E. Selective sensing of isoprene by Ti-doped ZnO for breath diagnostics. J. Mater. Chem. B 2016, 4, 5358–5366. [Google Scholar] [CrossRef]
- Güntner, A.T.; Koren, V.; Chikkadi, K.; Righettoni, M.; Pratsinis, S.E. E-nose sensing of low-ppb formaldehyde in gas mixtures at high relative humidity for breath screening of lung cancer? Acs Sens. 2016, 1, 528–535. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si: WO3 sensors for highly selective detection of acetone for easy diagnosis of diabetes by breath analysis. Anal. Chem. 2010, 82, 3581–3587. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Santhanam, S.; Schultz, L.; Jeffries-El, M.; Iovu, M.C.; Sauvé, G.; Cooper, J.; Zhang, R.; Revelli, J.C.; Kusne, A.G.; et al. Inkjet printed chemical sensor array based on polythiophene conductive polymers. Sens. Actuators B Chem. 2007, 123, 651–660. [Google Scholar] [CrossRef]
- Fu, D.; Chung, J.; Liu, Q.; Raziq, R.; Kee, J.S.; Park, K.; Valiyaveettil, S.; Lee, P. Polymer coated silicon microring device for the detection of sub-ppm volatile organic compounds. Sens. Actuators B Chem. 2018, 257, 136–142. [Google Scholar] [CrossRef]
- Okazaki, M.; Maruyama, K.; Tsuchida, M.; Tsubokawa, N. A novel gas sensor from poly (ethylene glycol)-grafted carbon black. Responsibility of electric resistance of poly (ethylene glycol)-grafted carbon black against humidity and solvent vapor. Polym. J. 1999, 31, 672. [Google Scholar] [CrossRef]
- Salehi-Khojin, A.; Khalili-Araghi, F.; Kuroda, M.A.; Lin, K.Y.; Leburton, J.P.; Masel, R.I. On the sensing mechanism in carbon nanotube chemiresistors. ACS Nano 2010, 5, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Siegal, M.P.; Yelton, W.G.; Overmyer, D.L.; Provencio, P.P. Nanoporous carbon films for gas microsensors. Langmuir 2004, 20, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Lorwongtragool, P.; Sowade, E.; Dinh, T.N.; Kanoun, O.; Kerdcharoen, T.; Baumann, R.R. Inkjet printing of chemiresistive sensors based on polymer and carbon nanotube networks[C]//Systems. In Proceedings of the 2012 9th International Multi-Conference on Signals and Devices (SSD), Chemnitz, Germany, 20–23 March 2012; pp. 1–4. [Google Scholar]
- Rajavel, K.; Lalitha, M.; Radhakrishnan, J.K.; Senthilkumar, L.; Rajendra Kumar, R.T. Multiwalled carbon nanotube oxygen sensor: Enhanced oxygen sensitivity at room temperature and mechanism of sensing. ACS Appl. Mater. Interfaces 2015, 7, 23857–23865. [Google Scholar] [CrossRef] [PubMed]
- Rajavel, K.; Dinesh, M.; Saranya, R.; Kumar, R.R. Enhanced vacuum sensing performance of multiwalled carbon nanotubes: Role of defects and carboxyl functionalization. RSC Adv. 2015, 5, 20479–20485. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, H.; Yu, A.; Perea, D.; Haddon, R.C. Synthesis and characterization of water soluble single-walled carbon nanotube graft copolymers. J. Am. Chem. Soc. 2005, 127, 8197–8203. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cadena, G.; Riu, J.; Rius, F.X. Gas sensors based on nanostructured materials. Analyst 2007, 132, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Lipatov, A.; Varezhnikov, A.; Wilson, P.; Sysoev, V.; Kolmakov, A.; Sinitskii, A. Highly selective gas sensor arrays based on thermally reduced graphene oxide. Nanoscale 2013, 5, 5426–5434. [Google Scholar] [CrossRef] [PubMed]
- Tasaltin, C.; Basarir, F. Preparation of flexible VOC sensor based on carbon nanotubes and gold nanoparticles. Sens. Actuators B Chem. 2014, 194, 173–179. [Google Scholar] [CrossRef]
- Maity, D.; Rajavel, K.; Kumar, R.T.R. Polyvinyl alcohol wrapped multiwall carbon nanotube (MWCNTs) network on fabrics for wearable room temperature ethanol sensor. Sens. Actuators B Chem. 2018, 261, 297–306. [Google Scholar] [CrossRef]
- Zhang, H.; Cen, Y.; Du, Y.; Ruan, S. Enhanced acetone sensing characteristics of ZnO/graphene composites. Sensors 2016, 16, 1876. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.K.; Wang, L.C.; Kuo, C.T. Development of a high performance integrated sensor chip with a multi-walled carbon nanotube assisted sensing array. Thin Solid Films 2013, 529, 205–208. [Google Scholar] [CrossRef]
- Nag, S.; Sachan, A.; Castro, M.; Choudhary, V.; Feller, J.F. Spray layer-by-layer assembly of POSS functionalized CNT quantum chemo-resistive sensors with tuneable selectivity and ppm resolution to VOC biomarkers. Sens. Actuators B Chem. 2016, 222, 362–373. [Google Scholar] [CrossRef]


| Spectrum | Composition |
|---|---|
| C | 32.14 |
| O | 67.86 |
| Total | 100 |
| Sensing Materials | Operating Temp. (°C) | Concentration (ppm) | Sensitivity (ppm−1) | Resp/Recov. Time (s) | LDL (ppm) | Ref. |
|---|---|---|---|---|---|---|
| PSE/MWCNTs | RT | 50–500 | 0.00007 | -- | 50 | [17] |
| Au/CNTs | RT | 50–800 | - | -- | 50 | [23] |
| PVA/MWCNTs | RT | 100–500 | 0.00005 | 26/30 | 100 | [24] |
| ZnO/Graphene | 280 | 10–10,000 | 0.123 | 2/1 | <10 | [25] |
| PEO/MWCNTs | RT | 43–356 | 0.00022 | 275/300 | 43 | [26] |
| IO-POSS/CNTs | RT | 1.5–7 | 0.0025 | -- | 1.5 | [27] |
| PEG/MWCNTs | RT | 10–1000 | 0.0006 | 110/152 | <10 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yang, T.; Dong, Y.; Wang, X. A Room Temperature VOCs Gas Sensor Based on a Layer by Layer Multi-Walled Carbon Nanotubes/Poly-ethylene Glycol Composite. Sensors 2018, 18, 3113. https://doi.org/10.3390/s18093113
Liu Z, Yang T, Dong Y, Wang X. A Room Temperature VOCs Gas Sensor Based on a Layer by Layer Multi-Walled Carbon Nanotubes/Poly-ethylene Glycol Composite. Sensors. 2018; 18(9):3113. https://doi.org/10.3390/s18093113
Chicago/Turabian StyleLiu, Zitao, Tuoyu Yang, Ying Dong, and Xiaohao Wang. 2018. "A Room Temperature VOCs Gas Sensor Based on a Layer by Layer Multi-Walled Carbon Nanotubes/Poly-ethylene Glycol Composite" Sensors 18, no. 9: 3113. https://doi.org/10.3390/s18093113




