Facile Chemical Bath Synthesis of SnS Nanosheets and Their Ethanol Sensing Properties
Abstract
:1. Introduction
2. Materials and Methods
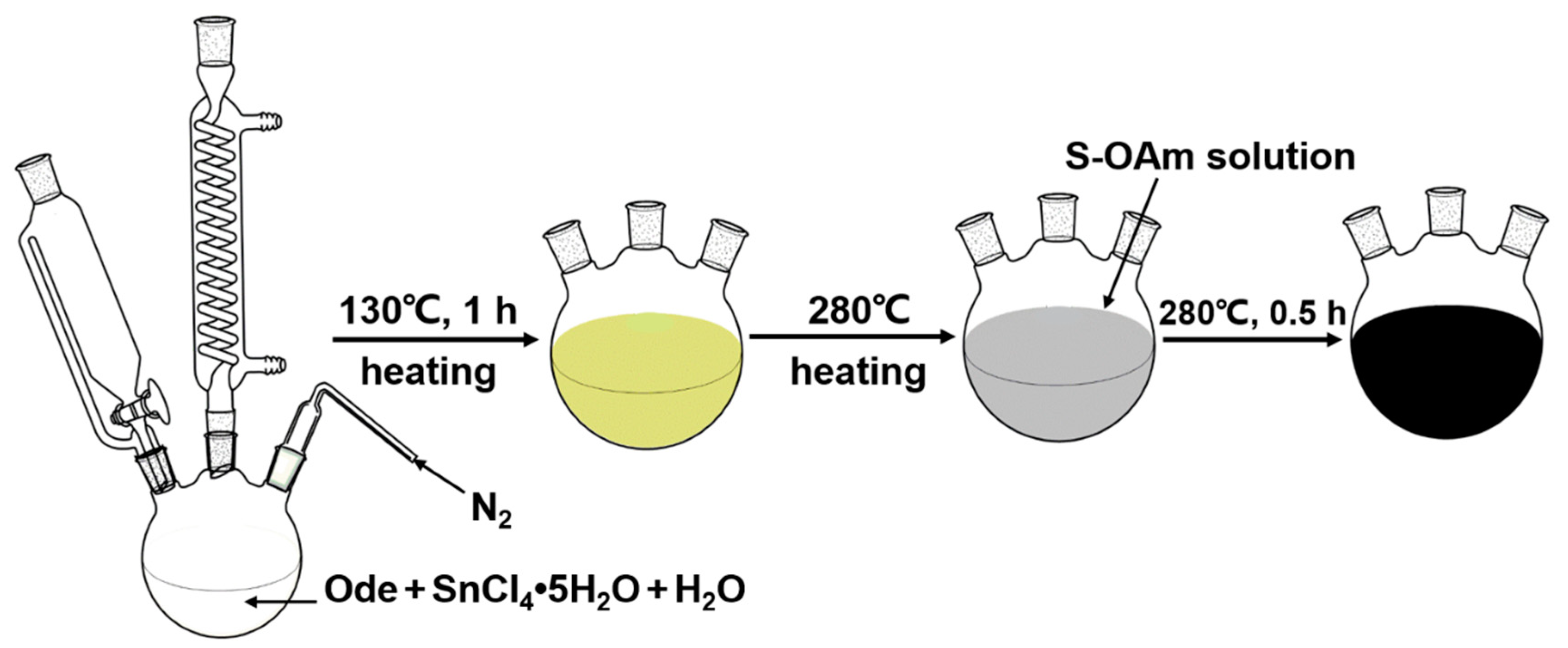
2.1. SnS Nanosheet Synthesis and Characterization
2.2. The Gas Sensing Test
3. Results and Discussion
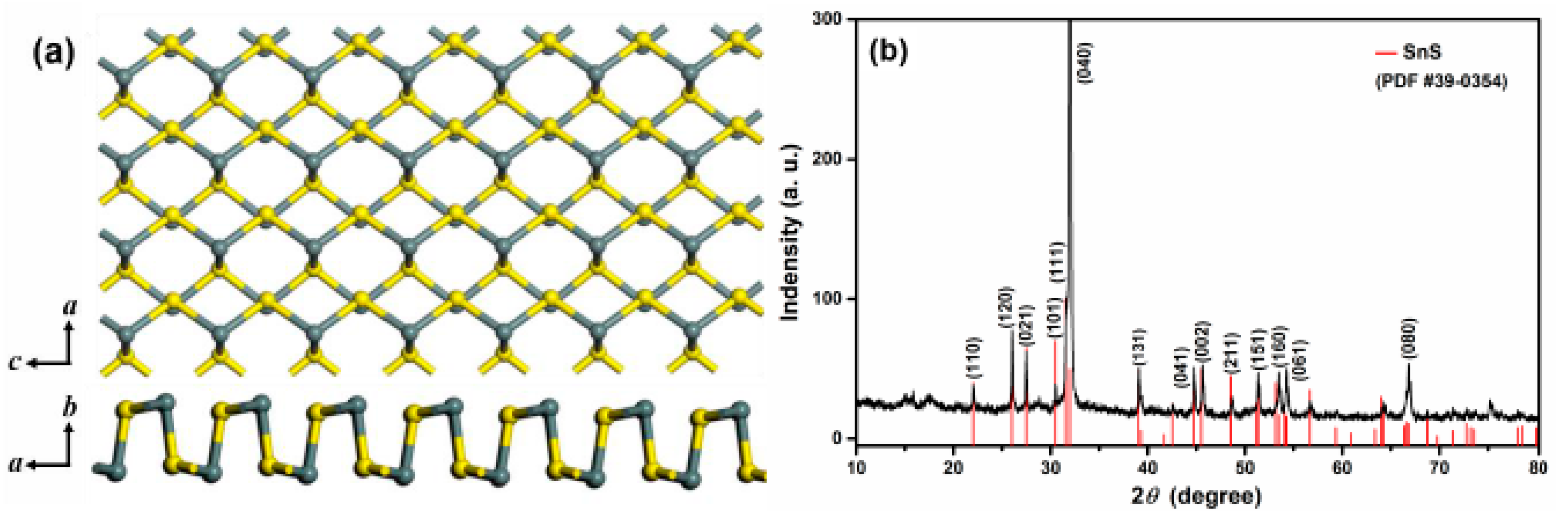
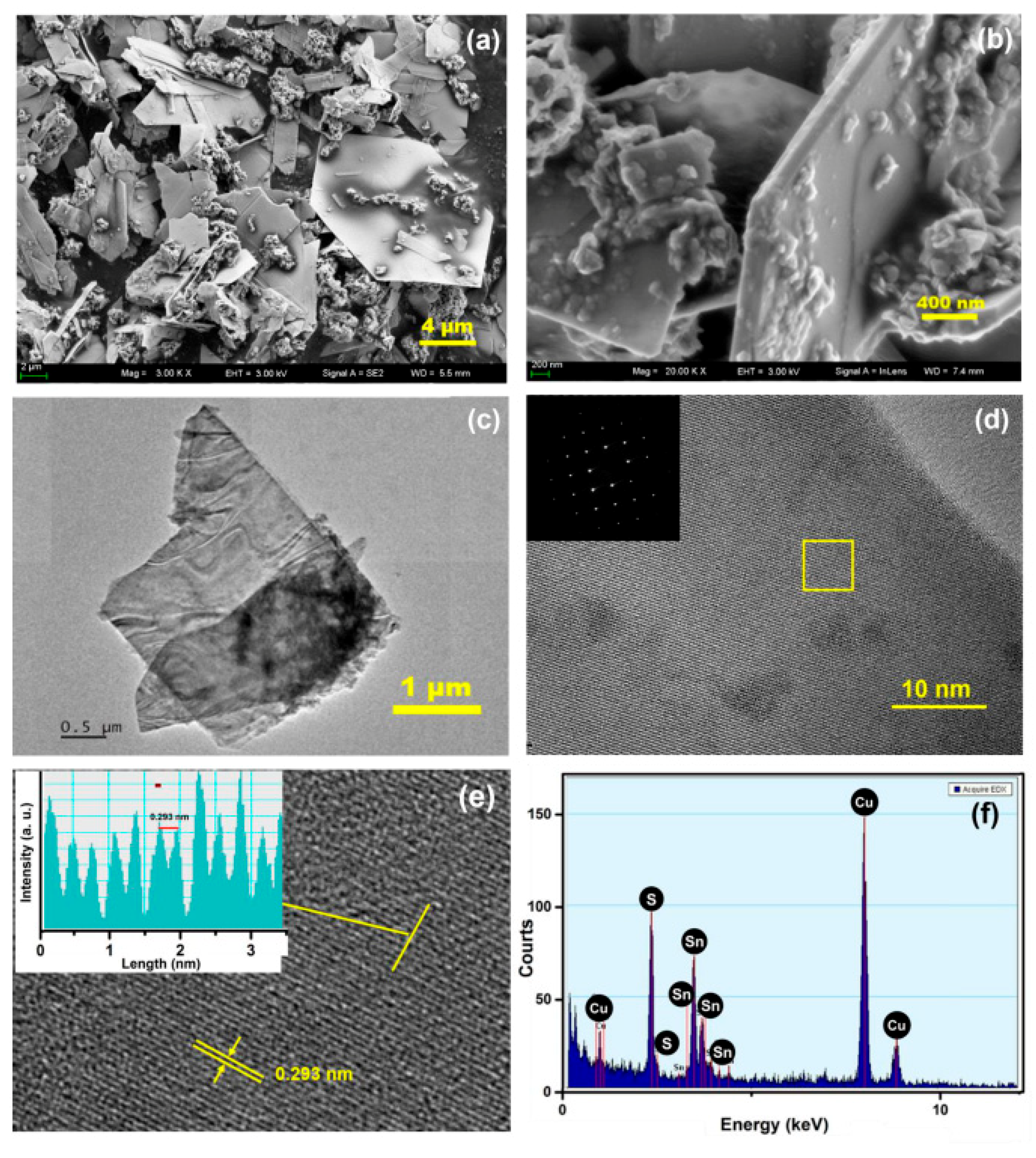
3.1. Microstructural Characterizations of the SnS Nanosheets
3.2. Synthesis Process and Mechanism
3.3. Gas Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mehta, S.S.; Tamboli, M.S.; Mulla, I.S.; Suryavanshi, S.S. Facile hydrothermal synthesis of nanobricks assembled WO3 microflowers and their ethanol sensing properties. Mater. Lett. 2017, 207, 80–84. [Google Scholar] [CrossRef]
- Saboor, F.H.; Khodadadi, A.A.; Mortazavi, Y.; Asgari, M. Microemulsion synthesized Silica/ZnO stable core/shell sensors highly selective to ethanol with minimum sensitivity to humidity. Sens. Actuators B Chem. 2017, 238, 1070–1083. [Google Scholar] [CrossRef]
- Chen, Y.J.; Xue, X.Y.; Wang, Y.G.; Wang, T.H. Synthesis and ethanol sensing characteristics of single crystalline SnO2 nanorods. Appl. Phys. Lett. 2005, 87, 233503. [Google Scholar] [CrossRef]
- Wang, L.W.; Kang, Y.F.; Liu, X.H.; Zhang, S.M.; Huang, W.P.; Wang, S.R. ZnO nanorod gas sensor for ethanol detection. Sens. Actuators B Chem. 2012, 162, 237–243. [Google Scholar] [CrossRef]
- Lai, H.Y.; Chen, T.H.; Chen, C.H. Architecture controlled synthesis of flower-like In2O3 nanobundles with significantly enhanced ultraviolet scattering and ethanol sensing. Cryst. Eng. Commun. 2012, 14, 5589–5595. [Google Scholar] [CrossRef]
- Li, X.L.; Lou, T.J.; Sun, X.M.; Li, Y.D. Highly sensitive WO3 hollow-sphere gas sensors. Inorg. Chem. 2004, 43, 5442–5449. [Google Scholar] [CrossRef] [PubMed]
- Baruwati, B.; Kumar, D.K.; Manorama, S.V. Hydrothermal synthesis of highly crystalline ZnO nanoparticles: A competitive sensor for LPG and EtOH. Sens. Actuators B Chem. 2006, 119, 676–682. [Google Scholar] [CrossRef]
- Bagheri, M.; Hamedania, N.F.; Mahjouba, A.R.; Khodadadi, A.A.; Mortazavi, Y. Highly sensitive and selective ethanol sensor based on Sm2O3-loaded flower-like ZnO nanostructure. Sens. Actuators B Chem. 2014, 191, 283–290. [Google Scholar] [CrossRef]
- Song, P.; Han, D.; Zhang, H.H.; Li, J.; Yang, Z.X.; Wang, Q. Hydrothermal synthesis of porous In2O3 nanospheres with superior ethanol sensing properties. Sens. Actuators B Chem. 2014, 196, 434–439. [Google Scholar] [CrossRef]
- Bhowmik, B.; Dutta, K.; Bhattacharyya, P. An efficient room temperature ethanol sensor device based on p-n homojunction of TiO2 nanostructures. IEEE Trans. Electron Devices 2019, 66, 1063–1068. [Google Scholar] [CrossRef]
- Bindra, P.; Hazra, A. Multi-layered TiO2 nanotubes array based highly sensitive room temperature vapor sensors. IEEE Trans. Nanotechnol. 2019, 18, 13–20. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, C.M.; Ma, P.; Li, L.; Zhu, K.K.; Kang, X.F.; Chen, W. Ethanol gas sensor based on γ-Fe2O3 nanoparticles working at room temperature with high sensitivity. Chin. J. Anal. Chem. 2018, 46, 1854–1862. [Google Scholar] [CrossRef]
- Carey, W.P.; Beebe, K.R.; Sanchez, E.; Geladi, E.P.; Kowalski, B.R. Chemometric analysis of multisensor arrays. Sens. Actuators B Chem. 1984, 9, 225–243. [Google Scholar] [CrossRef]
- Pineau, N.J.; Kompalla, J.F.; Guntner, A.T.; Pratsinis, S.E. Orthogonal gas sensor arrays by chemoresistive material design. Microchim. Acta 2018, 185, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2001, 7, 1876–1902. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.W.; Jang, J.T.; Park, S.W.; Kim, C.; Park, B.; Cheon, J. Two-dimensional SnS2 nanoplates with extraordinary high discharge capacity for lithium ion batteries. Adv. Mater. 2008, 20, 4269–4273. [Google Scholar] [CrossRef]
- Kim, Y.H.; Phan, D.T.; Ahn, S.; Namb, K.H.; Park, C.M.; Jeon, K.J. Two-dimensional SnS2 materials as high-performance NO2 sensors with fast response and high sensitivity. Sens. Actuators B Chem. 2018, 255, 616–621. [Google Scholar] [CrossRef]
- Ou, J.Z.; Ge, W.Y.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.C.; Fu, Z.Q.; Chrimes, A.F.; Wlodarski, W.; et al. Physisorption-based charge transfer in two-dimensional SnS2 for selective and reversible NO2 gas sensing. Am. Chem. Soc. Nano 2015, 9, 10313–10323. [Google Scholar]
- Perkins, F.K.; Friedman, A.L.; Cobas, E.; Campbell, P.M.; Jernigan, G.G.; Jonker, B.T. Chemical Vapor Sensing with Monolayer MoS2. Nano Lett. 2013, 13, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yuan, D.; Wang, Y.; Jiao, Z. Monolayer GeS as a potential candidate for NO2 gas sensors and capturers. J. Mater. Chem. C 2018, 6, 8082–8091. [Google Scholar] [CrossRef]
- Rana, C.; Bera, S.R.; Saha, S. Growth of SnS nanoparticles and its ability as ethanol gas sensor. J. Mater. Sci. Mater. Electron. 2019, 30, 2016–2029. [Google Scholar] [CrossRef]
- Barzegar, M.; Tudu, B. Two-dimensional materials for gas sensors: From first discovery to future possibilities. Surf. Innov. 2018, 6, 1–79. [Google Scholar] [CrossRef]
- Vidal, J.; Lany, S.; D’Avezac, M.; Zunger, A.; Zakutayev, A.; Francis, J.; Tate, J. Band-structure, optical properties, and defect physics of the photovoltaic semiconductor SnS. Appl. Phys. Lett. 2012, 100, 032104. [Google Scholar] [CrossRef] [Green Version]
- Sinsermsuksakul, P.; Heo, J.; Noh, W.; Hock, A.S.; Gordon, R.G. Atomic layer deposition of tin monosulfide thin films. Adv. Energy Mater. 2011, 1, 1116–1125. [Google Scholar] [CrossRef]
- Wangperawong, A.; Herron, S.M.; Runser, R.R.; Hagglund, C.; Tanskanen, J.T.; Lee, H.B.R.; Clemens, B.M.; Bent, S.F. Vapor transport deposition and epitaxy of orthorhombic SnS on glass and NaCl substrates. Appl. Phys. Lett. 2013, 103, 052105. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Ramasamy, K.; Kuznetsov, V.L.; Gopal, K.; Malik, M.A.; Raftery, J.; Edwards, P.P.; O’Brien, P. Organotin dithiocarbamates: Single-source precursors for tin sulfide thin films by aerosol assisted chemical vapor deposition (AACVD). Chem. Mater. 2013, 25, 266–276. [Google Scholar] [CrossRef]
- Herron, S.M.; Tanskanen, J.T.; Roelofs, K.E.; Bent, S.F. Highly textured tin(II) sulfide thin films formed from sheetlike nanocrystal inks. Chem. Mater. 2014, 26, 7106–7113. [Google Scholar] [CrossRef]
- Deng, Z.T.; Cao, D.; He, J.; Lin, S.; Lindsay, S.M.; Liu, Y. Solution synthesis of ultrathin single crystalline SnS nanoribbons for photodetectors via phase transition and surface processing. Am. Chem. Soc. Nano 2012, 6, 6197–6207. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lian, G.; Xu, Z.H.; Fu, C.; Lin, Z.J.; Li, L.Y.; Wang, Q.L.; Cui, D.L.; Wong, C.P. Growth of large-size SnS thin crystals driven by oriented attachment and applications to gas sensors and photodetectors. Am. Chem. Soc. Appl. Mater. Interfaces 2016, 8, 9545–9551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Lu, J.; Shen, S.L.; Xu, H.R.; Wang, Q.B. Ultralarge single crystal SnS rectangular nanosheets. Chem. Commun. 2011, 47, 5226–5228. [Google Scholar]
- Thomson, J.W.; Nagashima, K.; Macdonald, P.M.; Ozin, G.A. From sulfur-amine solutions to metal sulfide nanocrystals: Peering into the oleylamine-sulfur black box. J. Am. Chem. Soc. 2011, 133, 5036–5041. [Google Scholar] [CrossRef]
- Du, Y.P.; Yin, Z.Y.; Rui, X.H.; Zeng, Z.Y.; Wu, X.J.; Liu, J.Q.; Zhu, Y.Y.; Zhu, J.X.; Huang, X.; Yan, Q.Y.; et al. A facile, relative green, and inexpensive synthetic approach toward large-scale production of SnS2 nanoplates for high-performance lithium-ion batteries. Nanoscale 2013, 5, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.J.; Cheng, Z.X.; Gao, L.P.; Zhang, Y.; Xu, J.Q.; Zhao, H.B. Facile synthesis of reduced graphene oxide/hexagonal WO3 nanosheets composites with enhanced H2S sensing properties. Sens. Actuators B Chem. 2016, 230, 736–745. [Google Scholar] [CrossRef]
- Jing, Z.H.; Zhan, J.H. Fabrication and gas-sensing properties of porous ZnO nanoplates. Adv. Mater. 2008, 20, 4547–4551. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure App. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Yang, X.Y.; Tian, S.S.; Li, R.; Wang, W.; Zhou, S.M. Use of single-crystalline Bi2S3 nanowires as room temperature ethanol sensor synthesized by hydrothermal approach. Sens. Actuators B Chem. 2017, 241, 210–216. [Google Scholar] [CrossRef]
- Zoolfakar, A.S.; Ahmad, M.Z.; Rani, R.A.; Ou, J.Z.; Balendhran, S.; Zhuiykov, S.; Latham, K.; Wlodarskia, W.; Kalantar-zadeh, K. Nanostructured copper oxides as ethanol vapour sensors. Sens. Actuators B Chem. 2013, 185, 620–627. [Google Scholar] [CrossRef]
- Liu, J.F.; Wang, X.; Peng, Q.; Li, Y.D. Vanadium pentoxide nanobelts: Highly selective and stable ethanol sensor materials. Adv. Mater. 2005, 17, 764–767. [Google Scholar] [CrossRef]
- Wen, Z.; Tian-Mo, L. Gas-sensing properties of SnO2–TiO2-based sensor for volatile organic compound gas and its sensing mechanism. Physical. B 2010, 405, 1345–1348. [Google Scholar] [CrossRef]
- Wang, L.; Deng, J.; Fei, T. Template-free synthesized hollow NiO–SnO2 nanospheres with high gas-sensing performance. Sens. Actuators B Chem. 2012, 164, 90–95. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, W.; Fu, Z.; Ma, M.; Liu, Z.; Xue, Z.; Xu, J.; Zhang, F.; Li, Y. Facile Chemical Bath Synthesis of SnS Nanosheets and Their Ethanol Sensing Properties. Sensors 2019, 19, 2581. https://doi.org/10.3390/s19112581
Shan W, Fu Z, Ma M, Liu Z, Xue Z, Xu J, Zhang F, Li Y. Facile Chemical Bath Synthesis of SnS Nanosheets and Their Ethanol Sensing Properties. Sensors. 2019; 19(11):2581. https://doi.org/10.3390/s19112581
Chicago/Turabian StyleShan, Wei, Zhengqian Fu, Mingsheng Ma, Zhifu Liu, Zhenggang Xue, Jiaqiang Xu, Faqiang Zhang, and Yongxiang Li. 2019. "Facile Chemical Bath Synthesis of SnS Nanosheets and Their Ethanol Sensing Properties" Sensors 19, no. 11: 2581. https://doi.org/10.3390/s19112581





