Comparative Evaluation of the Autonomic Response to Cognitive and Sensory Stimulations through Wearable Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Signal Acquisition
- (i)
- Baseline (3 min): basal measurement. The subject, seated in a comfortable chair, was asked to relax during this phase;
- (ii)
- Task I (4 min): olfactory task I. The subject, comfortably seated, was asked to sniff a battery of six odors (as detailed in the following section);
- (iii)
- Task II (5–10 min, depending on the subject): cognitive task. The subject, comfortably seated, was asked to complete a cognitive task administered through a user interface on the laptop (later detailed);
- (iv)
- Task III (4 min): olfactory task II. Same procedure as Task I. The olfactory test was performed before and after the cognitive task in order to assess whether the prior administration of a cognitive task would affect the ANS response to the olfactory task;
- (v)
- Recovery (3 min): post-task basal measurement. The subject was asked to relax during this phase, similarly to the baseline.
2.3. Olfactory Stimulation and Related Questionnaires
2.4. Cognitive Stressor Administration
2.5. Signal Analysis
2.5.1. ECG
2.5.2. GSR
2.6. Statistical Analysis
3. Results
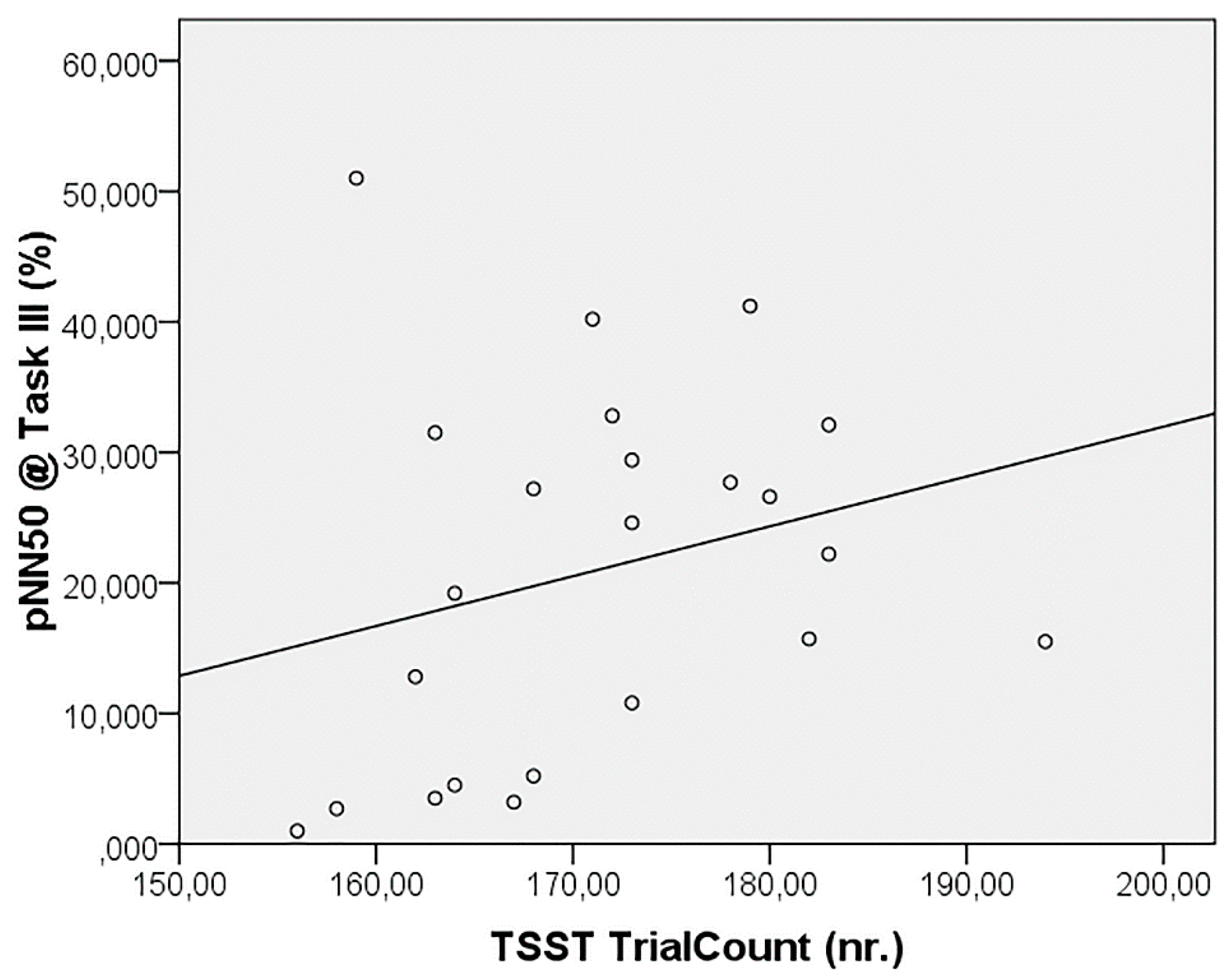
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Charmandari, E.; Kino, T.; Souvatzoglou, E.; Chrousos, G.P. Pediatric stress: hormonal mediators and human development. Horm. Res. 2003, 59, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. Stressors, Stress, and Neuroendocrine Integration of the Adaptive Response: The 1997 Hans Selye Memorial Lecture. Ann. New York Acad. Sci. 1998, 851, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Croy, I.; Laqua, K.; Süß, F.; Joraschky, P.; Ziemssen, T.; Hummel, T. The sensory channel of presentation alters subjective ratings and autonomic responses toward disgusting stimuli—Blood pressure, heart rate and skin conductance in response to visual, auditory, haptic and olfactory presented disgusting stimuli. Front. Hum. Neurosci. 2013, 7, 510. [Google Scholar] [CrossRef] [PubMed]
- Tonacci, A.; Calderoni, S.; Billeci, L.; Maestro, S.; Fantozzi, P.-L.; Ciuccoli, F.; Morales, M.A.; Narzisi, A.; Muratori, F. Autistic traits impact on olfactory processing in adolescent girls with Anorexia Nervosa restricting type. Psychiatry Res. Neuroimaging 2019, 274, 20–26. [Google Scholar] [CrossRef]
- Forte, G.; Favieri, F.; Casagrande, M. Heart Rate Variability and Cognitive Function: A Systematic Review. Front. Mol. Neurosci. 2019, 13, 710. [Google Scholar] [CrossRef]
- Billeci, L.; Tonacci, A.; Tartarisco, G.; Narzisi, A.; Di Palma, S.; Corda, D.; Baldus, G.; Cruciani, F.; Anzalone, S.M.; Calderoni, S.; et al. An Integrated Approach for the Monitoring of Brain and Autonomic Response of Children with Autism Spectrum Disorders during Treatment by Wearable Technologies. Front. Mol. Neurosci. 2016, 10, 799. [Google Scholar] [CrossRef]
- Di Palma, S.; Tonacci, A.; Narzisi, A.; Domenici, C.; Pioggia, G.; Muratori, F.; Billeci, L. Monitoring of autonomic response to sociocognitive tasks during treatment in children with Autism Spectrum Disorders by wearable technologies: A feasibility study. Comput. Boil. Med. 2017, 85, 143–152. [Google Scholar] [CrossRef]
- Mackersie, C.L.; Calderon-Moultrie, N. Autonomic Nervous System Reactivity During Speech Repetition Tasks: Heart Rate Variability and Skin Conductance. Ear Hearing 2016, 37, 118S–125S. [Google Scholar] [CrossRef]
- Tonacci, A.; Billeci, L.; Sansone, F.; Masci, A.; Pala, A.P.; Domenici, C.; Conte, R. An Innovative, Unobtrusive Approach to Investigate Smartphone Interaction in Nonaddicted Subjects Based on Wearable Sensors: A Pilot Study. Medicina 2019, 55, 37. [Google Scholar] [CrossRef]
- Kobal, G.; Hummel, T.; Sekinger, B.; Barz, S.; Roscher, S.; Wolf, S. “Sniffin’ sticks”: screening of olfactory performance. Rhinol. J. 1996, 34, 222–226. [Google Scholar]
- Sezille, C.; Fournel, A.; Rouby, C.; Rinck, F.; Bensafi, M. Hedonic appreciation and verbal description of pleasant and unpleasant odors in untrained, trainee cooks, flavorists, and perfumers. Front. Psychol. 2014, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Rumeau, C.; Nguyen, D.; Jankowski, R. How to assess olfactory performance with the Sniffin’ Sticks test ®. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Kennedy, P.J.; Dockray, S.; Cryan, J.F.; Dinan, T.G.; Clarke, G. The Trier Social Stress Test: Principles and practice. Neurobiol. Stress 2016, 6, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef]
- Billeci, L.; Tonacci, A.; Narzisi, A.; Manigrasso, Z.; Varanini, M.; Fulceri, F.; Lattarulo, C.; Calderoni, S.; Muratori, F. Heart Rate Variability During a Joint Attention Task in Toddlers With Autism Spectrum Disorders. Front. Physiol. 2018, 9, 467. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Heal. 2017, 5, 258. [Google Scholar] [CrossRef]
- Toichi, M.; Sugiura, T.; Murai, T.; Sengoku, A. A new method of assessing cardiac autonomic function and its comparison with spectral analysis and coefficient of variation of R–R interval. J. Auton. Nerv. Syst. 1997, 62, 79–84. [Google Scholar] [CrossRef]
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoustics 1967, 15, 70–73. [Google Scholar] [CrossRef] [Green Version]
- Benedek, M.; Kaernbach, C. A continuous measure of phasic electrodermal activity. J. Neurosci. Methods 2010, 190, 80–91. [Google Scholar] [CrossRef] [Green Version]
- Bach, D.R.; Friston, K.J.; Dolan, R.J. Analytic measures for quantification of arousal from spontaneous skin conductance fluctuations. Int. J. Psychophysiol. 2010, 76, 52–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensafi, M.; Rouby, C.; Farget, V.; Bertrand, B.; Vigouroux, M.; Holley, A. Influence of affective and cognitive judgments on autonomic parameters during inhalation of pleasant and unpleasant odors in humans. Neurosci. Lett. 2002, 319, 162–166. [Google Scholar] [CrossRef]
- Boesch, M.; Sefidan, S.; Ehlert, U.; Annen, H.; Wyss, T.; Steptoe, A.; La Marca, R. Mood and autonomic responses to repeated exposure to the Trier Social Stress Test for Groups (TSST-G). Psychoneuroendocrinology 2014, 43, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellhammer, J.; Schubert, M. The physiological response to Trier Social Stress Test relates to subjective measures of stress during but not before or after the test. Psychoneuroendocrinology 2012, 37, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Rohleder, N.; Gaab, J.; Berger, S.; Jud, A.; Kirschbaum, C.; Ehlert, U. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int. J. Psychophysiol. 2005, 55, 333–342. [Google Scholar] [CrossRef]
- Giuliano, R.J.; Karns, C.M.; Bell, T.A.; Petersen, S.; Skowron, E.A.; Neville, H.J.; Pakulak, E. Parasympathetic and sympathetic activity are associated with individual differences in neural indices of selective attention in adults. Psychophysiology 2018, 55, e13079. [Google Scholar] [CrossRef]

| Time Domain | ||
| Feature | Acronym | Physiological Significance |
| Heart rate | HR | Number of heart pounds per time unit, expressed in bpm. The HR is normally related to the SNS activation of the ANS |
| Standard deviation of normal-to-normal R–R intervals | SDNN | Measure of heart rate variability (HRV), expressed in ms. The SDNN is normally affected by both SNS and PNS components, but largely depends on testing conditions, with the PNS activity being predominant during short-term resting recordings [17] |
| Changes in successive normal sinus (NN) intervals exceeding 50 ms | pNN50 | Measure of HRV, expressed as a percentage. Overall, pNN50 is closely correlated to the PNS activity [17] |
| Cardiac sympathetic index | CSI | Measure of SNS activity derived from the Lorenz plot analysis [18] |
| Frequency Domain | ||
| Feature | Acronym | Physiological Significance |
| Normalized component of the power spectral density of the ECG signal at low frequency (0.04–0.15 Hz) | nLF | nLF is related to both SNS and PNS activity, though, in resting conditions, the LF band reflects baroreflex activity (see [17] and related works) |
| Normalized component of the power spectral density of the ECG spectrum at high frequency (0.15–0.4 Hz) | nHF | nHF is related to PNS activity, though its direct relationship with the vagal tone is not always evident |
| Low-to-high frequency component of the power spectral density of the ECG spectrum | LF/HF | To a first approximation, LF/HF ratio is considered a marker of the balance between SNS and PNS activity, despite this assumption is not necessarily true, especially during short, resting recordings, where, as stated, the LF often reflects baroreflex activity |
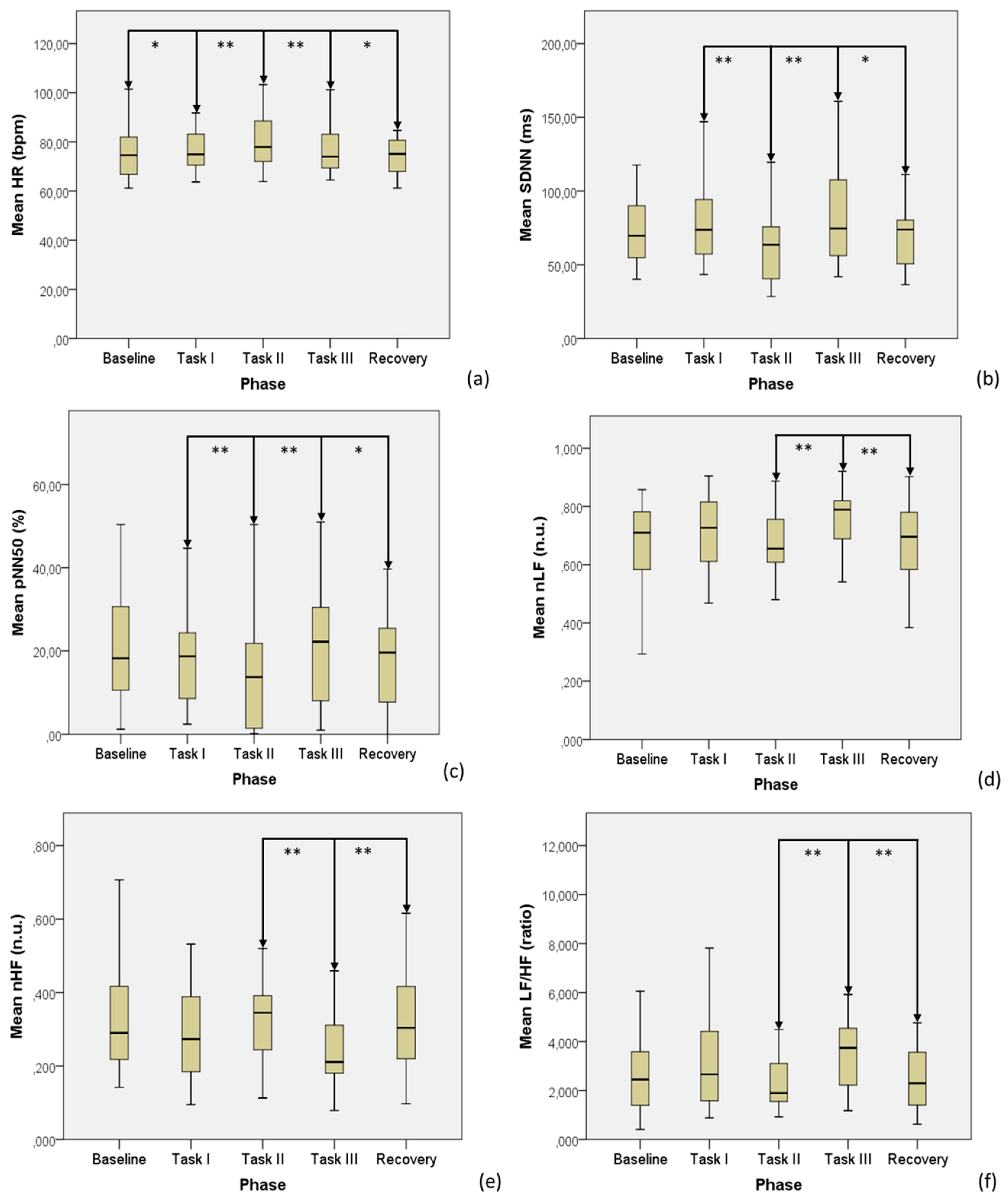
| Feature | Baseline | Task I | Task II | Task III | Recovery | p-Value (Friedman’s Test) | p-Value B vs. T I | p-Value T I vs. T II | p-Value T II vs. T III | p-Value T III vs. R |
|---|---|---|---|---|---|---|---|---|---|---|
| HR, bpm | 75.7 ± 10.6 | 77.1 ± 9.4 | 81.1 ± 11.8 | 76.3 ± 8.7 | 74.9 ± 9.4 | <0.001 ** | 0.040 * | 0.003 ** | <0.001 ** | 0.014 * |
| SDNN, ms | 74.1 ± 28.4 | 79.0 ± 28.0 | 62.5 ± 28.3 | 82.8 ± 31.7 | 74.2 ± 31.4 | <0.001 ** | 0.094 | <0.001 ** | <0.001 ** | 0.016 * |
| pNN50, % | 22.3 ± 16.6 | 19.8 ± 14.4 | 14.4 ± 13.0 | 20.9 ± 14.1 | 18.4 ± 14.3 | <0.001 ** | 0.077 | 0.001 ** | <0.001 ** | 0.021 * |
| CSI, ratio | 2.95 ± 0.72 | 3.19 ± 0.73 | 3.29 ± 1.18 | 3.11 ± 0.66 | 3.18 ± 0.70 | 0.352 | N/A | N/A | N/A | N/A |
| nLF, n.u. | 0.67 ± 0.16 | 0.71 ± 0.13 | 0.67 ± 0.11 | 0.75 ± 0.10 | 0.68 ± 0.14 | <0.001 ** | 0.465 | 0.117 | 0.001 ** | 0.005 ** |
| nHF, n.u. | 0.33 ± 0.16 | 0.29 ± 0.13 | 0.33 ± 0.11 | 0.25 ± 0.10 | 0.32 ± 0.14 | <0.001 ** | 0.465 | 0.117 | 0.001 ** | 0.005 ** |
| LF/HF, ratio | 2.69 ± 1.54 | 3.26 ± 2.20 | 2.61 ± 1.84 | 3.82 ± 2.41 | 3.02 ± 2.46 | <0.001 ** | 0.563 | 0.068 | 0.001 ** | 0.012 * |
| GSR Tonic, µS | 3.46 ± 2.90 | 4.92 ± 4.93 | 5.93 ± 4.68 | 6.73 ± 5.18 | 6.53 ± 5.36 | <0.001 ** | <0.001 ** | <0.001 ** | 0.001 ** | 0.031 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonacci, A.; Billeci, L.; Burrai, E.; Sansone, F.; Conte, R. Comparative Evaluation of the Autonomic Response to Cognitive and Sensory Stimulations through Wearable Sensors. Sensors 2019, 19, 4661. https://doi.org/10.3390/s19214661
Tonacci A, Billeci L, Burrai E, Sansone F, Conte R. Comparative Evaluation of the Autonomic Response to Cognitive and Sensory Stimulations through Wearable Sensors. Sensors. 2019; 19(21):4661. https://doi.org/10.3390/s19214661
Chicago/Turabian StyleTonacci, Alessandro, Lucia Billeci, Elisa Burrai, Francesco Sansone, and Raffaele Conte. 2019. "Comparative Evaluation of the Autonomic Response to Cognitive and Sensory Stimulations through Wearable Sensors" Sensors 19, no. 21: 4661. https://doi.org/10.3390/s19214661





