A limiting Current Oxygen Sensor Constituted of (CeO2)0.95(Y2O3)0.05 as Solid Electrolyte Layer and (CeO2)0.75(ZrO2)0.25 as Dense Diffusion Barrier Layer
Abstract
:1. Introduction
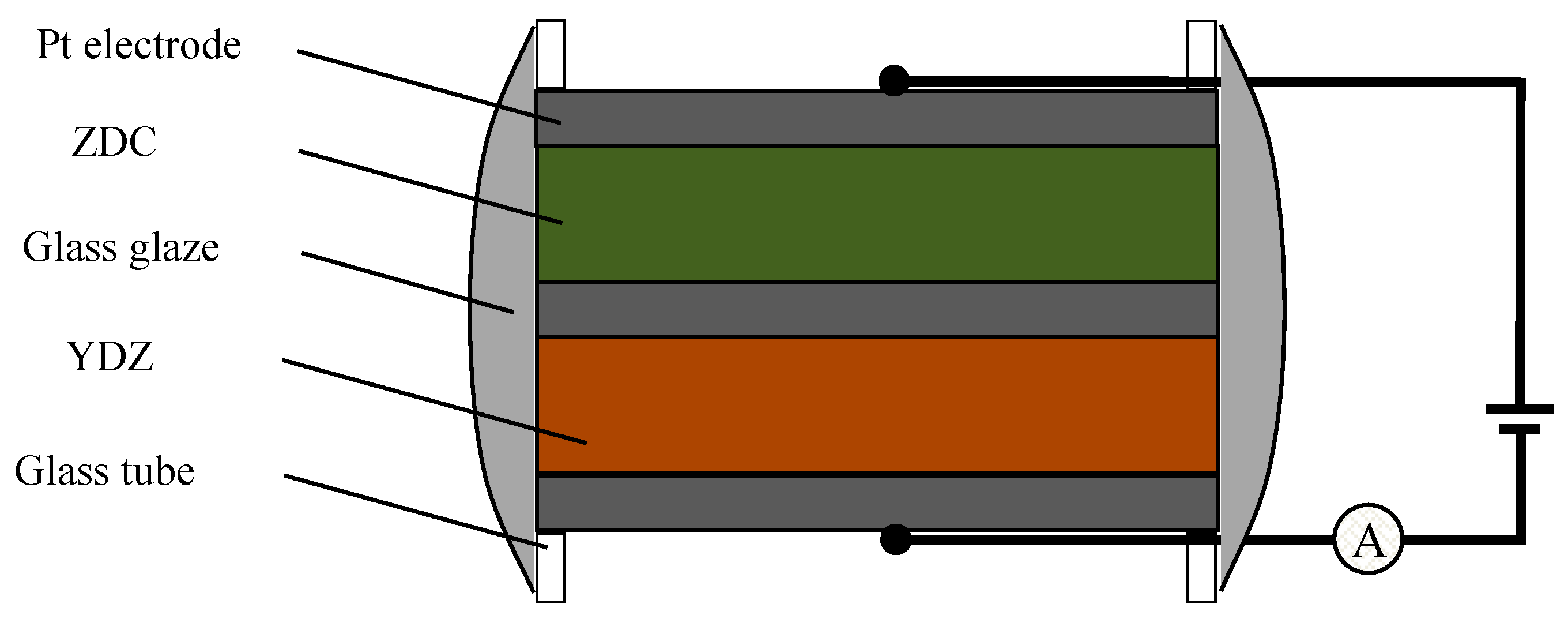
2. Experimental
3. Results and Discussion
3.1. YDC and ZDC
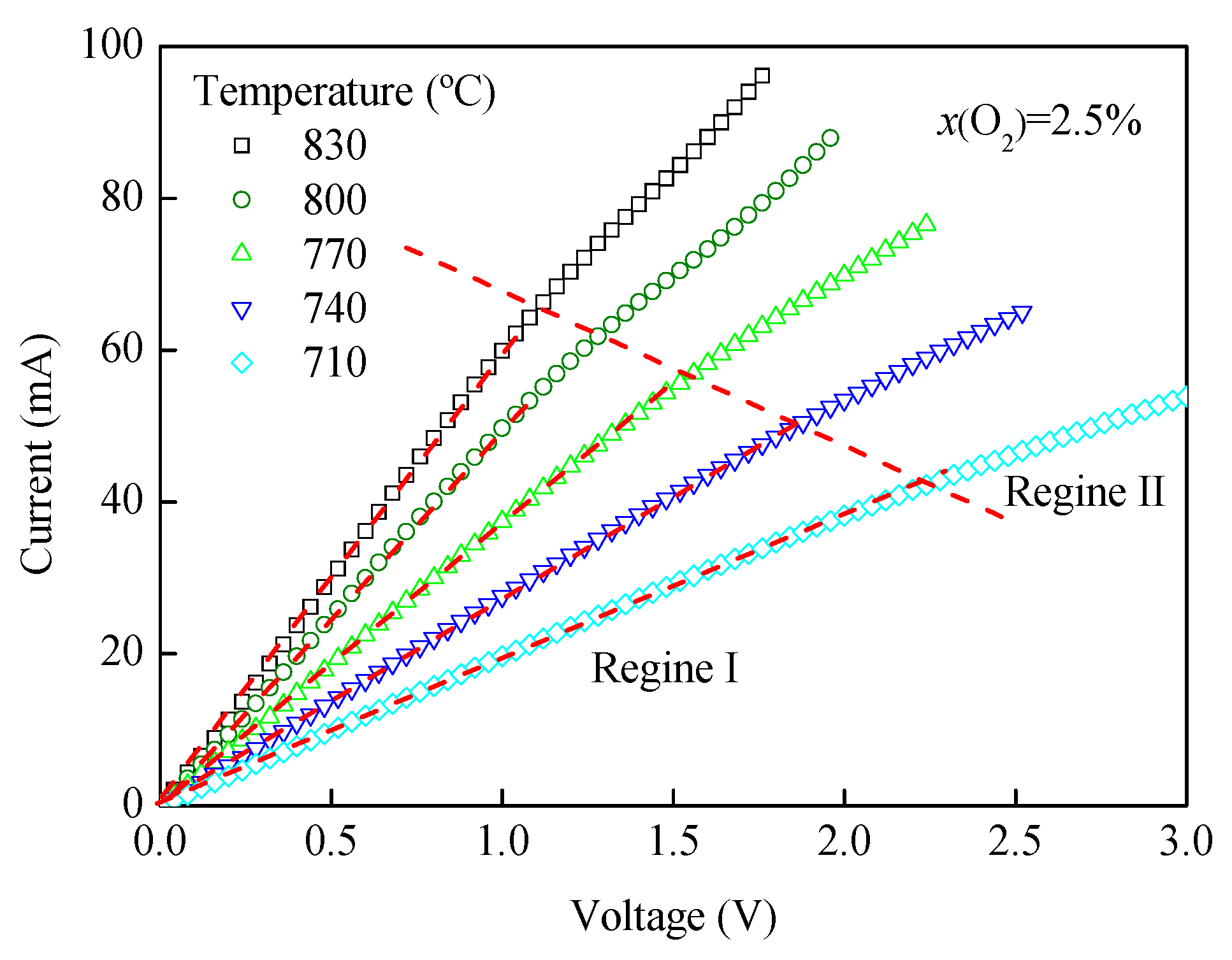
3.2. I-V and T
3.3. I-V and x(O2)
3.4. I-V and p(H2O)
3.5. Long-Term Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, T.; Zhang, X.F.; Yuan, L.; Yu, J.K. A review of high-temperature electrochemical sensors based on stabilized zirconia. Solid State Ion. 2015, 283, 91–102. [Google Scholar] [CrossRef]
- Han, J.X.; Zhou, F.; Bao, J.X.; Wang, X.J.; Song, X.W. A high performance limiting current oxygen sensor with Ce0.8Sm0.2O1.9 electrolyte and La0.8Sr0.2Co0.8Fe0.2O3 diffusion barrier. Electrochim. Acta 2013, 108, 763–768. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.F.; Wang, X.N.; Yu, J.K.; Li, L. A review of zirconia-based solid electrolytes. Ionics 2016, 22, 2249–2262. [Google Scholar] [CrossRef]
- Wang, X.N.; Liu, T.; Yu, J.K.; Mo, Y.C.; Yi, M.Y.; Li, J.Y.; Li, L. Preparation and electrical property of CaZr0.7M0.3O3 (M=Fe, Cr and Co) dense diffusion barrier for application in limiting current oxygen sensor. Sens. Actuator B Chem. 2018, 266, 455–462. [Google Scholar] [CrossRef]
- Brailsford, A.D.; Logothetis, E.M. Selected aspects of gas sensing. Sens. Actuator B Chem. 1998, 52, 195–203. [Google Scholar] [CrossRef]
- Ivers-Tiffée, E.; Härdtl, K.H.; Menesklou, W.; Riegel, J. Principles of solid state oxygen sensors for lean combustion gas control. Electrochim. Acta 2001, 47, 807–814. [Google Scholar] [CrossRef]
- Riegel, J.; Neumann, H.; Wiedenmann, H.M. Exhaust gas sensors for automotive emission control. Solid State Ion. 2002, 152–153, 783–800. [Google Scholar] [CrossRef]
- Xia, C.Y.; Lu, X.C.; Yan, Y.; Wang, T.Z.; Zhang, Z.M. Simulation of the transient response of limiting current oxygen sensor. Sens. Actuator B Chem. 2011, 156, 881–886. [Google Scholar] [CrossRef]
- Liu, T.; Gao, X.; He, B.G.; Yu, J.K. A limiting current oxygen sensor based on LSGM as solid electrolyte and LSGMN (N = Fe, Co) as dense diffusion barrier. J. Mater. Eng. Perform. 2016, 25, 2943–2950. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, T.; Yu, J.K.; Gao, X.; Jin, H.B.; Wang, X.N.; Wang, C. A limiting current oxygen sensor with La0.8Sr0.2(Ga0.8Mg0.2)1-xFexO3-δ dense diffusion barrier. J. Solid State Electrochem. 2017, 21, 1323–1328. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.N.; Zhang, X.F.; Gao, X.; Li, L.; Yu, J.K.; Yin, X.T. A limiting current oxygen sensor prepared by a co-pressing and co-sintering technique. Sens. Actuator B Chem. 2018, 277, 216–223. [Google Scholar] [CrossRef]
- Mo, Y.C.; Liu, T.; Wang, C. A limiting current oxygen sensor based on (La0.4Ce0.6O2-δ)0.96(FeO1.5)0.04 as dense diffusion barrier. Ceram. Int. 2019, 45, 8319–8324. [Google Scholar] [CrossRef]
- Garzon, F.; Raistrick, I.; Brosha, E.; Houlton, R.; Chung, B.W. Dense diffusion barrier limiting current oxygen sensors. Sens. Actuator B Chem. 1998, 50, 125–130. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Liu, M.L.; Balko, E. A new type of amperometric oxygen sensor based on a mixed-conducting composite membrane. Sens. Actuator B Chem. 2001, 72, 35–40. [Google Scholar] [CrossRef]
- Gao, X.; Liu, T.; Yu, J.K.; Li, L. Limiting current oxygen sensor based on La0.8Sr0.2Ga0.8Mg0.2O3-δ as both dense diffusion barrier and solid electrolyte. Ceram. Int. 2017, 43, 6329–6332. [Google Scholar] [CrossRef]
- Wang, C.Z. Solid Electrolytes and Chemical Sensors; Metallurgical Industry Press: Beijing, China, 2000. [Google Scholar]
- Wang, X.N.; Liu, T.; Wang, C.; Yu, J.K.; Li, L. Crystal structure, microstructure, thermal expansion and electrical conductivity of CeO2-ZrO2 solid solution. Adv. Appl. Ceram. 2017, 116, 477–481. [Google Scholar] [CrossRef]
- Wang, X.N.; Liu, T.; Yu, J.K.; Li, L.; Zhang, X.F. A new application of CexZr1-xO2 as dense diffusion barrier in limiting current oxygen sensor. Sens. Actuator B Chem. 2019, 285, 391–397. [Google Scholar] [CrossRef]
- Gokcen, N.A. Vapor pressure of water above saturated lithium chloride solution. J. Am. Chem. Soc. 1951, 73, 3789–3790. [Google Scholar] [CrossRef]
- Chung, T.W.; Luo, C.M. Vapor pressures of the aqueous desiccants. J. Chem. Eng. Data 1999, 44, 1024–1027. [Google Scholar] [CrossRef]
- Kolár, P.; Nakata, H.; Tsuboi, A.; Wang, P.; Anderko, A. Measurement and modeling of vapor-liquid equilibria at high salt concentrations. Fluid Phase Equilib. 2005, 228–229, 493–497. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Cryst. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Usui, T.; Asada, A.; Nakazawa, M.; Osanai, H. Gas polarographic oxygen sensor using an oxygen / zirconia electrolyte. J. Electrochem. Soc. 1989, 136, 534–542. [Google Scholar] [CrossRef]
- Gao, X.; Liu, T.; Zhang, X.F.; He, B.G.; Yu, J.K. Properties of limiting current oxygen sensor with La0.8Sr0.2Ga0.8Mg0.2O3-δ solid electrolyte and La0.8Sr0.2(Ga0.8Mg0.2)1-xCrxO3-δ dense diffusion barrier. Solid State Ion. 2017, 304, 135–144. [Google Scholar] [CrossRef]
- Mari, C.M.; Rabotti, G. Humidity determination by solid state limiting current sensor. Solid State Ion. 1999, 124, 309–315. [Google Scholar] [CrossRef]
- Akasaka, S. Thin film YSZ-based limiting current-type oxygen and humidity sensor on thermally oxidized silicon substrates. Sens. Actuator B Chem. 2016, 236, 499–505. [Google Scholar] [CrossRef]
- Wang, X.N.; Liu, T.; Yu, Y.K.; Li, L. The effect of Fe doping on the electrical conductivities of CaZrO3 and its sensing performance in limiting current oxygen sensor. J. Alloy. Compd. 2018, 768, 838–846. [Google Scholar] [CrossRef]
- Wang, X.N.; Liu, T.; Yu, Y.K. An application of (4YSZ)0.93(Fe2O3)0.07 in limiting current oxygen sensor. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, T.; Yu, J. A limiting Current Oxygen Sensor Constituted of (CeO2)0.95(Y2O3)0.05 as Solid Electrolyte Layer and (CeO2)0.75(ZrO2)0.25 as Dense Diffusion Barrier Layer. Sensors 2019, 19, 3511. https://doi.org/10.3390/s19163511
Wang X, Liu T, Yu J. A limiting Current Oxygen Sensor Constituted of (CeO2)0.95(Y2O3)0.05 as Solid Electrolyte Layer and (CeO2)0.75(ZrO2)0.25 as Dense Diffusion Barrier Layer. Sensors. 2019; 19(16):3511. https://doi.org/10.3390/s19163511
Chicago/Turabian StyleWang, Xiangnan, Tao Liu, and Jingkun Yu. 2019. "A limiting Current Oxygen Sensor Constituted of (CeO2)0.95(Y2O3)0.05 as Solid Electrolyte Layer and (CeO2)0.75(ZrO2)0.25 as Dense Diffusion Barrier Layer" Sensors 19, no. 16: 3511. https://doi.org/10.3390/s19163511



