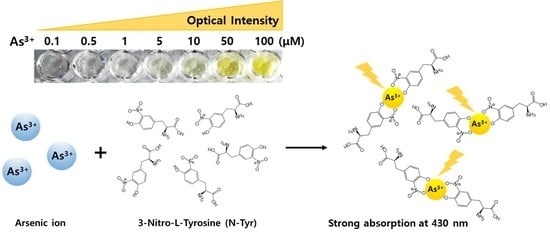
A Simple and Label-Free Detection of As3+ using 3-nitro-L-tyrosine as an As3+-chelating Ligand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Absorbance Measurement
2.3. Infrared (IR) Measurement
2.4. Isothermal Titration Calorimetry (ITC) Measurement
2.5. Cyclic Voltammetry (CV) Measurement
2.6. Spiking test
3. Results
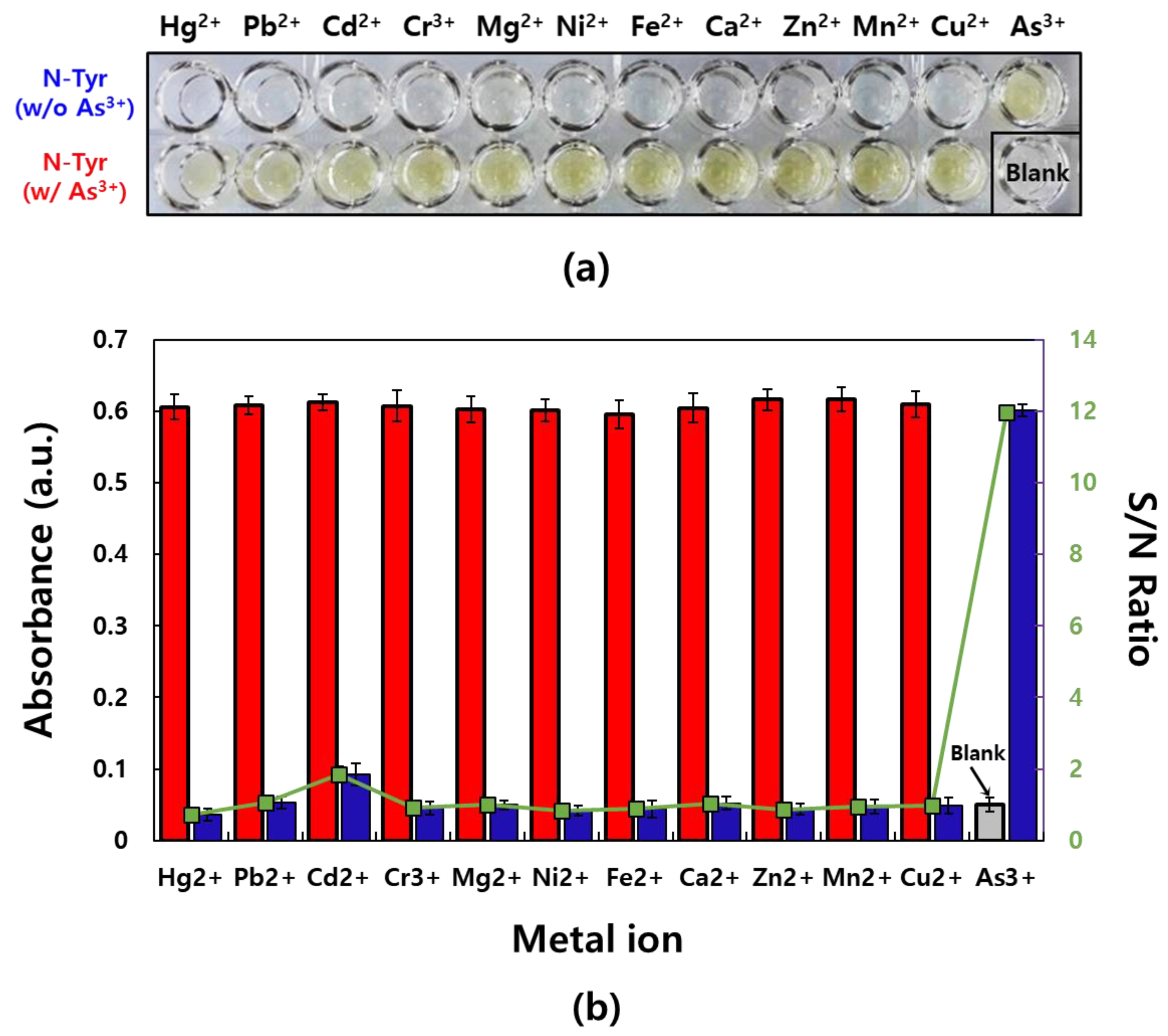
3.1. Selective Reaction Between N-Tyr and As3+
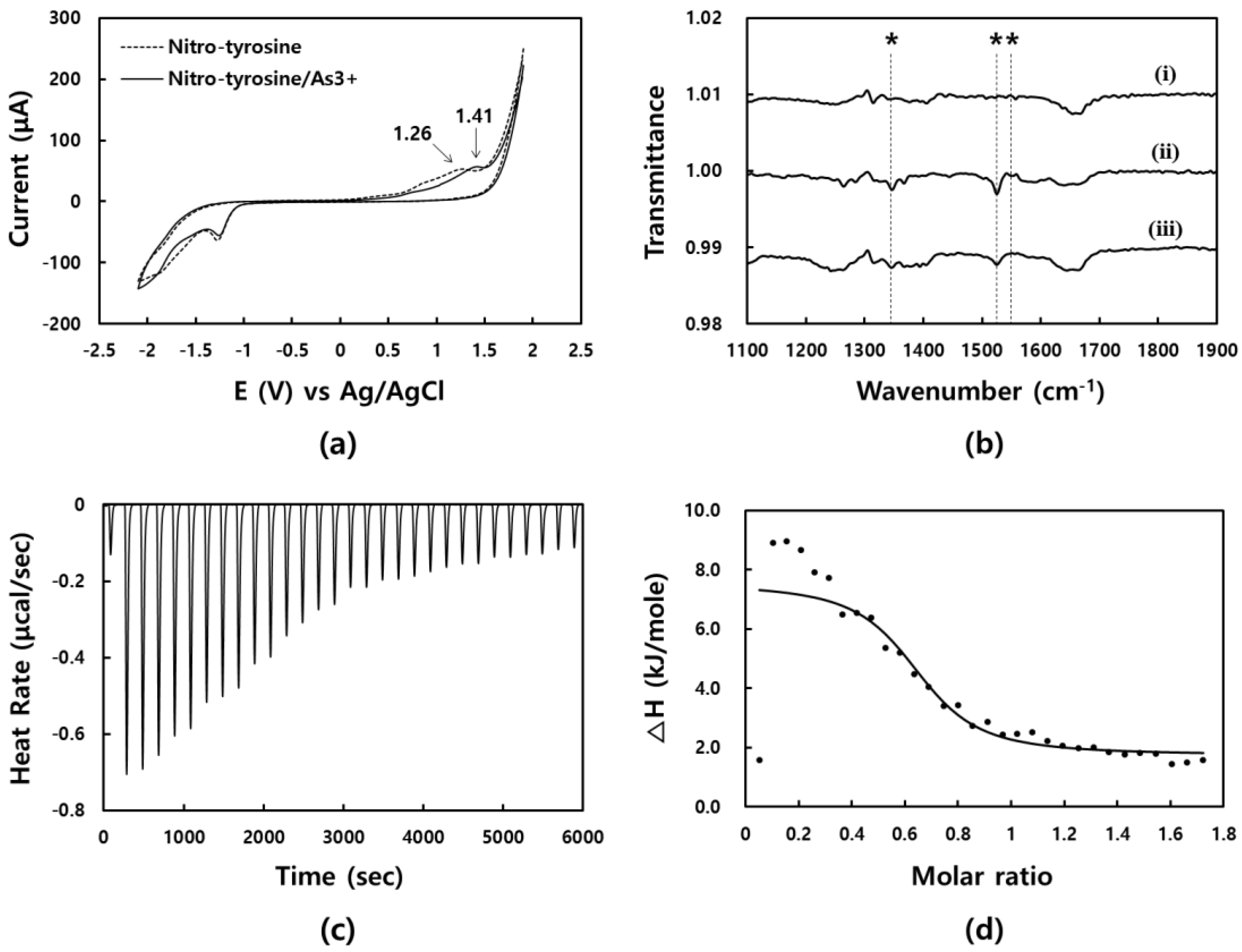
3.2. Investigation of Binding Site, Affinity, and Stoichiometry
3.3. Optimization for As3+ Sensor
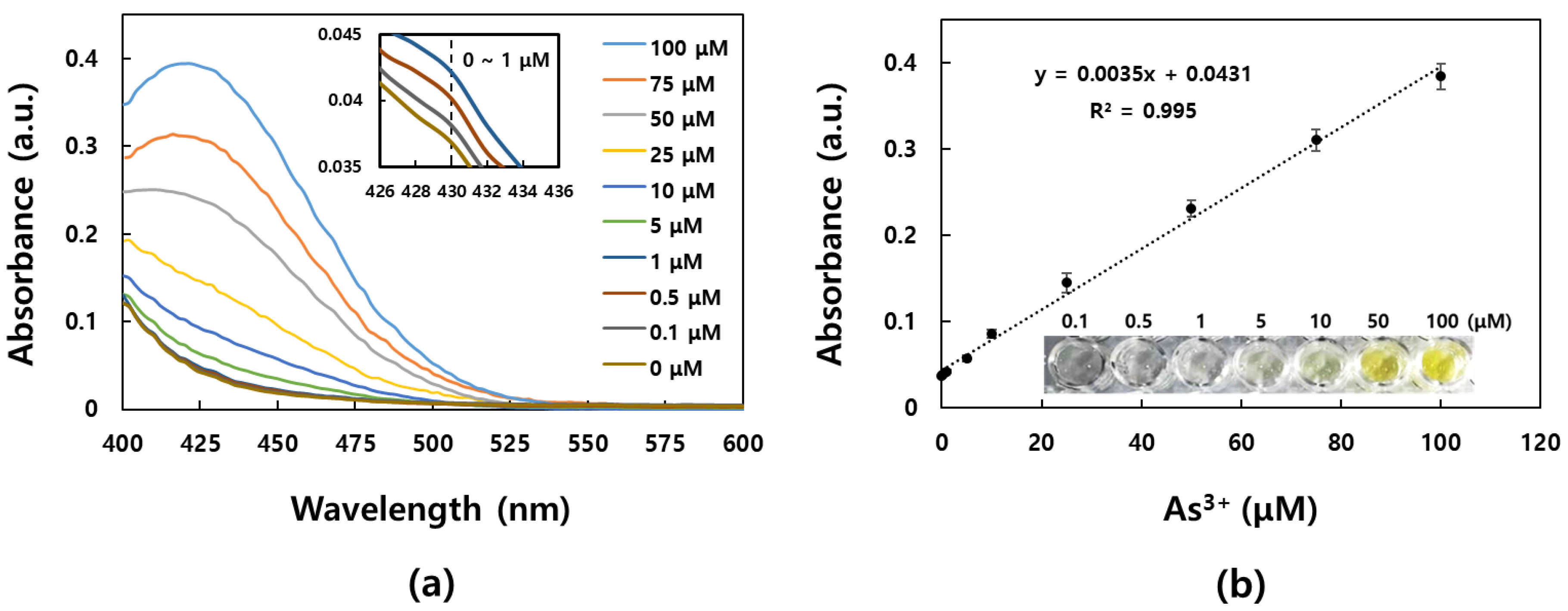
3.4. Quantitative Analysis of As3+
3.5. Application to Analysis of As3+ in Real Samples
4. Conclusions
5. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yogarajah, N.; Tsai, S.S.H. Detection of trace arsenic in drinking water: Challenges and opportunities for microfluidics. Environ. Sci. Water Res. Technol. 2015, 1, 426–447. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Chang, C.-C.; Ho, S.-C.; Chiu, H.-F. Is colon cancer mortality related to arsenic exposure? J. Toxicol. Environ. Health Part A 2008, 71, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ferreccio, C.; Gonzalez, C.; Milosavjlevic, V.; Marshall, G.; Sancha, A.M.; Smith, A.H. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology 2000, 11, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.R.; Allan, A.M. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: A review. Curr. Environ. Health Rep. 2014, 1, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Biyani, M.; Biyani, R.; Tsuchihashi, T.; Takamura, Y.; Ushijima, H.; Tamiya, E.; Biyani, M. DEP-On-Go for simultaneous sensing of multiple heavy metals pollutants in environmental samples. Sensors 2017, 17, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Cullen, W.R.; Reimer, K.J. Arsenic speciation in the environment. Chem. Rev. 1989, 89, 713–764. [Google Scholar] [CrossRef] [Green Version]
- Saha, J.; Roy, A.D.; Dey, D.; Nath, J.; Bhattacharjee, D.; Hussain, S.A. Development of arsenic(v) sensor based on fluorescence resonance energy transfer. Sens. Actuators B-Chem. 2017, 241, 1014–1023. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, R.; Babu, J.N.; Mittal, S. Advances in arsenic biosensor development—A comprehensive review. Biosens. Bioelectron. 2015, 63, 533–545. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, A.K.; Bhardwaj, S.; Singhal, D. Electroanalytical studies of chromone based ionophores for the selective determination of arsenite ion. Electroanalysis 2015, 27, 1166–1175. [Google Scholar] [CrossRef]
- Chowdhury, U.K.; Biswas, B.K.; Chowdhury, T.R.; Samanta, G.; Mandal, B.K.; Basu, G.C.; Chanda, C.R.; Lodh, D.; Saha, K.C.; Mukherjee, S.K.; et al. Groundwater arsenic contamination in bangladesh and west bengal, india. Environ. Health Perspect. 2000, 108, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Habib-Ur-Rehman, M.; Khanam, T.; Sheer, A.; Kebin, Z.; Jianjun, Y. Health risk assessment of different heavy metals dissolved in drinking water. Int. J. Environ. Res. Public Health 2019, 16, 1737–1750. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mulligan, C.N. Effect of natural organic matter on arsenic release from soils and sediments into groundwater. Environ. Geochem. Health 2006, 28, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Sharma, C.; Kumar, P.; Kumar, M.; Bansod, B.K.S.; Nayak, M.K.; Singla, M.L. Selective electrochemical sensing for arsenite using rGO/Fe3O4 nanocomposites. J. Hazard. Mater. 2017, 322, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Behari, J.R.; Prakash, R. Determination of total arsenic content in water by atomic absorption spectroscopy (AAS) using vapour generation assembly (VGA). Chemosphere 2006, 63, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Slejkovec, Z.; van Elteren, J.T.; Byrne, A.R. Determination of arsenic compounds in reference materials by HPLC-(UV)-HG-AFS. Talanta 1999, 49, 619–627. [Google Scholar] [CrossRef]
- Hirata, S.; Toshimitsu, H.; Aihara, M. Determination of arsenic species in marine samples by HPLC-ICP-MS. Anal. Sci. 2006, 22, 39–43. [Google Scholar] [CrossRef]
- Pretty, J.R.; Blubaough, E.A.; Caruso, J.A. Determination of arsenic(III) and selenium(IV) using an on-line anodic stripping voltammetry flow cell with detection by inductively coupled plasma atomic emission spectrometry and inductively coupled plasma mass spectrometry. Anal. Chem. 1993, 65, 3396–3403. [Google Scholar] [CrossRef]
- Petursdottir, A.H.; Gunnlaugsdottir, H.; Jorundsdottir, H.; Mestrot, A.; Krupp, E.M.; Feldmann, J. HPLC-HG-ICP-MS: A sensitive and selective method for inorganic arsenic in seafood. Anal. Bioanal. Chem. 2012, 404, 2185–2191. [Google Scholar] [CrossRef]
- Mulvihill, M.; Tao, A.; Benjauthrit, K.; Arnold, J.; Yang, P. Surface-enhanced Raman spectroscopy for trace arsenic detection in contaminated water. Angew. Chem. Int. Ed. 2008, 47, 6456–6460. [Google Scholar] [CrossRef]
- Forzani, E.S.; Foley, K.; Westerhoff, P.; Tao, N. Detection of arsenic in groundwater using a surface plasmon resonance sensor. Sens. Actuators B-Chem. 2007, 123, 82–88. [Google Scholar] [CrossRef]
- Gong, L.; Du, B.; Pan, L.; Liu, Q.; Yang, K.; Wang, W.; Zhao, H.; Wu, L.; He, Y. Colorimetric aggregation assay for arsenic(III) using gold nanoparticles. Microchim. Acta 2017, 184, 1185–1190. [Google Scholar] [CrossRef]
- Melinte, G.; Hosu, O.; Lettieri, M.; Crisea, C.; Marrazza, G. Electrochemical fingerprint of arsenic (III) by using hybrid nanocomposite-based platforms. Sensors 2019, 19, 2779–2791. [Google Scholar] [CrossRef] [PubMed]
- Sallorente-Méndez, S.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Immobilization of acetylcholinesterase on screen-printed electrodes. application to the determination of arsenic(III). Sensors 2010, 10, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Ezeh, V.C.; Harrop, T.C. A sensitive and selective fluorescence sensor for the detection of arsenic(III) in organic media. Inorg. Chem. 2012, 51, 1213–1215. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.-P.; Wang, Z.-X.; Zhang, L.; Qiu, J.-D. Label-free colorimetric detection of arsenite utilizing G-/T-rich oligonucleotides and unmodified Au nanoparticles. Chem. Eur. J. 2013, 19, 5029–5033. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, X.-Z.; Kuang, L.; Xu, A.-Z.; Liang, R.-P.; Qiu, J.-D. Simple and highly selective detection of arsenite based on the assembly induced fluorescence enhancement of DNA quantum dots. Biosens. Bioelectron. 2017, 94, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Singh, A.K. Dual anion colorimetric and fluorometric sensing of arsenite and cyanide ions. RSC Adv. 2016, 6, 100136–100144. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Yang, C.; Nie, Y.; Zhou, C.; Wang, Y. Design and synthesis of a molecule with aggregation-induced emission effects and its application in the detection of arsenite in groundwater. J. Mater. Chem. C 2017, 5, 3669–3672. [Google Scholar]
- Neupane, L.N.; Hwang, G.W.; Lee, K.-H. Tuning of the selectivity of fluorescent peptidyl bioprobe using aggregation induced emission for heavy metal ions by buffering agents in 100% aqueous solutions. Biosens. Bioelectron. 2017, 92, 179–185. [Google Scholar] [CrossRef]
- Knight, A.S.; Zhou, E.Y.; Fancis, M.B. Development of peptoid-based ligands for the removal of cadmium from biological media. Chem. Sci. 2015, 6, 4042–4048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morsali, A.; Abedini, J. Syntheses and characterization of some new mixed-ligand lead(II) complexes, Pb(en)(CH3COO)X (X=NCS−, ClO− or NO3−); crystal structure of [Pb(en)(CH3COO)(NO3)]n (en=ethane-1,2-diamine). J. Coord. Chem. 2004, 57, 1629–1636. [Google Scholar] [CrossRef]
- Mahajan, P.G.; Bhopate, D.P.; Kolekar, G.B.; Patil, S.R. N-methyl isatin nanoparticles as a novel probe for selective detectionof Cd2+ ion in aqueous medium based on chelation enhanced fluorescence and application to environmental sample. Sens. Actuators B-Chem. 2015, 220, 864–872. [Google Scholar] [CrossRef]
- Ogino, K.; Wang, D.-H. Biomarkers of oxidative/nitrosative stress: An approach to disease prevention. Acta Med. Okayama 2007, 61, 181–189. [Google Scholar] [PubMed]
- Ahsan, H. 3-Nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum. Immunol. 2013, 74, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Protein tyrosine nitration: Biochemical mechanisms and structural basis of functional effects. Acc. Chem. Res. 2013, 46, 550–559. [Google Scholar] [CrossRef]
- Park, J.-H.; Byun, J.-Y.; Yim, S.-Y.; Kim, M.-G. A localized surface plasmon resonance (LSPR)-based, simple, receptor-free and regeneratable Hg2+ detection system. J. Hazard. Mater. 2016, 307, 137–144. [Google Scholar] [CrossRef]
- Flora, S.J.S.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef]
- Bhowmik, D.; Fiorillo, G.; Lombardi, P.; Kumar, G.S. Recognition of human telomeric Gquadruplex DNA by berberine analogs: Effect of substitution at the 9 and 13 positions of the isoquinoline moiety. J. Mol. Recognit. 2105, 28, 722–730. [Google Scholar] [CrossRef]
- Gendusa, R.; Scalia, C.R.; Buscone, S.; Cattoretti, G. Elution of high-affinity (>10−9 KD) antibodies from tissue sections: Clues to the molecular mechanism and use in sequential immunostaining. J. Histochem. Cytochem. 2014, 62, 519–531. [Google Scholar] [CrossRef]


| Spiked (μM) | Measured (Abs.) | Found (μM) | Recovery (%) | RSD (±) | |
|---|---|---|---|---|---|
| Tap water | 0.1 | 0.0365 | 0.096 | 95.55 | 0.0004 |
| 1 | 0.0465 | 1.091 | 109.15 | 0.0008 | |
| 10 | 0.0831 | 9.640 | 96.40 | 0.0015 | |
| Yeongsan River | 0.1 | 0.0397 | 0.104 | 103.93 | 0.0007 |
| 1 | 0.0442 | 1.037 | 103.76 | 0.0007 | |
| 10 | 0.0813 | 9.432 | 94.32 | 0.0024 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-H.; Yeom, G.; Hong, D.; Jo, E.-J.; Park, C.-J.; Kim, M.-G. A Simple and Label-Free Detection of As3+ using 3-nitro-L-tyrosine as an As3+-chelating Ligand. Sensors 2019, 19, 2857. https://doi.org/10.3390/s19132857
Park J-H, Yeom G, Hong D, Jo E-J, Park C-J, Kim M-G. A Simple and Label-Free Detection of As3+ using 3-nitro-L-tyrosine as an As3+-chelating Ligand. Sensors. 2019; 19(13):2857. https://doi.org/10.3390/s19132857
Chicago/Turabian StylePark, Jin-Ho, Gyuho Yeom, Donggu Hong, Eun-Jung Jo, Chin-Ju Park, and Min-Gon Kim. 2019. "A Simple and Label-Free Detection of As3+ using 3-nitro-L-tyrosine as an As3+-chelating Ligand" Sensors 19, no. 13: 2857. https://doi.org/10.3390/s19132857