The Influence of Nb on the Synthesis of WO3 Nanowires and the Effects on Hydrogen Sensing Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Nanostructures
2.2. Morphological and Structural Characterization
2.3. Chemical Sensing Measurements
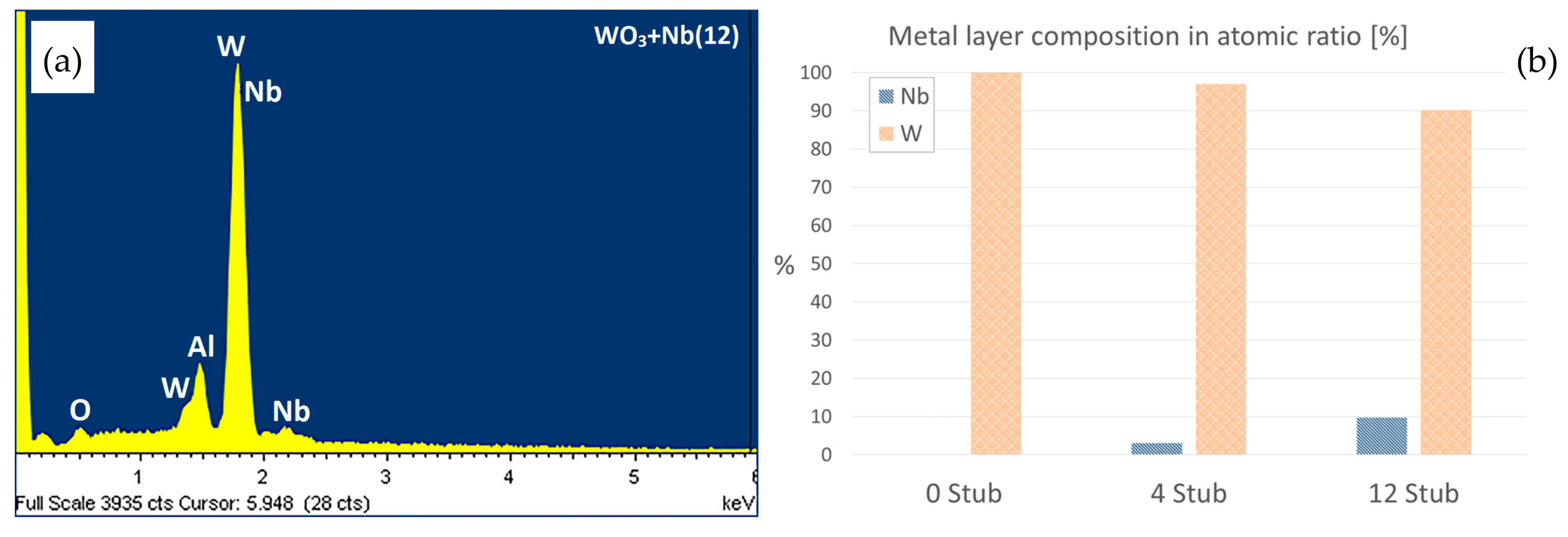
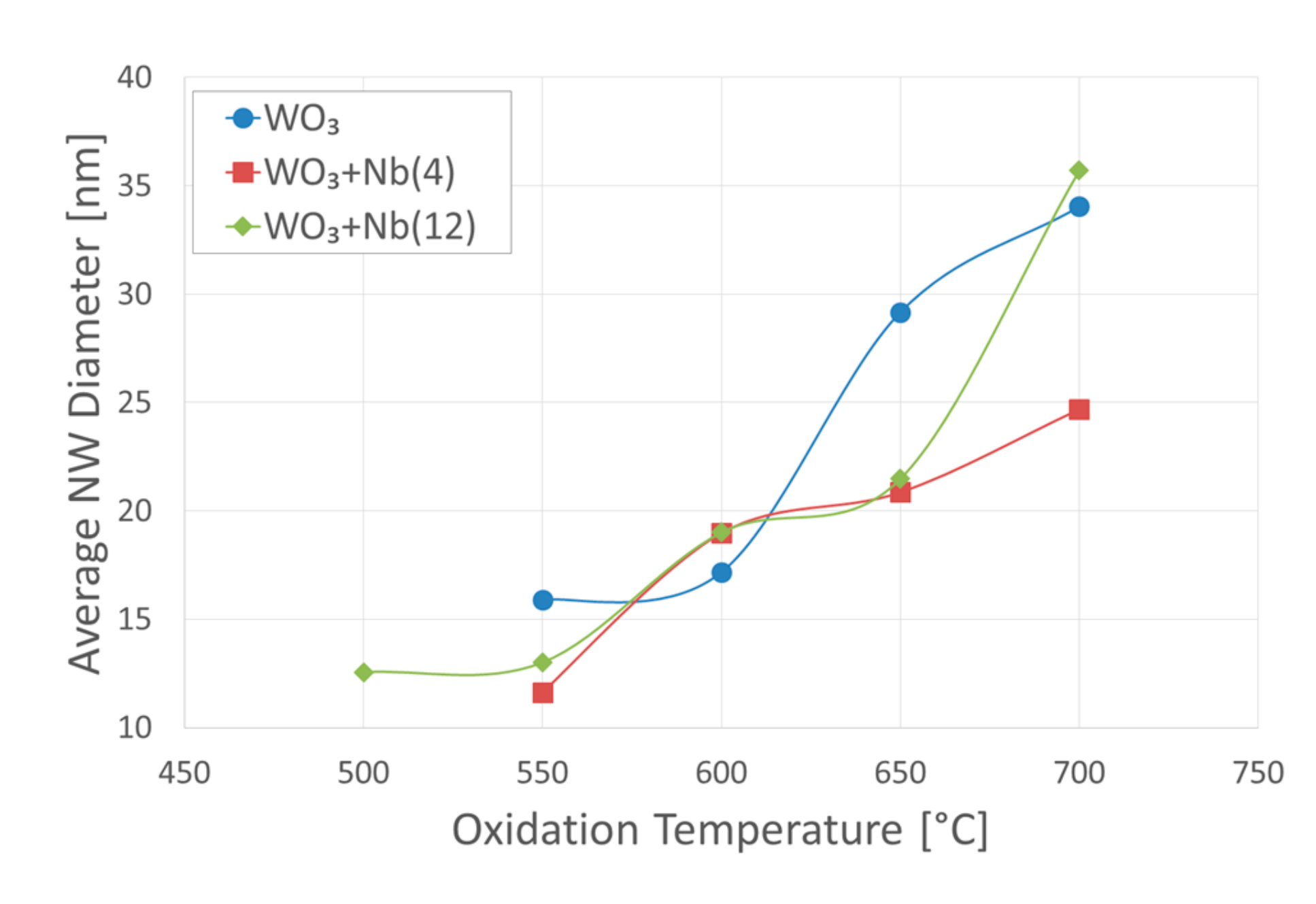
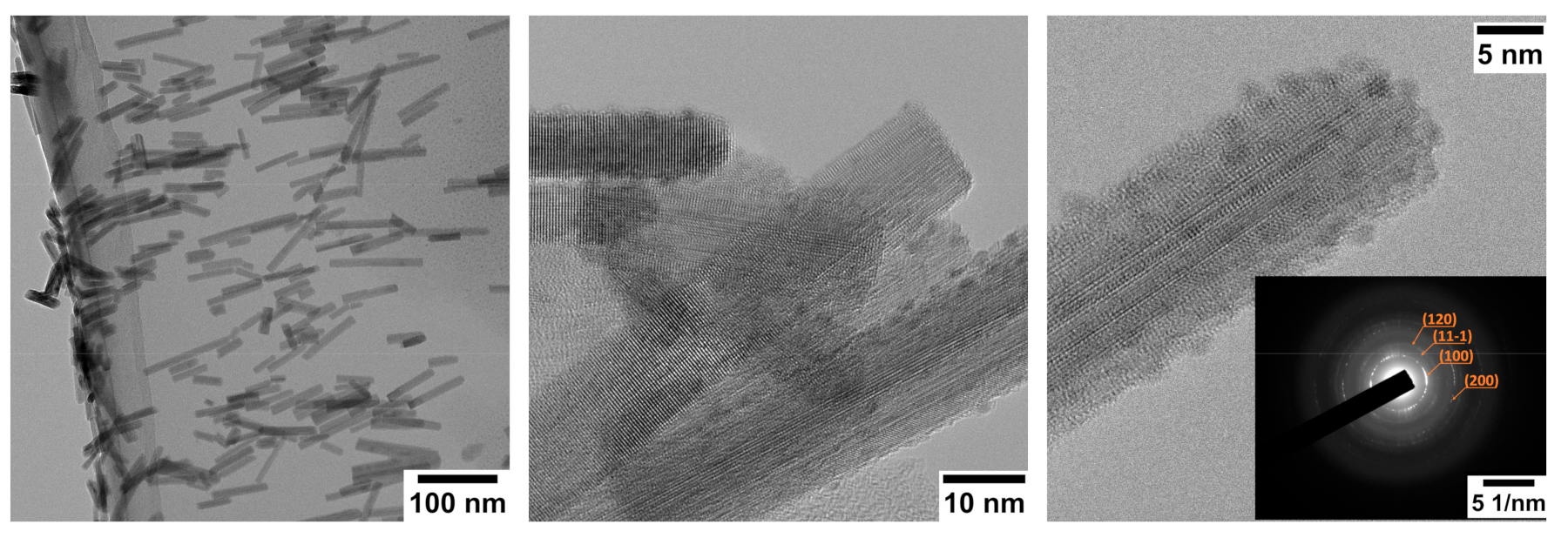
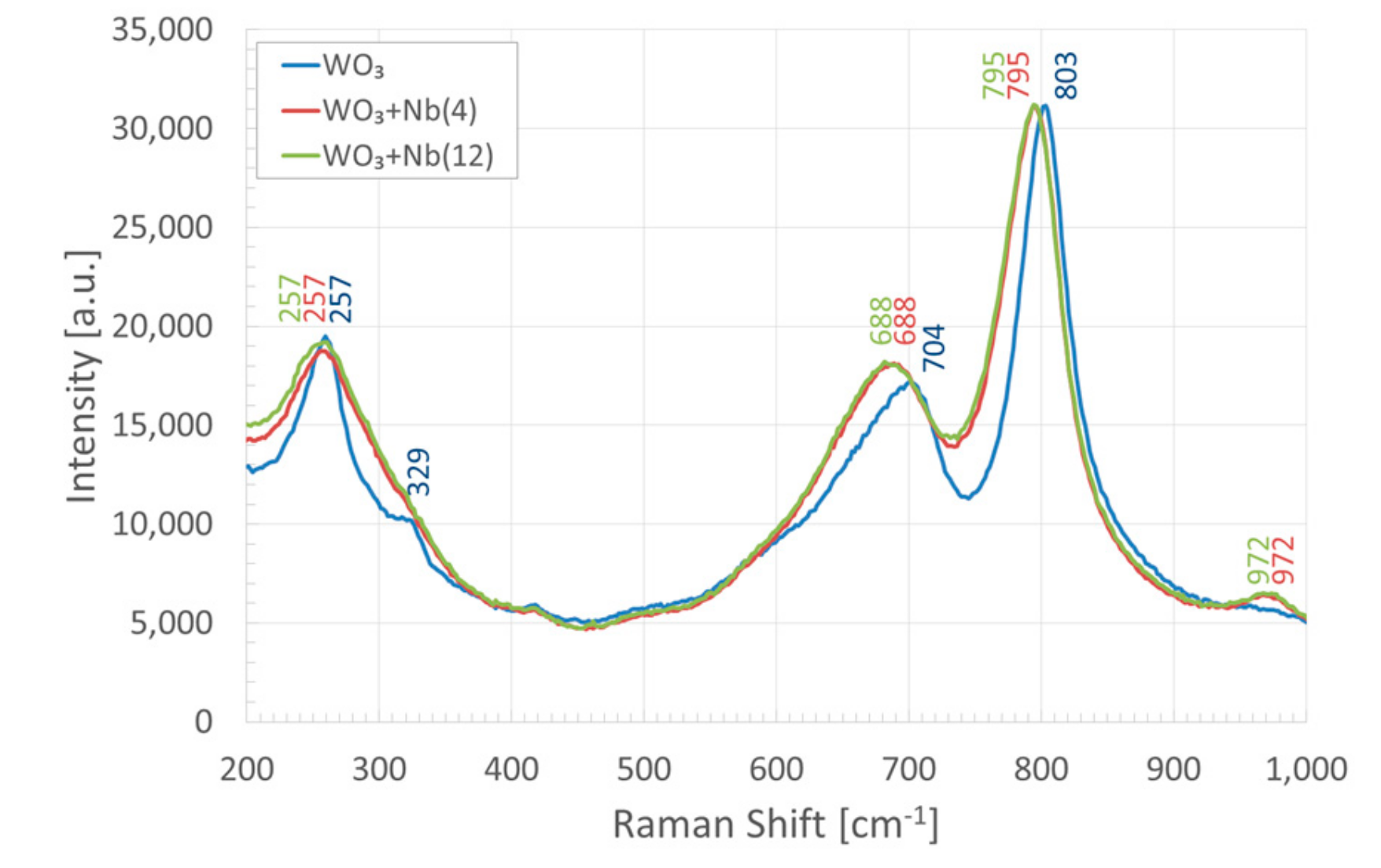
3. Morphologic and Structural Investigations
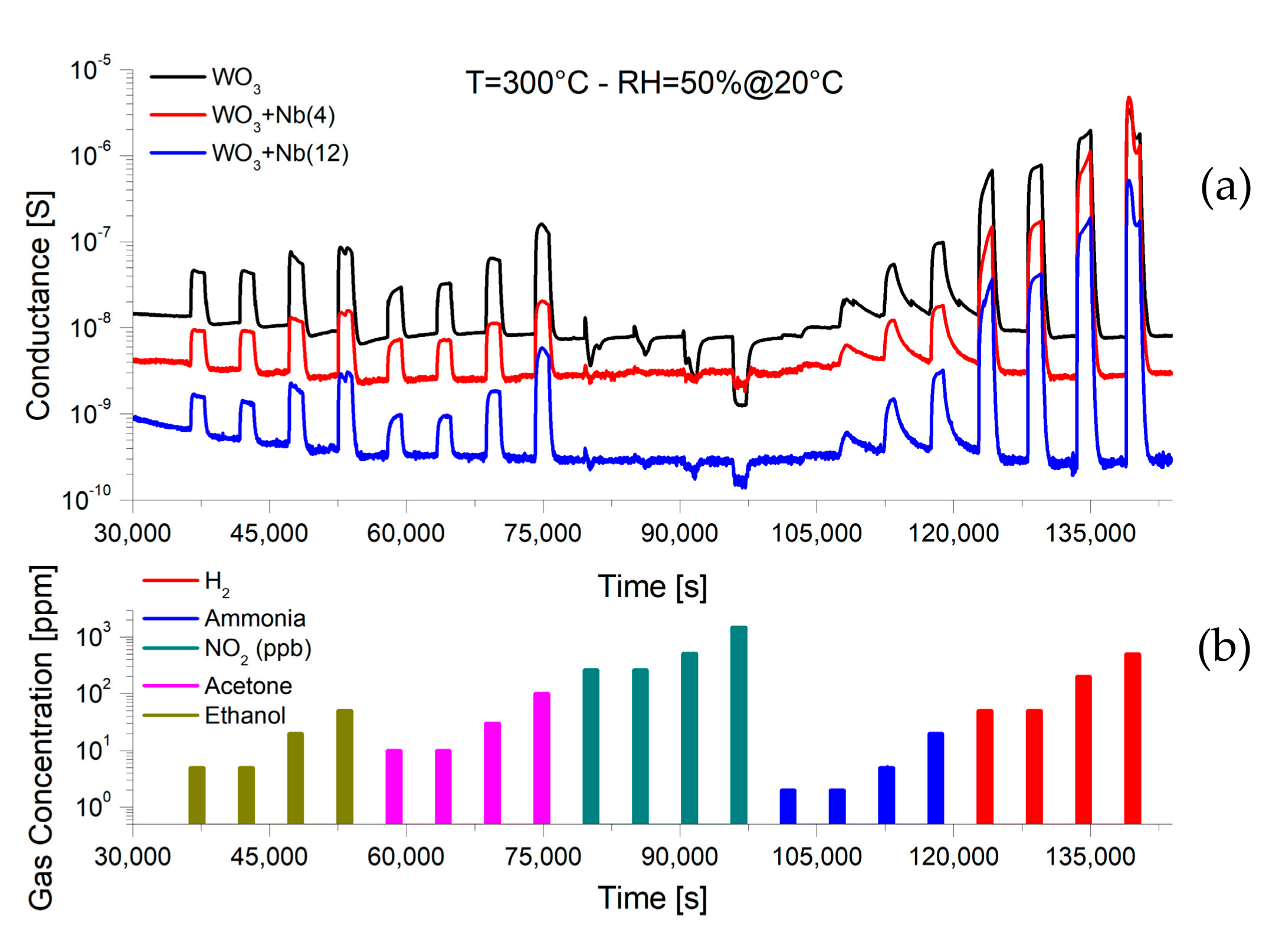
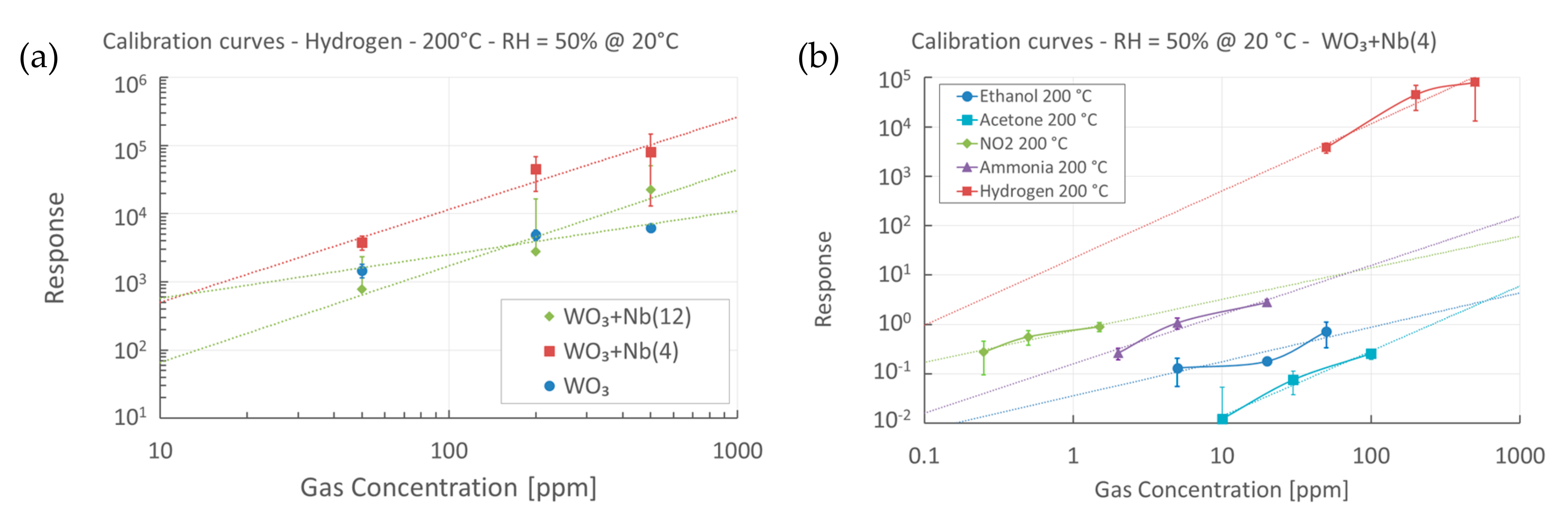
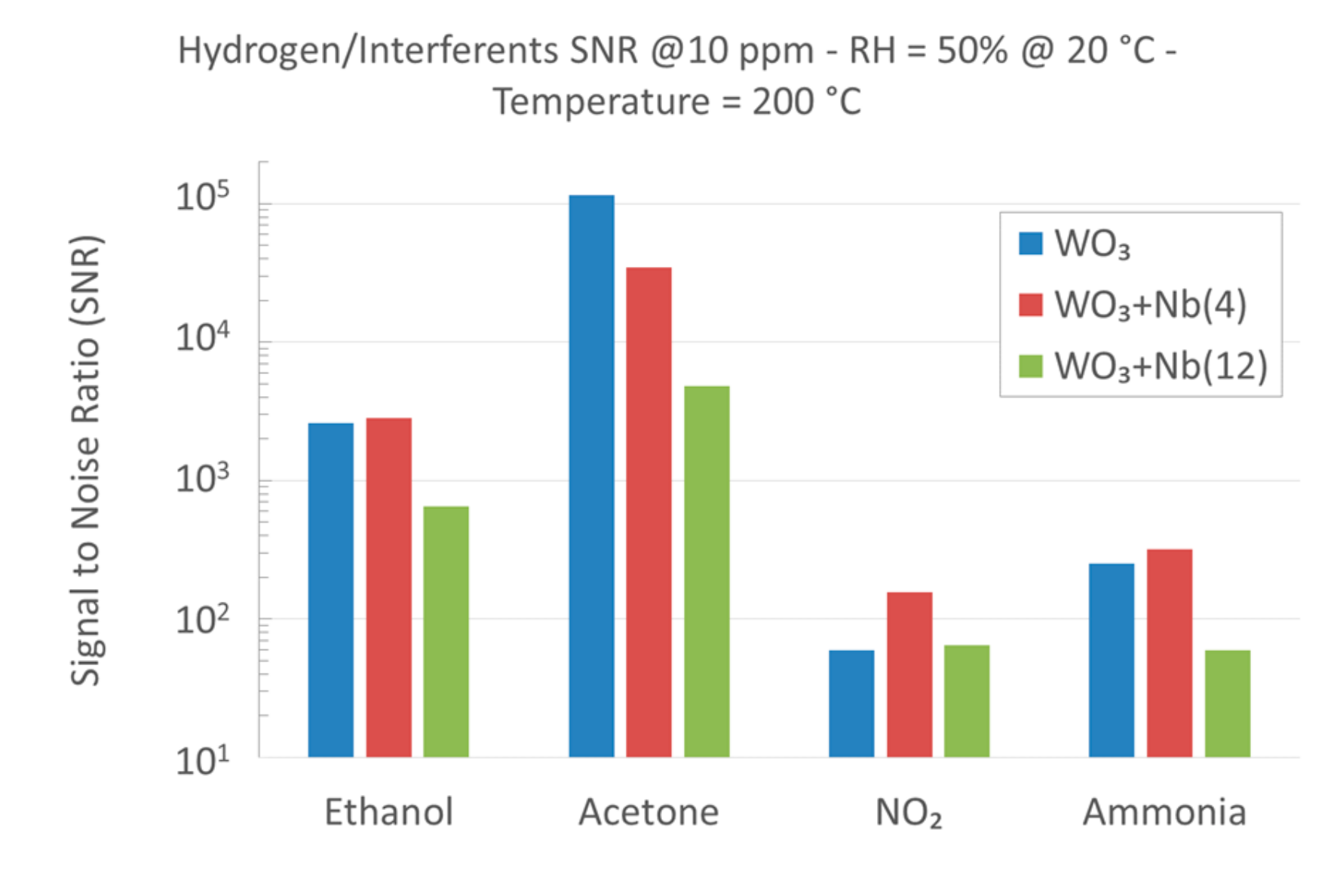
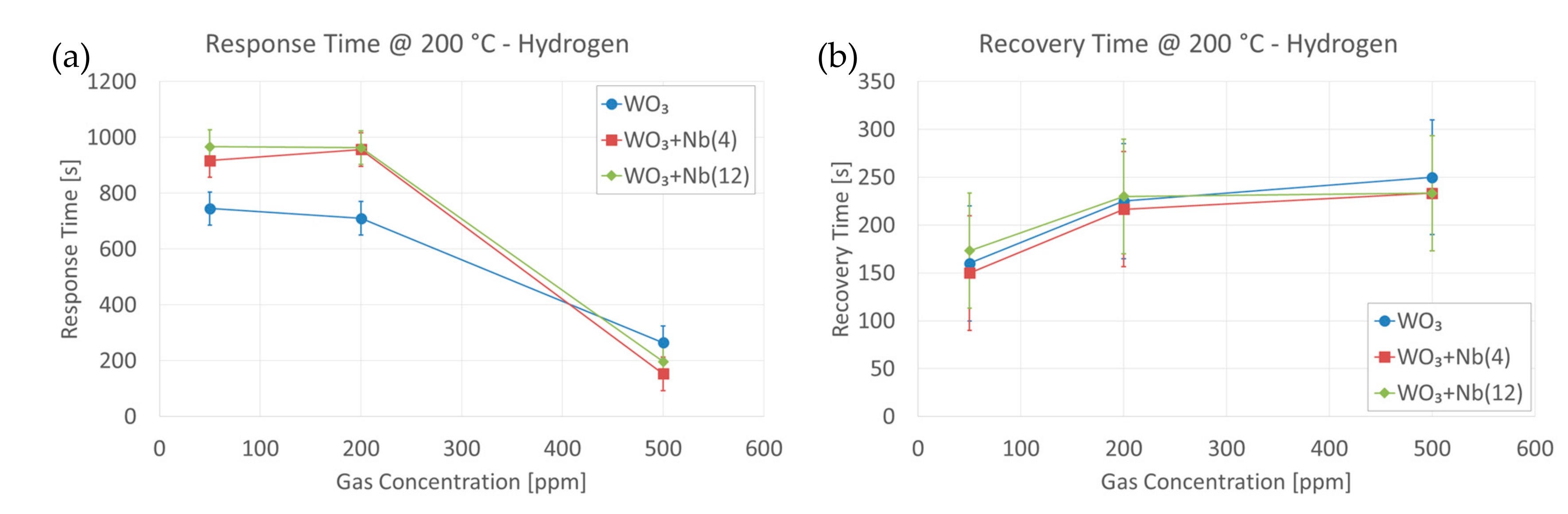
4. Functional Characterizations as Chemical Sensor
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Pearton, S. Recent progress in processing and properties of ZnO. Prog. Mater. Sci. 2005, 50, 293–340. [Google Scholar] [CrossRef]
- Look, D.C. Recent advances in ZnO materials and devices. Mater. Sci. Eng. B 2001, 80, 383–387. [Google Scholar] [CrossRef]
- Güntner, A.T.; Abegg, S.; Königstein, K.; Gerber, P.A.; Schmidt-Trucksäss, A.; Pratsinis, S.E. Breath sensors for health monitoring. ACS Sensors 2019, 4, 268–280. [Google Scholar] [CrossRef]
- Nasiri, N.; Clarke, C. Nanostructured chemiresistive gas sensors for medical applications. Sensors 2019, 19, 462. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-W.; Lee, J.-H. Toward breath analysis on a chip for disease diagnosis using semiconductor-based chemiresistors: Recent progress and future perspectives. Lab Chip 2017, 17, 3537–3557. [Google Scholar] [CrossRef] [PubMed]
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide materials for development of integrated gas sensors—A comprehensive review. Crit. Rev. Solid State Mater. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Williams, D.E. Semiconducting oxides as gas-sensitive resistors. Sens. Actuators B Chem. 1999, 57, 1–16. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Schneider, K.; Maziarz, W. V2O5 thin films as nitrogen dioxide Sensors. Sensors 2018, 18, 4177. [Google Scholar] [CrossRef] [PubMed]
- Comini, E.; Faglia, G.; Sberveglieri, G.; Pan, Z.; Wang, Z.L. Stable and highly sensitive gas sensors based on semiconducting oxide nanobelts. Appl. Phys. Lett. 2002, 81, 1869–1871. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Comini, E. Metal oxide nano-crystals for gas sensing. Anal. Chim. Acta 2006, 568, 28–40. [Google Scholar] [CrossRef]
- Comini, E.; Sberveglieri, G. Metal oxide nanowires as chemical sensors. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Varghese, O.K.; Gong, D.; Paulose, M.; Ong, K.G.; Grimes, C.A. Hydrogen sensing using titania nanotubes. Sens. Actuators B Chem. 2003, 93, 338–344. [Google Scholar] [CrossRef]
- Wang, H.T.; Kang, B.S.; Ren, F.; Tien, L.C.; Sadik, P.W.; Norton, D.P.; Pearton, S.J.; Lin, J. Hydrogen-selective sensing at room temperature with ZnO nanorods. Appl. Phys. Lett. 2005, 86, 243503. [Google Scholar] [CrossRef]
- Sayago, I.; Terrado, E.; Lafuente, E.; Horrillo, M.C.; Maser, W.K.; Benito, A.M.; Navarro, R.; Urriolabeitia, E.P.; Martinez, M.T.; Gutierrez, J. Hydrogen sensors based on carbon nanotubes thin films. Synth. Met. 2005, 148, 15–19. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Latimer, M.L.; Xiao, Z.L.; Panuganti, S.; Welp, U.; Kwok, W.K.; Xu, T. Hydrogen gas sensing with networks of ultrasmall palladium nanowires formed on filtration membranes. Nano Lett. 2011, 11, 262–268. [Google Scholar] [CrossRef]
- Crabtree, G.W.; Dresselhaus, M.S.; Buchanan, M.V. The Hydrogen Economy. Phys. Today 2004, 57, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Kamat, P.V. Meeting the clean energy demand: Nanostructure architectures for solar energy conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Herring, J.S.; O’Brien, J.E.; Stoots, C.M.; Hawkes, G.L.; Hartvigsen, J.J.; Shahnam, M. Progress in high-temperature electrolysis for hydrogen production using planar SOFC technology. Int. J. Hydrogen Energy 2007, 32, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Meng, G.; Jiang, C.; Ma, J.; Ma, Q.; Liu, X. Comparative study on the performance of a SDC-based SOFC fueled by ammonia and hydrogen. J. Power Sources 2007, 173, 189–193. [Google Scholar] [CrossRef]
- Chen, X.J.; Liu, Q.L.; Chan, S.H.; Brandon, N.P.; Khor, K.A. High performance cathode-supported SOFC with perovskite anode operating in weakly humidified hydrogen and methane. Electrochem. Commun. 2007, 9, 767–772. [Google Scholar] [CrossRef]
- Bessler, W.; Warnatz, J.; Goodwin, D. The influence of equilibrium potential on the hydrogen oxidation kinetics of SOFC anodes. Solid State Ionics 2007, 177, 3371–3383. [Google Scholar] [CrossRef]
- Schröder, V.; Emonts, B.; Janßen, H.; Schulze, H.-P. Explosion limits of hydrogen/oxygen mixtures at initial pressures up to 200 bar. Chem. Eng. Technol. 2004, 27, 847–851. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Bai, S.; Ma, Y.; Shu, X.; Sun, J.; Feng, Y.; Luo, R.; Li, D.; Chen, A. Doping metal elements of WO3 for enhancement of NO2-sensing performance at room temperature. Ind. Eng. Chem. Res. 2017, 56, 2616–2623. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Schneider, K.; Lubecka, M.; Czapla, A. V2O5 thin films for gas sensor applications. Sens. Actuators B Chem. 2016, 236, 970–977. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Haddi, Z.; Ling, M.; Di Maggio, F.; Vallejos, S.; Vilic, T.; Zhu, Y.; Shujah, T.; Umek, P.; Bittencourt, C.; et al. Aerosol-assisted CVD-grown PdO nanoparticle-decorated tungsten oxide nanoneedles extremely sensitive and selective to hydrogen. ACS Appl. Mater. Interfaces 2016, 8, 10413–10421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ippolito, S.J.; Kandasamy, S.; Kalantar-zadeh, K.; Wlodarski, W. Hydrogen sensing characteristics of WO3 thin film conductometric sensors activated by Pt and Au catalysts. Sens. Actuators B Chem. 2005, 108, 154–158. [Google Scholar] [CrossRef]
- Ghadiri, E.; Zad, A.I.; Razi, F. Hydrogen sensing properties of pure and Pd activated WO3 nanostructured films. Synth. React. Inorganic Met. Nano Metal Chem. 2007, 37, 453–456. [Google Scholar] [CrossRef]
- Chan, C.-C.; Hsu, W.-C.; Chang, C.-C.; Hsu, C.-S. Preparation and characterization of gasochromic Pt/WO3 hydrogen sensor by using the Taguchi design method. Sens. Actuators B Chem. 2010, 145, 691–697. [Google Scholar] [CrossRef]
- Hsu, W.-C.; Chan, C.-C.; Peng, C.-H.; Chang, C.-C. Hydrogen sensing characteristics of an electrodeposited WO3 thin film gasochromic sensor activated by Pt catalyst. Thin Solid Films 2007, 516, 407–411. [Google Scholar] [CrossRef]
- Esfandiar, A.; Irajizad, A.; Akhavan, O.; Ghasemi, S.; Gholami, M.R. Pd–WO3/reduced graphene oxide hierarchical nanostructures as efficient hydrogen gas sensors. Int. J. Hydrogen Energy 2014, 39, 8169–8179. [Google Scholar] [CrossRef]
- Horprathum, M.; Srichaiyaperk, T.; Samransuksamer, B.; Wisitsoraat, A.; Eiamchai, P.; Limwichean, S.; Chananonnawathorn, C.; Aiempanakit, K.; Nuntawong, N.; Patthanasettakul, V.; et al. Ultrasensitive hydrogen sensor based on Pt-decorated WO3 nanorods prepared by glancing-angle dc magnetron sputtering. ACS Appl. Mater. Interfaces 2014, 6, 22051–22060. [Google Scholar] [CrossRef] [PubMed]
- Boudiba, A.; Roussel, P.; Zhang, C.; Olivier, M.-G.; Snyders, R.; Debliquy, M. Sensing mechanism of hydrogen sensors based on palladium-loaded tungsten oxide (Pd–WO3). Sens. Actuators B Chem. 2013, 187, 84–93. [Google Scholar] [CrossRef]
- Griffith, K.J.; Wiaderek, K.M.; Cibin, G.; Marbella, L.E.; Grey, C.P. Niobium tungsten oxides for high-rate lithium-ion energy storage. Nature 2018, 559, 556–563. [Google Scholar] [CrossRef]
- Liu, B.; Mu, W.; Xie, X.; Li, X.; Wei, H.; Tan, Z.; Jian, Y.; Luo, S. Enhancing the adsorption capacity of Sr2+ and Cs+ onto hexagonal tungsten oxide by doped niobium. RSC Adv. 2015, 5, 15603–15611. [Google Scholar] [CrossRef]
- Takagaki, A.; Tagusagawa, C.; Takanabe, K.; Kondo, J.N.; Tatsumi, T.; Domen, K. Effect of post-calcination thermal treatment on acid properties and pores structure of a mesoporous niobium–tungsten oxide. Catal. Today 2012, 192, 144–148. [Google Scholar] [CrossRef]
- Yue, C.; Zhu, X.; Rigutto, M.; Hensen, E. Acid catalytic properties of reduced tungsten and niobium-tungsten oxides. Appl. Catal. B Environ. 2015, 163, 370–381. [Google Scholar] [CrossRef]
- Kruefu, V.; Wisitsoraat, A.; Phanichphant, S. Effects of niobium-loading on sulfur dioxide gas-sensing characteristics of hydrothermally prepared tungsten oxide thick film. J. Nanomater. 2015, 2015, 5. [Google Scholar] [CrossRef]
- Yu, J.; Cheung, K.W.; Yan, W.H.; Li, Y.X.; Ho, D. High-sensitivity low-power tungsten doped niobium oxide nanorods sensor for nitrogen dioxide air pollution monitoring. Sens. Actuators B Chem. 2017, 238, 204–213. [Google Scholar] [CrossRef]
- Yu, J.; Wen, H.; Shafiei, M.; Field, M.R.; Liu, Z.F.; Wlodarski, W.; Motta, N.; Li, Y.X.; Kalantar-zadeh, K.; Lai, P.T. A hydrogen/methane sensor based on niobium tungsten oxide nanorods synthesised by hydrothermal method. Sens. Actuators B Chem. 2013, 184, 118–129. [Google Scholar] [CrossRef]
- Yu, J.; Yuan, L.; Wen, H.; Shafiei, M.; Field, M.R.; Liang, J.; Yang, J.; Liu, Z.F.; Wlodarski, W.; Motta, N.; et al. Hydrothermally formed functional niobium oxide doped tungsten nanorods. Nanotechnology 2013, 24, 495501. [Google Scholar] [CrossRef]
- Romain, A.C.; Nicolas, J. Long term stability of metal oxide-based gas sensors for e-nose environmental applications: An overview. Sens. Actuators B Chem. 2010, 146, 502–506. [Google Scholar] [CrossRef] [Green Version]
- Aleixandre, M.; Lozano, J.; Gutiérrez, J.; Sayago, I.; Fernández, M.J.; Horrillo, M.C. Portable e-nose to classify different kinds of wine. Sens. Actuators B Chem. 2008, 131, 71–76. [Google Scholar] [CrossRef]
- Castro, M.; Kumar, B.; Feller, J.F.; Haddi, Z.; Amari, A.; Bouchikhi, B. Novel e-nose for the discrimination of volatile organic biomarkers with an array of carbon nanotubes (CNT) conductive polymer nanocomposites (CPC) sensors. Sens. Actuators B Chem. 2011, 159, 213–219. [Google Scholar] [CrossRef]
- Abbatangelo, M.; Núñez-Carmona, E.; Sberveglieri, V.; Zappa, D.; Comini, E.; Sberveglieri, G. Application of a novel S3 nanowire gas sensor device in parallel with GC-MS for the identification of rind percentage of grated Parmigiano Reggiano. Sensors (Switzerland) 2018, 18, 1617. [Google Scholar] [CrossRef]
- Zappa, D.; Bertuna, A.; Comini, E.; Molinari, M.; Poli, N.; Sberveglieri, G. Tungsten oxide nanowires for chemical detection. Anal. Methods 2015, 7, 2203–2209. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Faglia, G.; Perego, C.; Nelli, P.; Marks, R.N.; Virgili, T.; Taliani, C.; Zamboni, R. Hydrogen and humidity sensing properties of C60 thin films. Synth. Met. 1996, 77, 273–275. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Zappa, D.; Comini, E.; Zamani, R.; Arbiol, J.; Morante, J.R.; Sberveglieri, G. Preparation of copper oxide nanowire-based conductometric chemical sensors. Sens. Actuators B Chem. 2013, 182, 7–15. [Google Scholar] [CrossRef]
- Thummavichai, K.; Wang, N.; Xu, F.; Rance, G.; Xia, Y.; Zhu, Y. In situ investigations of the phase change behaviour of tungsten oxide nanostructures. R. Soc. Open Sci. 2018, 5, 171932. [Google Scholar] [CrossRef] [Green Version]
- Cantalini, C.; Sun, H.T.; Faccio, M.; Pelino, M.; Santucci, S.; Lozzi, L.; Passacantando, M. NO2 sensitivity of WO3 thin film obtained by high vacuum thermal evaporation. Sens. Actuators B Chem. 1996, 31, 81–87. [Google Scholar] [CrossRef]
- Penza, M.; Tagliente, M.; Mirenghi, L.; Gerardi, C.; Martucci, C.; Cassano, G. Tungsten trioxide (WO3) sputtered thin films for a NOx gas sensor. Sens. Actuators B Chem. 1998, 50, 9–18. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Y.; Kong, F.; Wang, S.; Zhu, B.; Guo, X.; Zhang, J.; Wang, Y.; Wu, S. Au-doped WO3-based sensor for NO2 detection at low operating temperature. Sens. Actuators B Chem. 2008, 134, 133–139. [Google Scholar] [CrossRef]
- Terohid, S.A.A.; Heidari, S.; Jafari, A.; Asgary, S. Effect of growth time on structural, morphological and electrical properties of tungsten oxide nanowire. Appl. Phys. A 2018, 124, 567. [Google Scholar] [CrossRef]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Toyoshima, R.; Yoshida, M.; Monya, Y.; Kousa, Y.; Suzuki, K.; Abe, H.; Mun, B.S.; Mase, K.; Amemiya, K.; Kondoh, H. In Situ ambient pressure XPS study of CO oxidation reaction on Pd(111) surfaces. J. Phys. Chem. C 2012, 116, 18691–18697. [Google Scholar] [CrossRef]
- Schnadt, J.; Knudsen, J.; Andersen, J.N.; Siegbahn, H.; Pietzsch, A.; Hennies, F.; Johansson, N.; Mårtensson, N.; Ohrwall, G.; Bahr, S.; et al. The new ambient-pressure X-ray photoelectron spectroscopy instrument at MAX-lab. J. Synchrotron Radiat. 2012, 19, 701–704. [Google Scholar] [CrossRef] [Green Version]
- Atashbar, M.Z.; Sun, H.T.; Gong, B.; Wlodarski, W.; Lamb, R. XPS study of Nb-doped oxygen sensing TiO2 thin films prepared by sol-gel method. Thin Solid Films 1998, 326, 238–244. [Google Scholar] [CrossRef]
- Briggs, D. Handbook of X-ray Photoelectron Spectroscopy C. D. Wanger, W. M. Riggs, L. E. Davis, J. F. Moulder and G. E. Muilenberg Perkin-Elmer Corp., Physical Electronics Division, Eden Prairie, Minnesota, USA, 1979. 190 pp. $195. Surf. Interface Anal. 1981, 3. [Google Scholar] [CrossRef]
- Rougier, A.; Portemer, F.; Quédé, A.; El Marssi, M. Characterization of pulsed laser deposited WO3 thin films for electrochromic devices. Appl. Surf. Sci. 1999, 153, 1–9. [Google Scholar] [CrossRef]
- Santato, C.; Odziemkowski, M.; Ulmann, M.; Augustynski, J. Crystallographically oriented mesoporous WO3 films: Synthesis, characterization, and applications. J. Am. Chem. Soc. 2001, 123, 10639–10649. [Google Scholar] [CrossRef] [PubMed]
- Gatehouse, B.M.; Wadsley, A.D. The crystal structure of the high temperature form of niobium pentoxide. Acta Crystallogr. 1964, 17, 1545–1554. [Google Scholar] [CrossRef] [Green Version]
- Raba, A.M.; Bautista-Ruíz, J.; Joya, M.R.; Raba, A.M.; Bautista-Ruíz, J.; Joya, M.R. Synthesis and structural properties of niobium pentoxide powders: A comparative study of the growth process. Mater. Res. 2016, 19, 1381–1387. [Google Scholar] [CrossRef]
- Jehng, J.M.; Wachs, I.E. Structural chemistry and Raman spectra of niobium oxides. Chem. Mater. 1991, 3, 100–107. [Google Scholar] [CrossRef]
- Cava, R.J.; Batlogg, B.; Krajewski, J.J.; Poulsen, H.F.; Gammel, P.; Peck, W.F.; Rupp, L.W. Electrical and magnetic properties of Nb2O5-delta crystallographic shear structures. Phys. Rev. B. Condens. Matter 1991, 44, 6973–6981. [Google Scholar] [CrossRef] [PubMed]
- Shigesato, Y.; Hayashi, Y.; Masui, A.; Haranou, T. The structural changes of indium-tin oxide and a-WO3 films by introducing water to the deposition processes. Jpn. J. Appl. Phys. 1991, 30, 814–819. [Google Scholar] [CrossRef]
- Woo, H.-S.; Na, C.; Lee, J.-H. Design of highly selective gas sensors via physicochemical modification of oxide nanowires: overview. Sensors 2016, 16, 1531. [Google Scholar] [CrossRef]
- Zampolli, S.; Elmi, I.; Stürmann, J.; Nicoletti, S.; Dori, L.; Cardinali, G.C. Selectivity enhancement of metal oxide gas sensors using a micromachined gas chromatographic column. Sens. Actuators B Chem. 2005, 105, 400–406. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Instability of metal oxide-based conductometric gas sensors and approaches to stability improvement (short survey). Sens. Actuators B Chem. 2011, 156, 527–538. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, J.X.; Ong, C.W. Diffusion-controlled H2 sensors composed of Pd-coated highly porous WO3 nanocluster films. Sens. Actuators B Chem. 2014, 191, 711–718. [Google Scholar] [CrossRef]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceramics 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Lupan, O.; Ursaki, V.V.; Chai, G.; Chow, L.; Emelchenko, G.A.; Tiginyanu, I.M.; Gruzintsev, A.N.; Redkin, A.N. Selective hydrogen gas nanosensor using individual ZnO nanowire with fast response at room temperature. Sens. Actuators B Chem. 2010, 144, 56–66. [Google Scholar] [CrossRef]
- Wongchoosuk, C.; Wisitsoraat, A.; Phokharatkul, D.; Tuantranont, A.; Kerdcharoen, T. Multi-walled carbon nanotube-doped tungsten oxide thin films for hydrogen gas sensing. Sensors 2010, 10, 7705–7715. [Google Scholar] [CrossRef] [PubMed]
- Kurmaev, E.Z.; Moewes, A.; Bureev, O.G.; Nekrasov, I.A.; Cherkashenko, V.M.; Korotin, M.A.; Ederer, D.L. Electronic structure of niobium oxides. J. Alloys Compd. 2002, 347, 213–218. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shim, G.; Park, J.; Seo, H. Tunable polaron-induced coloration of tungsten oxide: Via a multi-step control of the physicochemical property for the detection of gaseous F. Phys. Chem. Chem. Phys. 2018, 20, 16932–16938. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, K. Material and gas-sensing properties of tungsten oxide nanorod thin-films. J. Nanosci. Nanotechnol. 2009, 9, 2463–2468. [Google Scholar] [CrossRef] [PubMed]
- Malyshev, V.V.; Pislyakov, A. V Investigation of gas-sensitivity of sensor structures to hydrogen in a wide range of temperature, concentration and humidity of gas medium. Sens. Actuators B Chem. 2008, 134, 913–921. [Google Scholar] [CrossRef]




| No Flow | 10 sccm O2 | 10 sccm Ar | |
|---|---|---|---|
| SEM |  |  |  |
| WO3 | WO3 + Nb(4) | WO3 + Nb(12) | |
|---|---|---|---|
| 500 °C | 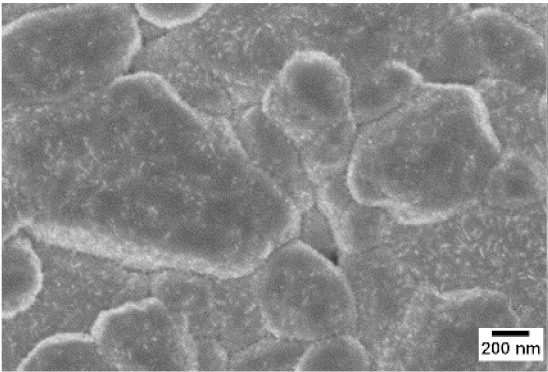 |  | 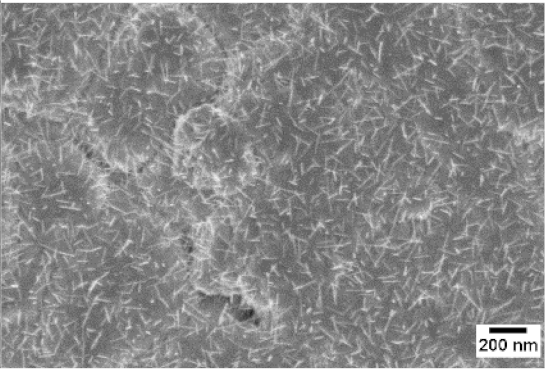 |
| 550 °C | 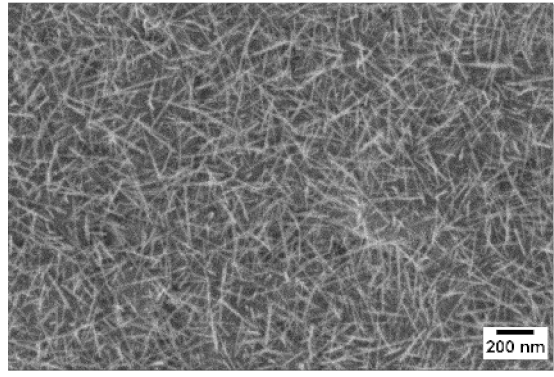 |  | 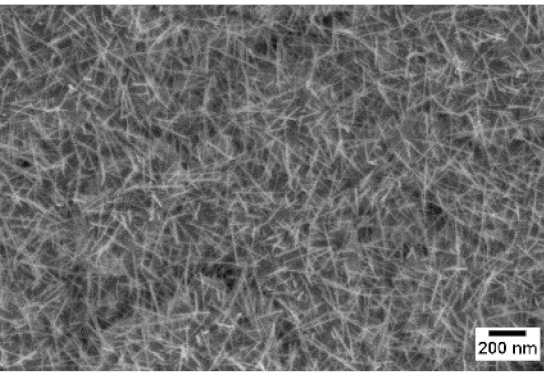 |
| 600 °C | 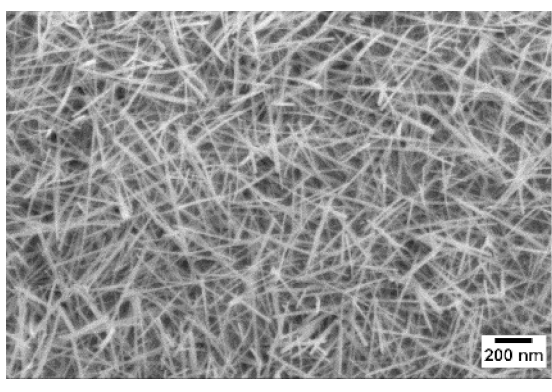 | 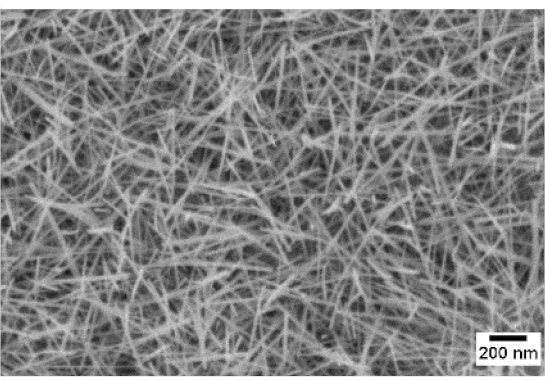 | 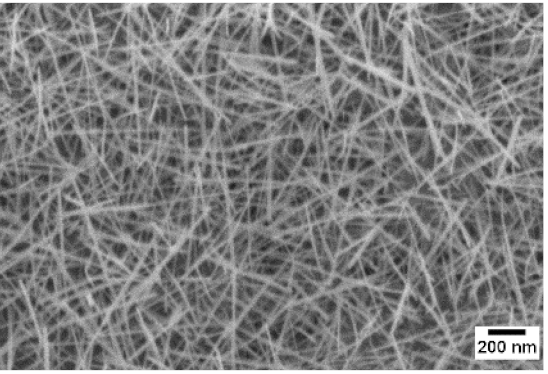 |
| 650 °C |  |  | 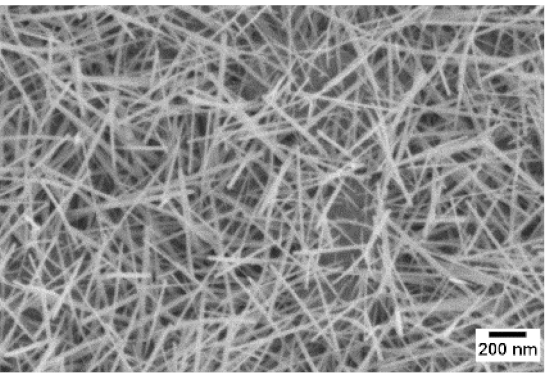 |
| 700 °C |  | 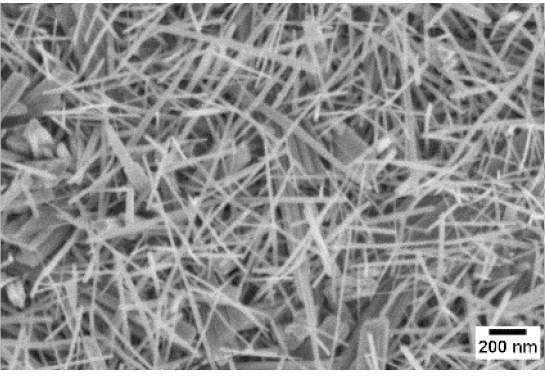 | 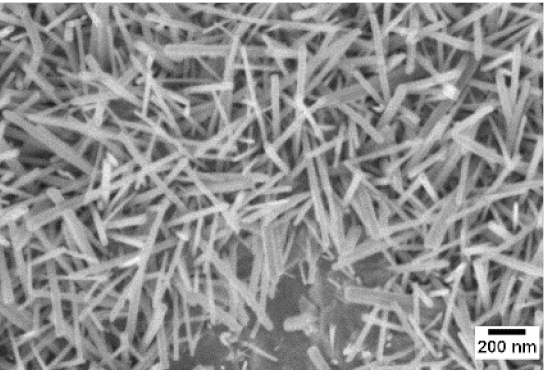 |
| Material | Morphology | H2 Gas Concentration | Temperature | Humidity | Response | Ref. |
|---|---|---|---|---|---|---|
| Pd-WO3 | Ribbon-like | 500 ppm | 100 °C | Dry air | ∆G/G = 80 | [36] |
| Pd-WO3/PRGO | Irregular | 500 ppm | 100 °C | Dry air | ∆G/G = 150 | [36] |
| WO3 | Thin film | 0.50% | 180 °C | Dry air | ∆G/G = 6 | [35] |
| Pt-WO3 | Thin film | 0.50% | 70 °C | Dry air | ∆G/G = 450 | [35] |
| Au-WO3 | Thin film | 0.50% | 262 °C | Dry air | ∆G/G = 250 | [35] |
| WO3 | Nanorods | 500 ppm | 200 °C | Dry air | Rair/Rgas ≈ 1 | [37] |
| Pt-WO3 | Nanorods | 500 ppm | 200 °C | Dry air | Rair/Rgas = 3 × 104 | [37] |
| Pd-WO3 | Cluster film | 2% | 80 °C | Dry air | ∆G/G = 2.4 × 104 | [75] |
| Pd-WO3 | Nanoparticles | 200 ppm | 200 °C | RH = 50% | ∆G/G = 20 | [38] |
| Pd-WO3 | Nanoneedles | 500 ppm | 150 °C | RH = 50% | ∆G/G = 1670 | [31] |
| WO3 | Nanowires | 500 ppm | 200 °C | RH = 50% | ∆G/G = 6 × 103 | This work |
| WO3 + Nb(4) | Nanowires | 500 ppm | 200 °C | RH = 50% | ∆G/G = 8 × 104 | This work |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zappa, D. The Influence of Nb on the Synthesis of WO3 Nanowires and the Effects on Hydrogen Sensing Performance. Sensors 2019, 19, 2332. https://doi.org/10.3390/s19102332
Zappa D. The Influence of Nb on the Synthesis of WO3 Nanowires and the Effects on Hydrogen Sensing Performance. Sensors. 2019; 19(10):2332. https://doi.org/10.3390/s19102332
Chicago/Turabian StyleZappa, Dario. 2019. "The Influence of Nb on the Synthesis of WO3 Nanowires and the Effects on Hydrogen Sensing Performance" Sensors 19, no. 10: 2332. https://doi.org/10.3390/s19102332





