Aptamer Conformation Switching-Induced Two-Stage Amplification for Fluorescent Detection of Proteins
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Equipment
2.3. Procedure for Fluorescence Detection of the Platelet-Derived Growth Factor
3. Results and Discussion
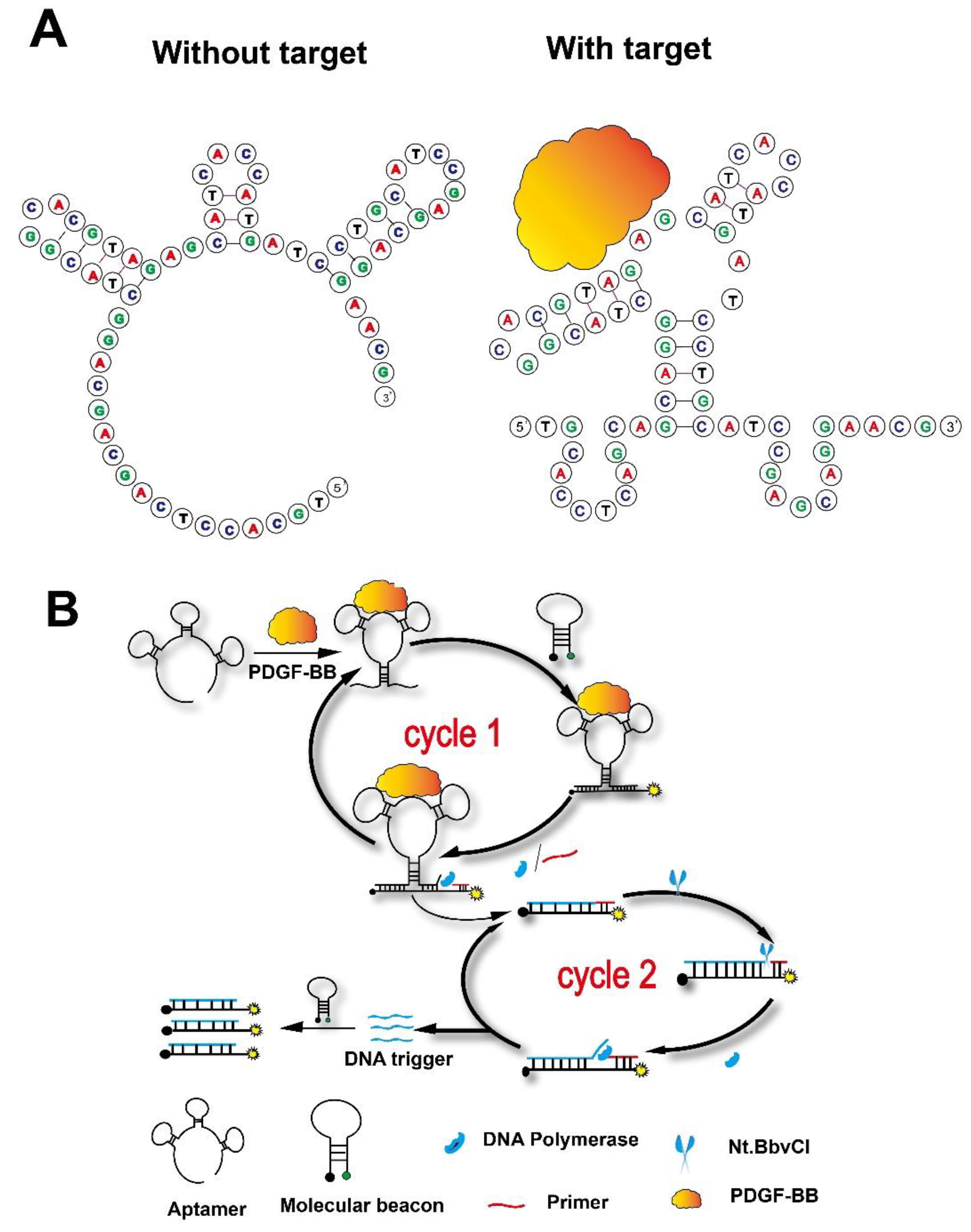
3.1. The Design of an Amplified Aptasensing Platform for Fluorescence Detection of the Platelet-Derived Growth Factor
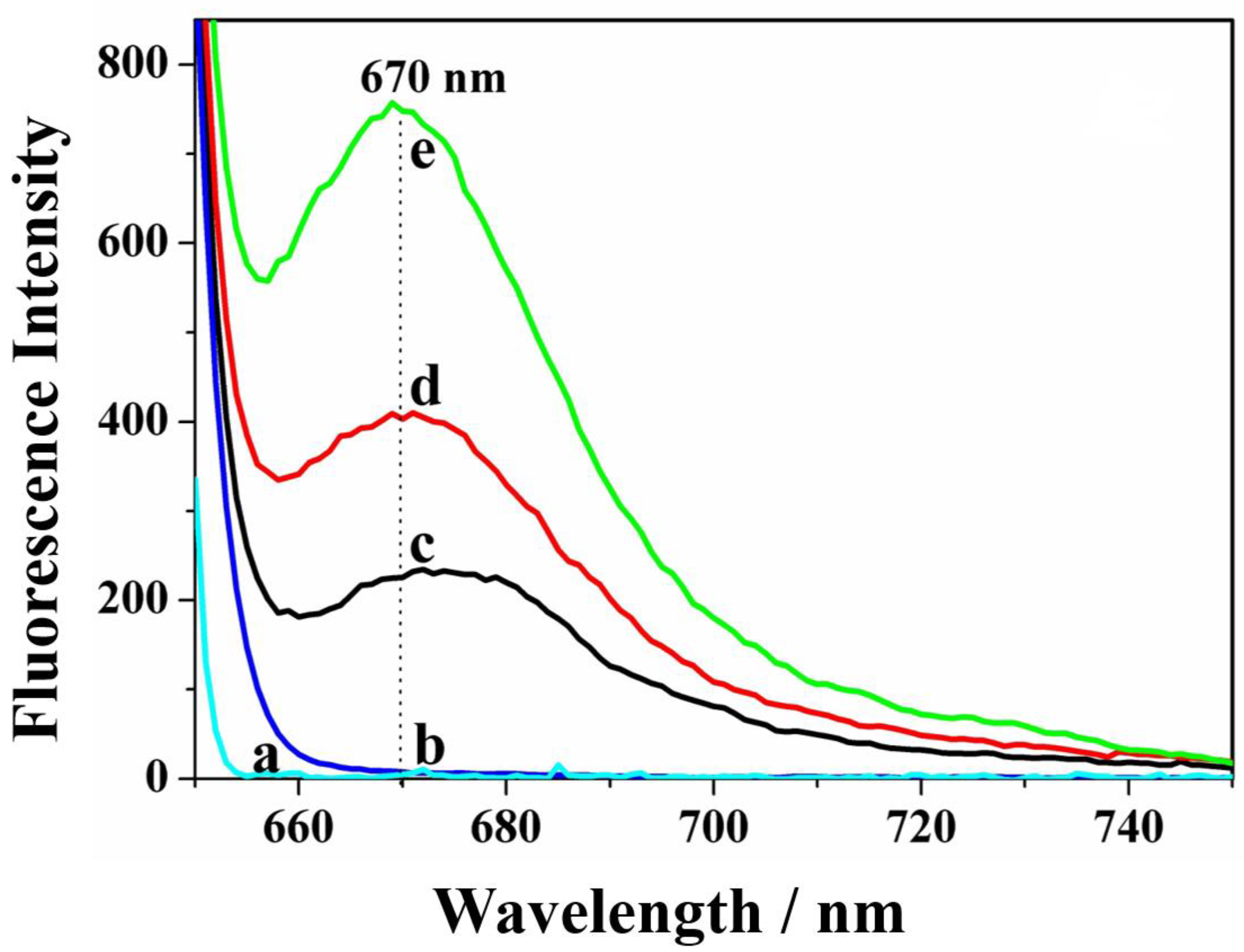
3.2. The Feasibility of This Strategy
3.3. Fluorescence Measurement of the Platelet-Derived Growth Factor
3.4. Detection Specificity
3.5. Application of the Proposed Biosensor in Human Serum Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, L.; Qu, X.G. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Rocha-Santos, T.A.; Duarte, A.C. Review of analytical figures of merit of sensors and biosensors in clinical applications. TrAC-Trends Anal. Chem. 2010, 29, 1172–1183. [Google Scholar] [CrossRef]
- Wang, X.M.; Cui, M.R.; Zhou, H.; Zhang, S.S. DNA-hybrid-gated functional mesoporous silica for sensitive DNA methyltransferase SERS detection. Chem. Commun. 2015, 51, 13983–13985. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.P.; Wu, Z.S.; Shen, G.L.; Yu, R.Q. Highly sensitive and selective bifunctional oligonucleotide probe for homogeneous parallel fluorescence detection of protein and nucleotide sequence. Anal. Chem. 2011, 83, 3050–3057. [Google Scholar] [CrossRef] [PubMed]
- Li, B.X.; Liu, J.; Zhou, H. Amplified fluorescence detection of serum prostate specific antigen based on metal-dependent DNAzyme assistant nanomachine. Anal. Chim. Acta 2018, 1008, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Wu, J.; Zhang, Y.; Yan, F.; Ju, H. Proximity Hybridization Regulated DNA Biogate for Sensitive Electrochemical Immunoassay. Anal. Chem. 2014, 86, 7494–7499. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Han, T.Q.; Wei, Q.; Zhang, S.S. Efficient Enhancement of Electrochemiluminescence from Cadmium Sulfide Quantum Dots by Glucose Oxidase Mimicking Gold Nanoparticales for Highly Sensitive Assay of Methyltransferase Activity. Anal. Chem. 2016, 88, 2976–2983. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, M.; Wang, C.Z.; Li, W.; Qiang, W.B.; Xu, D. Silver nanoparticle-enhanced fluorescence resonance energy transfer sensor for human platelet-derived growth factor-BB detection. Anal. Chem. 2013, 85, 4492–4499. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wang, X.; Wu, D.; Li, C.; Yin, Y.; Li, G. Proximity ligation-induced assembly of DNAzymes for simple and cost-effective colourimetric detection of proteins with high sensitivity. Chem. Commun. 2016, 52, 5633–5636. [Google Scholar] [CrossRef]
- Mohammadzadeh-Asl, S.; Keshtkar, A.; Dolatabadi, J.E.N.; Guardia, M. Nanomaterials and phase sensitive based signal enhancment in surface plasmon resonance. Biosens. Bioelectron. 2018, 110, 118–131. [Google Scholar] [CrossRef]
- Chiu, N.F.; Lin, T.L.; Kuo, C.T. Highly sensitive carboxyl-graphene oxide-based surface plasmon resonance immunosensor for the detection of lung cancer for cytokeratin 19 biomarker in human plasma. Sens. Actuators B Chem 2018, 265, 264–272. [Google Scholar] [CrossRef]
- Liang, H.; Chen, S.; Li, P.; Wang, L.; Li, J.; Yang, H.H.; Tan, W.H. Approach for Imaging Protein Dimerization by Aptamer Recognition and Proximity-Induced DNA Assembly. J. Am. Chem. Soc. 2018, 140, 4186–4190. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.M.; Liu, H.; Kuai, H.L.; Peng, R.Z.; Mo, L.T.; Zhang, X.B. Aptamer-integrated DNA nanostructures for biosensing, bioimaging and cancer therapy. Chem. Soc. Rev. 2016, 45, 2583–2602. [Google Scholar] [CrossRef] [PubMed]
- Famulok, M.; Harting, J.S.; Mayer, G. Functional Aptamers and Aptazymes in Biotechnology, Diagnostics and Therapy. Chem. Rev. 2007, 107, 3715–3743. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, X.; Yuan, R.; Chai, Y. “Off” to “On” Surface-Enhanced Raman Spectroscopy Platform with Padlock Probe-Based Exponential Rolling Circle Amplification for Ultrasensitive Detection of MicroRNA 155. Anal. Chem. 2017, 89, 2866–2872. [Google Scholar] [CrossRef]
- Qiu, Z.; Shu, J.; Tang, D.P. Bioresponsive Release System for Visual Fluorescence Detection of Carcinoembryonic Antigen from Mesoporous Silica Nanocontainers Mediated Optical Color on Quantum Dot-Enzyme-Impregnated Paper. Anal. Chem. 2017, 89, 89,5152–5160. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, J.; Xu, J.J.; Zhang, S.S.; Chen, H.Y. Optical nano-biosensing interface via nucleic acid amplification strategy: Construction and application. Chem. Soc. Rev. 2018, 47, 1996–2019. [Google Scholar] [CrossRef]
- Willner, I.; Zayats, M. Electronic aptamer-based sensors. Angew. Chem. Int. Ed. 2007, 46, 6408–6418. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, R.H.; Shi, M.L.; Wu, C.; Fang, X.H.; Li, Y.; Li, J.; Tan, W.H. Rationally designed molecular beacons for bioanalytical and biomedical applications. Chem. Soc. Rev. 2015, 44, 3036–3055. [Google Scholar] [CrossRef]
- Xie, N.; Huang, J.; Yang, X.H.; Yang, Y.J.; Quan, K.; Ou, M.; Fang, H.M.; Wang, K.M. Competition-Mediated FRET-Switching DNA Tetrahedron Molecular Beacon for Intracellular Molecular Detection. ACS Sens. 2016, 1, 1445–1452. [Google Scholar] [CrossRef]
- Li, J.S.; Jia, Y.H.; Zheng, J.; Zhong, W.W.; Shen, G.L.; Yang, R.H.; Tan, W.H. Aptamer degradation inhibition combined with DNAzyme cascade-based signal amplification for colorimetric detection of proteins. Chem. Commun. 2013, 49, 6137–6139. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.idtdna.com/analyzer/Applications/OligoAnalyzer (accessed on 1 December 2016).
- Zhang, Y.L.; Huang, Y.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. Electrochemical Aptasensor Based on Proximity-Dependent Surface Hybridization Assay for Single-Step, Reusable, Sensitive Protein Detection. J. Am. Chem. Soc. 2007, 129, 15448–15449. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, Y.; Dong, S.; Qiang, W.; Sun, L.; Xu, D. Fast functionalization of silver decahedral nanoparticles with aptamers for colorimetric detection of human platelet-derived growth factor-BB. Anal. Chim. Acta 2014, 829, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Xu, S.; Sun, Y. A highly sensitive LED-induced chemiluminescence platform for aptasensing of platelet-derived growth factor. Analyst 2014, 139, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhou, X.; Xing, D. A new kind of aptamer-based immunomagnetic electrochem iluminescence assay for quantitative detection of protein. Biosens. Bioelectron. 2010, 26, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhu, K.; Cao, Z.; Lau, C.; Lu, J. Hybridization chain reaction-based aptameric system for the highly selective and sensitive detection of protein. Analyst 2012, 137, 1396–1401. [Google Scholar] [CrossRef] [PubMed]



| Sample | PDGF-BB Added (ng/mL) | PDGF-BB Found (ng/mL) | Recovery (%) |
|---|---|---|---|
| 5% BSA | 1 | 0.97 | 97 |
| 10 | 10.06 | 100.6 | |
| 20 | 20.09 | 100.5 | |
| 5% Human Serum | 1 | 1.08 | 108 |
| 10 | 11.04 | 110.4 | |
| 20 | 21.33 | 106.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Zhai, F.; Zhou, H.; Wang, Z. Aptamer Conformation Switching-Induced Two-Stage Amplification for Fluorescent Detection of Proteins. Sensors 2019, 19, 77. https://doi.org/10.3390/s19010077
Yu Q, Zhai F, Zhou H, Wang Z. Aptamer Conformation Switching-Induced Two-Stage Amplification for Fluorescent Detection of Proteins. Sensors. 2019; 19(1):77. https://doi.org/10.3390/s19010077
Chicago/Turabian StyleYu, Qiao, Fenfen Zhai, Hong Zhou, and Zonghua Wang. 2019. "Aptamer Conformation Switching-Induced Two-Stage Amplification for Fluorescent Detection of Proteins" Sensors 19, no. 1: 77. https://doi.org/10.3390/s19010077
APA StyleYu, Q., Zhai, F., Zhou, H., & Wang, Z. (2019). Aptamer Conformation Switching-Induced Two-Stage Amplification for Fluorescent Detection of Proteins. Sensors, 19(1), 77. https://doi.org/10.3390/s19010077





