Synergistic Myoelectrical Activities of Forearm Muscles Improving Robust Recognition of Multi-Fingered Gestures
Abstract
:1. Introduction
2. Methods
2.1. Participants
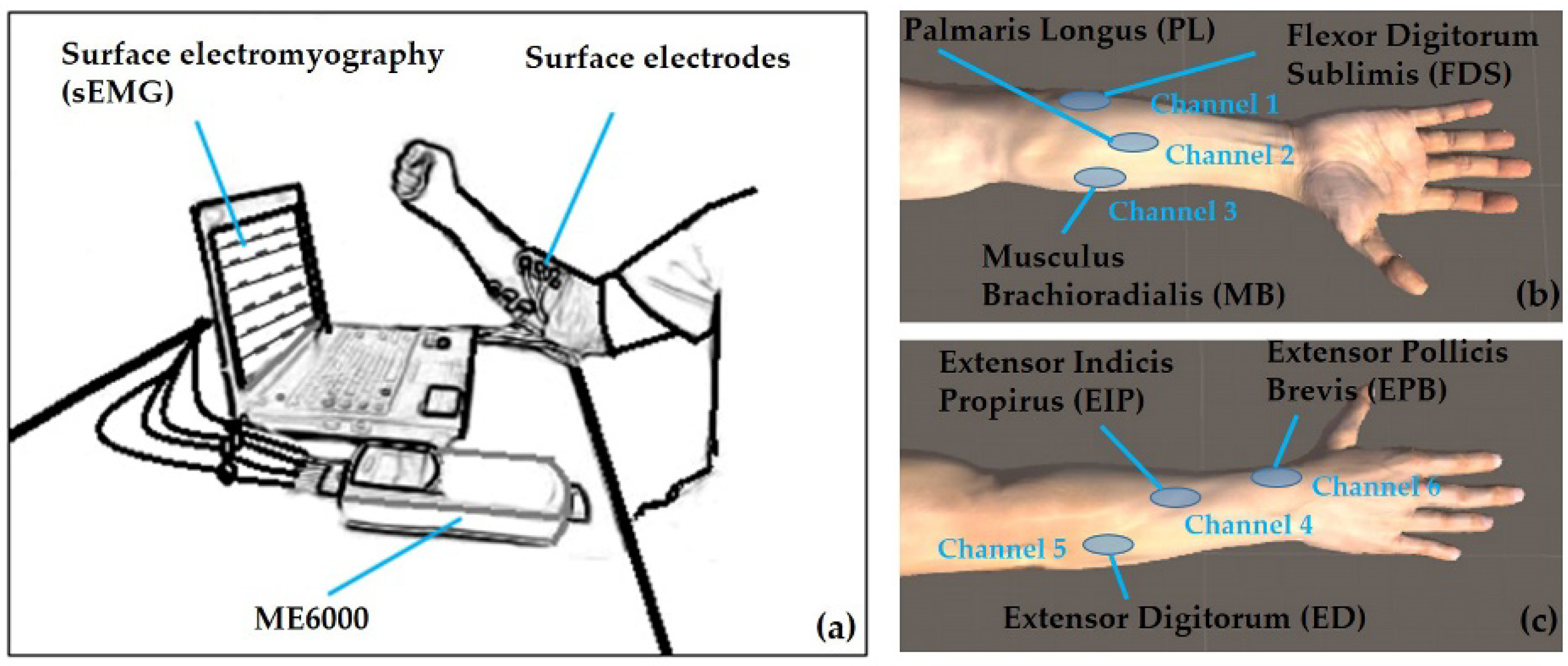
2.2. Experimental Protocol
2.3. Electromyography
2.4. Data Analysis
2.4.1. EMG Preprocessing
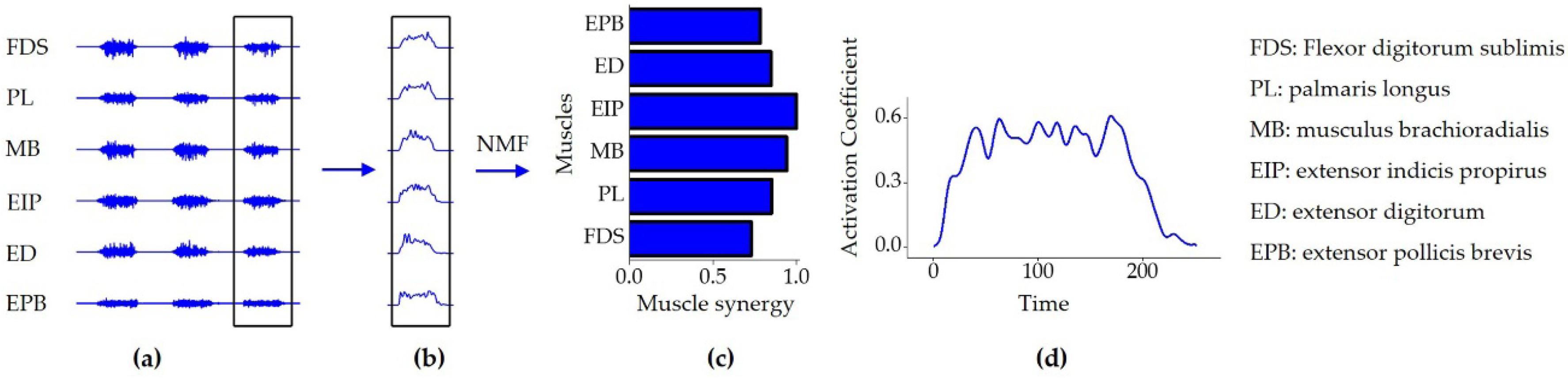
2.4.2. Non-Negative Matrix Factorization (NMF) Algorithm
2.4.3. EMG Feature Vector Construction and Classification
2.4.4. The Distance of Different Gesture Feature Sets
3. Results
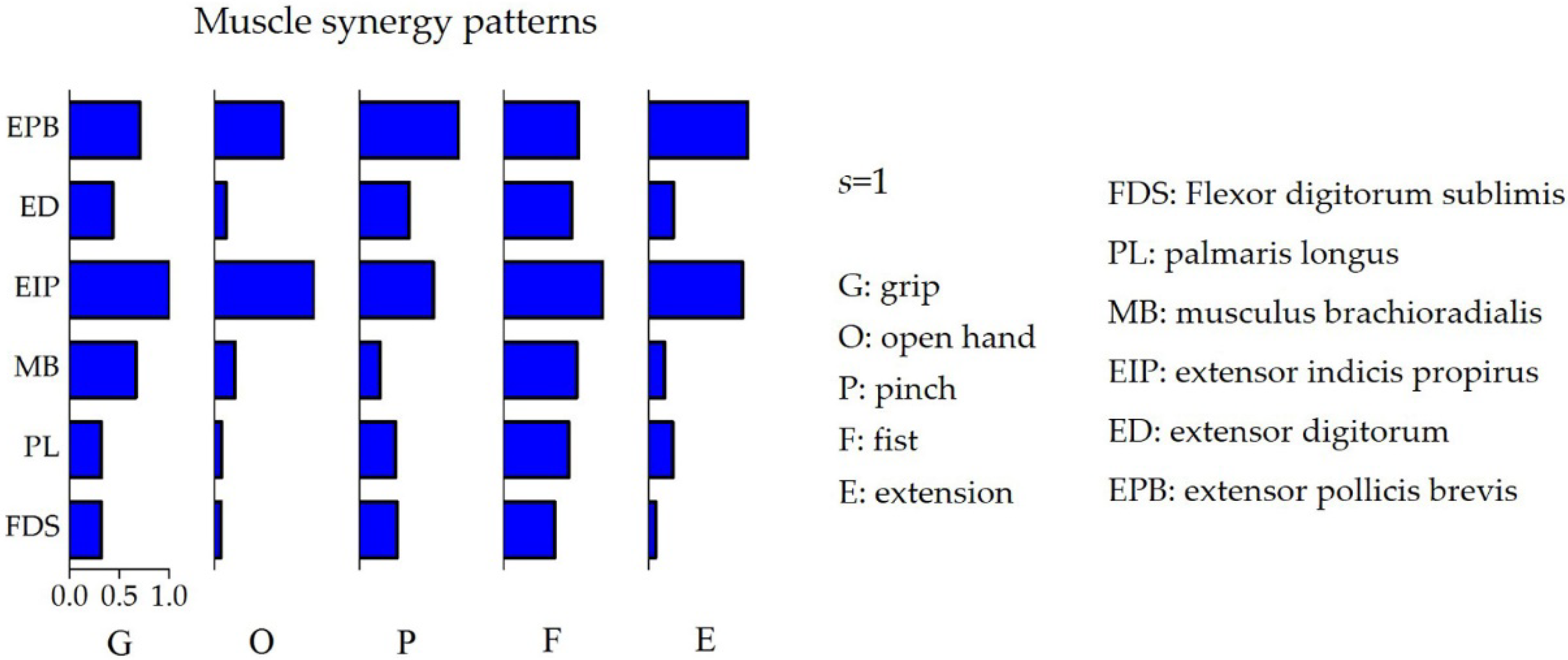
3.1. Selection of the Optimal Number of Muscle Synergies
3.2. Clustering Effect of the Feature in Feature Space
3.3. Classification Performance of Features
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nadzri, A.A.B.; Ahmad, S.A.; Marhaban, M.H.; Jaafar, H. Characterization of surface electromyography using time domain features for determining hand motion and stages of contraction. Aust. Phys. Eng. Sci. Med. 2014, 37, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Li, Y.; Lantz, V.; Wang, K.; Yang, J. A Framework for Hand Gesture Recognition Based on Accelerometer and EMG Sensors. IEEE Trans. Syst. Man Cybern. Part A Syst. Hum. 2011, 41, 1064–1076. [Google Scholar] [CrossRef]
- Kim, J.; Mastnik, S.; André, E. EMG-based hand gesture recognition for realtime biosignal interfacing. In Proceedings of the International Conference on Intelligent User Interfaces, Gran Canaria, Spain, 13–16 January 2008. [Google Scholar]
- Yang, X.D.; Chen, X.A.; Cao, X.A.; Wei, S.J.; Zhang, X. Chinese Sign Language Recognition Based on an Optimized Tree-Structure Framework. IEEE J. Biomed. Health 2017, 21, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chen, X.; Liu, A.P.; Peng, H. A Novel Phonology- and Radical-Coded Chinese Sign Language Recognition Framework Using Accelerometer and Surface Electromyography Sensors. Sensors 2015, 15, 23303–23324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardis, D.; Barsotti, M.; Loconsole, C.; Solazzi, M.; Troncossi, M.; Mazzotti, C.; Castelli, V.P.; Procopio, C.; Lamola, G.; Chisari, C.; et al. An EMG-Controlled Robotic Hand Exoskeleton for Bilateral Rehabilitation. IEEE Trans. Haptics 2015, 8, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.A.M.; Coelho, A.L.V.; Madeo, R.C.B.; Peres, S.M. Classification of electromyography signals using relevance vector machines and fractal dimension. Neural Comput. Appl. 2016, 27, 791–804. [Google Scholar] [CrossRef]
- Young, A.J.; Hargrove, L.J.; Kuiken, T.A. The Effects of Electrode Size and Orientation on the Sensitivity of Myoelectric Pattern Recognition Systems to Electrode Shift. IEEE Trans. Biomed. Eng. 2011, 58, 2537–2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheme, E.; Fougner, A.; Stavdahl, O.; Chan, A.D.C.; Englehart, K. Examining the Adverse Effects of Limb Position on Pattern Recognition Based Myoelectric Control. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 6337–6340. [Google Scholar]
- Chen, X.; Wang, Z.J. Pattern recognition of number gestures based on a wireless surface EMG system. Biomed. Signal Process. Control 2013, 8, 184–192. [Google Scholar] [CrossRef]
- Tang, L.; Li, F.; Cao, S.; Zhang, X.; Wu, D.; Chen, X. Muscle synergy analysis in children with cerebral palsy. J. Neural Eng. 2015, 12, 046017. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liao, Y.J.; Wu, X.Y.; Chen, L.; Xiong, Q.L.; Gao, Z.X.; Zheng, X.L.; Li, G.L.; Hou, W.S. Non-Uniform Sample Assignment in Training Set Improving Recognition of Hand Gestures Dominated with Similar Muscle Activities. Front. Neurorobot. 2018, 12, 3. [Google Scholar] [CrossRef]
- Englehart, K.; Hudgins, B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 2003, 50, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Khezri, M.; Jahed, M. Real-time intelligent pattern recognition algorithm for surface EMG signals. Biomed. Eng. Online 2007, 6, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Y.J.; Zhang, X.F.; Zhang, Y.T.; Li, G.L. A novel channel selection method for multiple motion classification using high-density electromyography. Biomed. Eng. Online 2014, 13, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huebner, A.; Faenger, B.; Schenk, P.; Scholle, H.C.; Anders, C. Alteration of Surface EMG amplitude levels of five major trunk muscles by defined electrode location displacement. J. Electromyogr. Kinesiol. 2015, 25, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Jiang, N.; Rehbaum, H.; Holobar, A.; Graimann, B.; Dietl, H.; Aszmann, O.C. The Extraction of Neural Information from the Surface EMG for the Control of Upper-Limb Prostheses: Emerging Avenues and Challenges. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; van der Smagt, P. Surface EMG in advanced hand prosthetics. Biol. Cybern. 2009, 100, 35–47. [Google Scholar] [CrossRef]
- D’Avella, A.; Portone, A.; Fernandez, L.; Lacquaniti, F. Control of fast-reaching movements by muscle synergy combinations. J. Neurosci. 2006, 26, 7791–7810. [Google Scholar] [CrossRef]
- D’Avella, A.; Saltiel, P.; Bizzi, E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat. Neurosci. 2003, 6, 300–308. [Google Scholar] [CrossRef]
- Isa, T.; Ohki, Y.; Alstermark, B.; Pettersson, L.G.; Sasaki, S. Direct and indirect cortico-motoneuronal pathways and control of Hand/Arm movements. Physiology 2007, 22, 145–152. [Google Scholar] [CrossRef]
- Drew, T.; Kalaska, J.; Krouchev, N. Muscle synergies during locomotion in the cat: A model for motor cortex control. J. Physiol. Lond. 2008, 586, 1239–1245. [Google Scholar] [CrossRef]
- Cheung, V.C.K.; Turolla, A.; Agostini, M.; Silvoni, S.; Bennis, C.; Kasi, P.; Paganoni, S.; Bonato, P.; Bizzi, E. Muscle synergy patterns as physiological markers of motor cortical damage. Proc. Natl. Acad. Sci. USA 2012, 109, 14652–14656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvietti, G. Replicating Human Hand Synergies onto Robotic Hands: A Review on Software and Hardware Strategies. Front. Neurorobot. 2018, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.X.; Thakor, N.V.; Matsuno, F. Hand and Wrist Movement Control of Myoelectric Prosthesis Based on Synergy. IEEE Trans. Hum. Mach. Syst. 2015, 45, 74–83. [Google Scholar] [CrossRef]
- Jiang, N.; Englehart, K.B.; Parker, P.A. Extracting Simultaneous and Proportional Neural Control Information for Multiple-DOF Prostheses from the Surface Electromyographic Signal. IEEE Trans. Biomed. Eng. 2009, 56, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Rehbaum, H.; Vujaklija, I.; Graimann, B.; Farina, D. Intuitive, Online, Simultaneous, and Proportional Myoelectric Control Over Two Degrees-of-Freedom in Upper Limb Amputees. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 501–510. [Google Scholar] [CrossRef]
- Israely, S.; Leisman, G.; Machluf, C.; Shnitzer, T.; Carmeli, E. Direction Modulation of Muscle Synergies in a Hand-Reaching Task. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 2427–2440. [Google Scholar] [CrossRef] [PubMed]
- Lunardini, F.; Casellato, C.; d’Avella, A.; Sanger, T.D.; Pedrocchi, A. Robustness and Reliability of Synergy-Based Myocontrol of a Multiple Degree of Freedom Robotic Arm. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamayleh, A.S.; Ahmad, R.; Abushariah, M.A.M.; Alam, K.A.; Jomhari, N. A systematic literature review on vision based gesture recognition techniques. Multimed. Tools Appl. 2018, 77, 28121–28184. [Google Scholar] [CrossRef]
- Raheja, J.L.; Rajsekhar, G.A.; Chaudhary, A. Controlling a remotely located Robot using Hand Gestures in real time: A DSP implementation. In Proceedings of the 2016 5th International Conference on Wireless Networks and Embedded Systems (WECON), Rajpura, India, 14–16 October 2016; pp. 8–12. [Google Scholar]
- Li, C.J.; Ren, J.; Huang, H.Q.; Wang, B.; Zhu, Y.F.; Hu, H.S. PCA and deep learning based myoelectric grasping control of a prosthetic hand. Biomed. Eng. Online 2018, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Dalley, S.A.; Varol, H.A.; Goldfarb, M. A Method for the Control of Multigrasp Myoelectric Prosthetic Hands. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Robust Hand Gesture Recognition for Robotic Hand Control; Springer: Singapore, 2018.
- Xie, B.; He, X.Y.; Li, Y. RGB-D static gesture recognition based on convolutional neural network. J. Eng. 2018, 2018, 1515–1520. [Google Scholar] [CrossRef]
- Chen, X.P.; Zhu, X.Y.; Zhang, D.G. A discriminant bispectrum feature for surface electromyogram signal classification. Med. Eng. Phys. 2010, 32, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Oskoei, M.A.; Hu, H.S. Myoelectric control systems—A survey. Biomed. Signal Process Control 2007, 2, 275–294. [Google Scholar] [CrossRef]
- Lee, D.D.; Seung, H.S. Learning the parts of objects by non-negative matrix factorization. Nature 1999, 401, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of Healthy Motor Modules Predicts Reduced Locomotor Performance and Muscle Coordination Complexity Post-Stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.; Rymer, W.Z.; Perreault, E.J.; Yoo, S.B.; Beer, R.F. Alterations in upper limb muscle synergy structure in chronic stroke survivors. J. Neurophysiol. 2013, 109, 768–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuiken, T.A.; Li, G.L.; Lock, B.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A.; Englehart, K.B. Targeted Muscle Reinnervation for Real-time Myoelectric Control of Multifunction Artificial Arms. JAMA J. Am. Med. Assoc. 2009, 301, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Oskoei, M.A.; Hu, H.S. Support vector machine-based classification scheme for myoelectric control applied to upper limb. IEEE Trans. Biomed. Eng. 2008, 55, 1956–1965. [Google Scholar] [CrossRef]
- Hargrove, L.J.; Li, G.L.; Englehart, K.B.; Hudgins, B.S. Principal Components Analysis Preprocessing for Improved Classification Accuracies in Pattern-Recognition-Based Myoelectric Control. IEEE Trans. Biomed. Eng. 2009, 56, 1407–1414. [Google Scholar] [CrossRef]
- D’Avella, A.; Tresch, M.C. Modularity in the motor system: Decomposition of muscle patterns as combinations of time-varying synergies. Adv. Neural Inf. Process. Syst. 2002, 14, 141–148. [Google Scholar]
- Latash, M.L.; Shim, J.K.; Zatsiorsky, V.M. Is there a timing synergy during multi-finger production of quick force pulses? Exp. Brain Res. 2004, 159, 65–71. [Google Scholar] [CrossRef] [PubMed]
- D’Avella, A.; Portone, A.; Lacquaniti, F. Superposition and modulation of muscle synergies for reaching in response to a change in target location. J. Neurophysiol. 2011, 106, 2796–2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas, H.; Veeger, H.E.J.; Stegeman, D.F. Understanding the constraints of finger motor control. J. Electromyogr. Kinesiol. 2018, 38, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Katirji, B. Practical Electromyography; Pitman Medical & Scientific: London, UK, 1998; pp. 53–64. [Google Scholar]
- Yan, Z.G.; Wang, Z.Z.; Xie, H.B. The application of mutual information-based feature selection and fuzzy LS-SVM-based classifier in motion classification. Comput. Methods Programs Biomed. 2008, 90, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Hushaba, R.N.; Al-Jumaily, A.; Al-Ani, A. Evolutionary fuzzy discriminant analysis feature projection technique in myoelectric control. Pattern Recogn. Lett. 2009, 30, 699–707. [Google Scholar] [CrossRef]
- Shuman, B.R.; Schwartz, M.H.; Steele, K.M. Electromyography Data Processing Impacts Muscle Synergies during Gait for Unimpaired Children and Children with Cerebral Palsy. Front. Comput. Neurosci. 2017, 11, 50. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Wu, X.; Chen, L.; Zhao, Y.; Zhang, L.; Li, G.; Hou, W. Synergistic Myoelectrical Activities of Forearm Muscles Improving Robust Recognition of Multi-Fingered Gestures. Sensors 2019, 19, 610. https://doi.org/10.3390/s19030610
Luo X, Wu X, Chen L, Zhao Y, Zhang L, Li G, Hou W. Synergistic Myoelectrical Activities of Forearm Muscles Improving Robust Recognition of Multi-Fingered Gestures. Sensors. 2019; 19(3):610. https://doi.org/10.3390/s19030610
Chicago/Turabian StyleLuo, Xiuying, Xiaoying Wu, Lin Chen, Yun Zhao, Li Zhang, Guanglin Li, and Wensheng Hou. 2019. "Synergistic Myoelectrical Activities of Forearm Muscles Improving Robust Recognition of Multi-Fingered Gestures" Sensors 19, no. 3: 610. https://doi.org/10.3390/s19030610



