Co3O4/Al-ZnO Nano-composites: Gas Sensing Properties
Abstract
:1. Introduction
- The bulk electronic structure of the metal oxide, which determines the electronic properties and hence the electronic conduction.
- The film morphology and structure: the size and the shape of the grains forming the film are particularly important in establishing the sensitivity of the sensor. The electronic conduction in ceramic, in fact, involves free carriers moving through the bulk of each grain, but also across the boundaries of the neighbor grains.
- The surface reactions. Obviously, the basic process that determines the sensor response is the chemisorption (adsorption and ionization of a gas molecule). Since the surface defect population is usually one of the two reactants (the other is the gas), the surface activity also depends on the specific preparation route. Moreover, the surface activity toward a given gas depends heavily on the possible presence of catalysts and, in composite materials, one of the two components can act as a catalyst and activate/favor some specific reaction on the surface of the other component. The catalytic effect is one of the explanations for the enhanced sensitivity and response speed or the reduced operating temperature of metal oxides that are doped with noble metals.
2. Materials and Methods
2.1. Instrumentation for Material Characterization
2.2. Gas Sensing Properties Characterization System
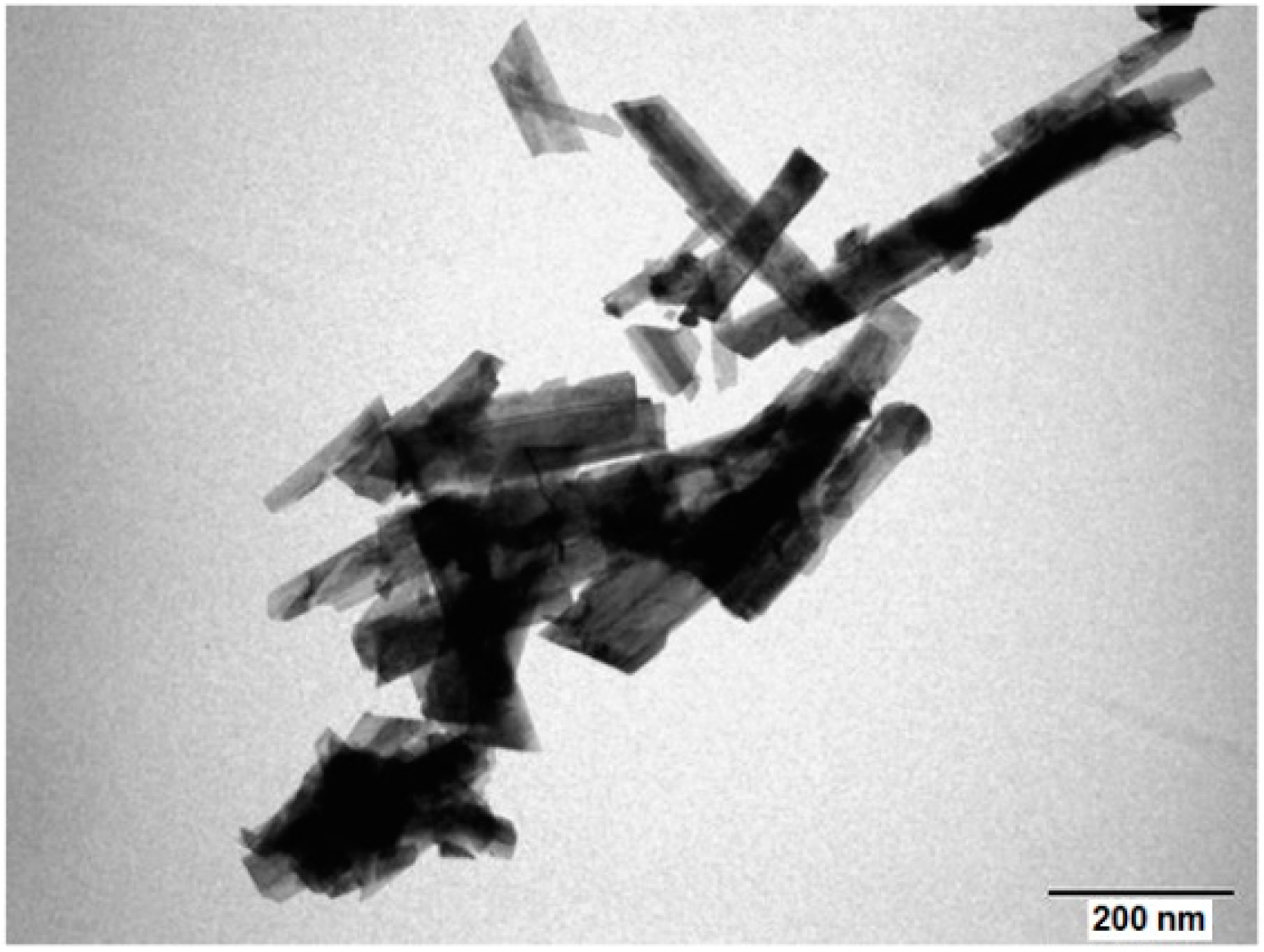
3. Materials Preparation and Characterization
3.1. Synthesis of ZnO-Al5% Nanoparticles
3.2. Preparation of Co3O4 Nanoparticles
4. Sensor Preparation
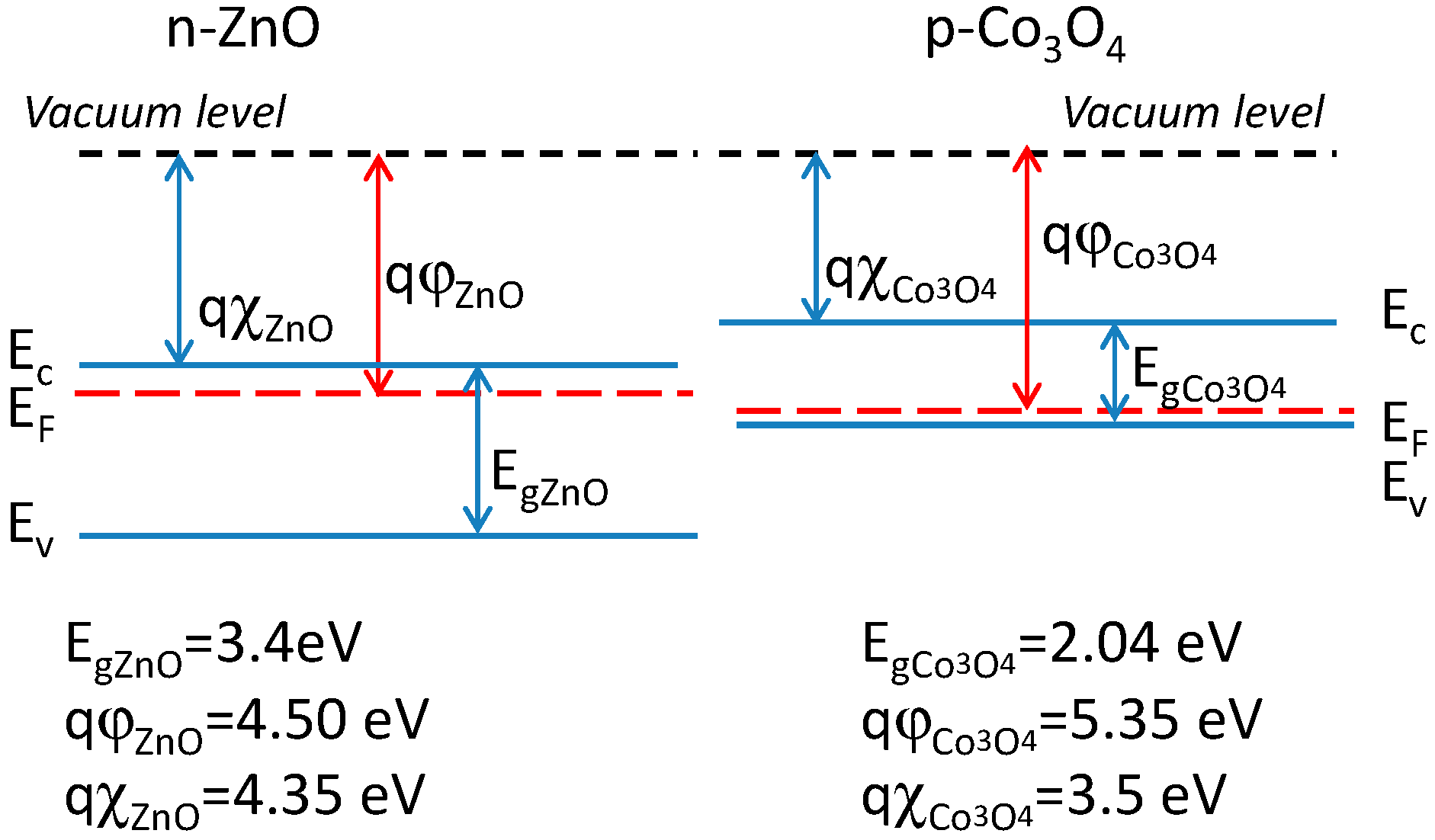
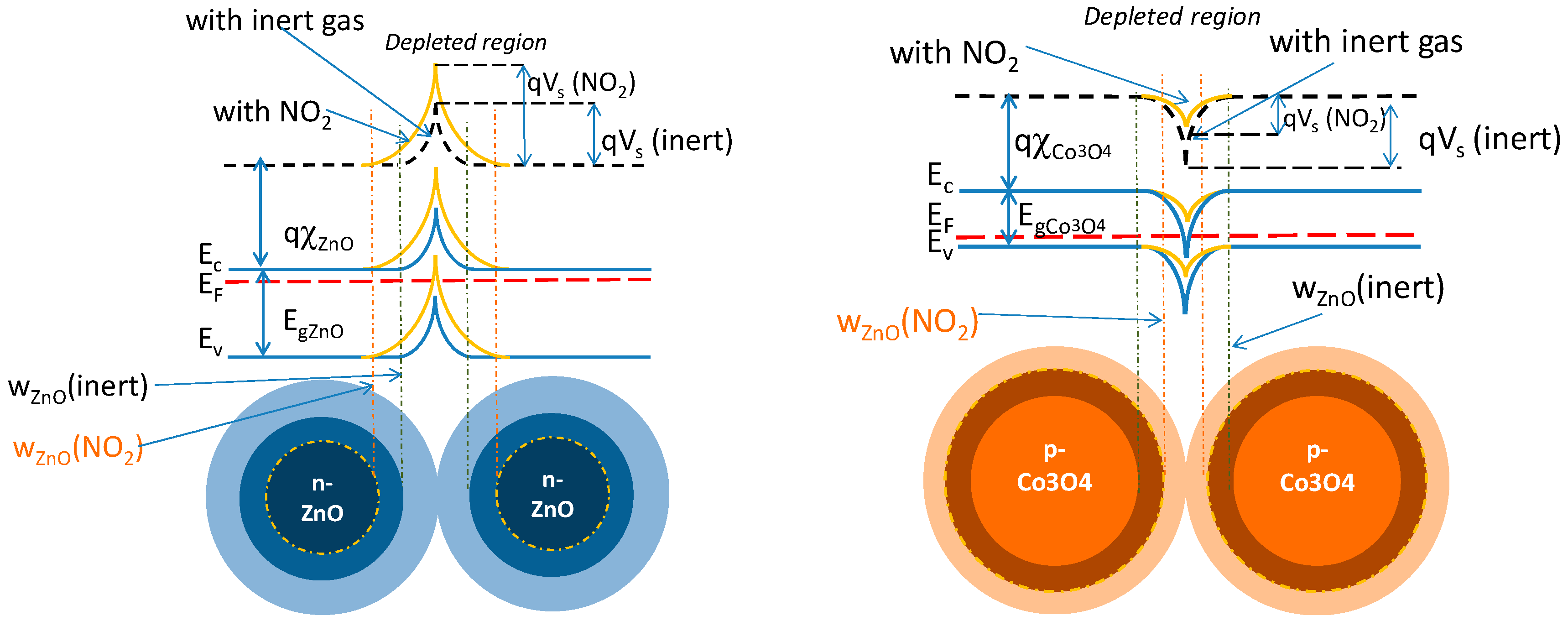
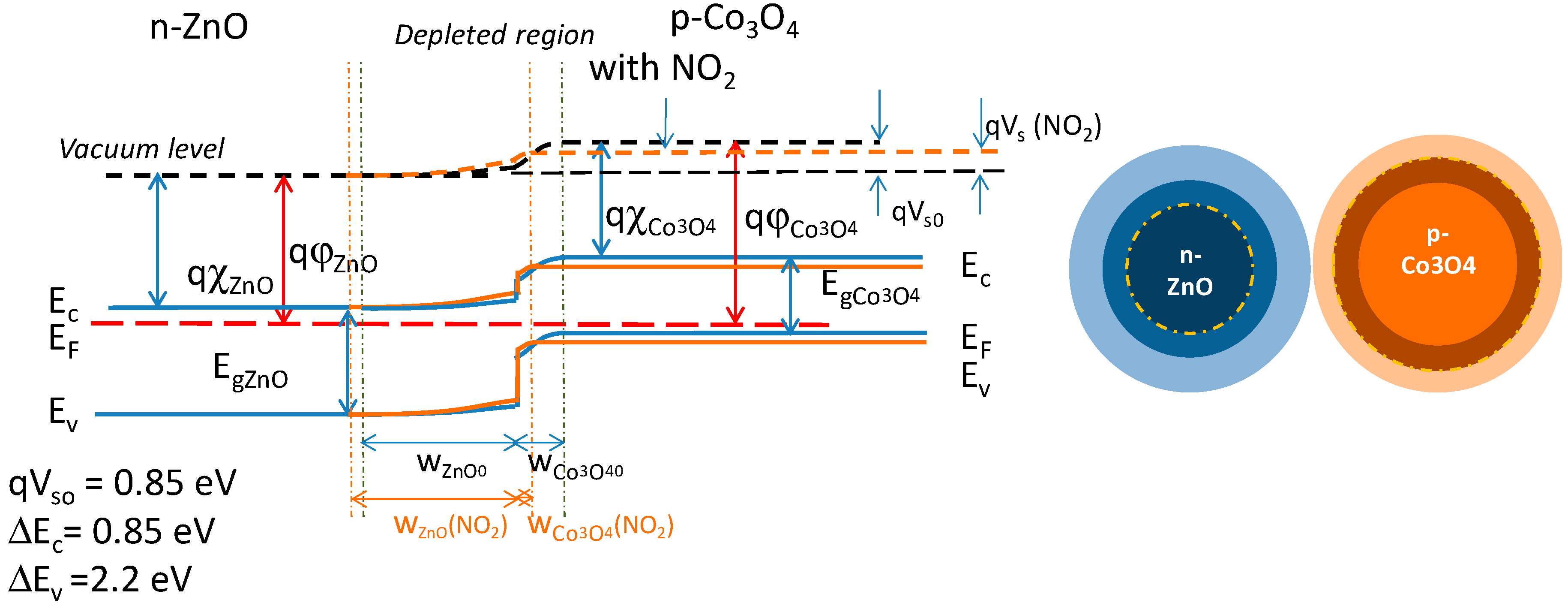
5. Gas Sensing Modeling
6. Experimental Results
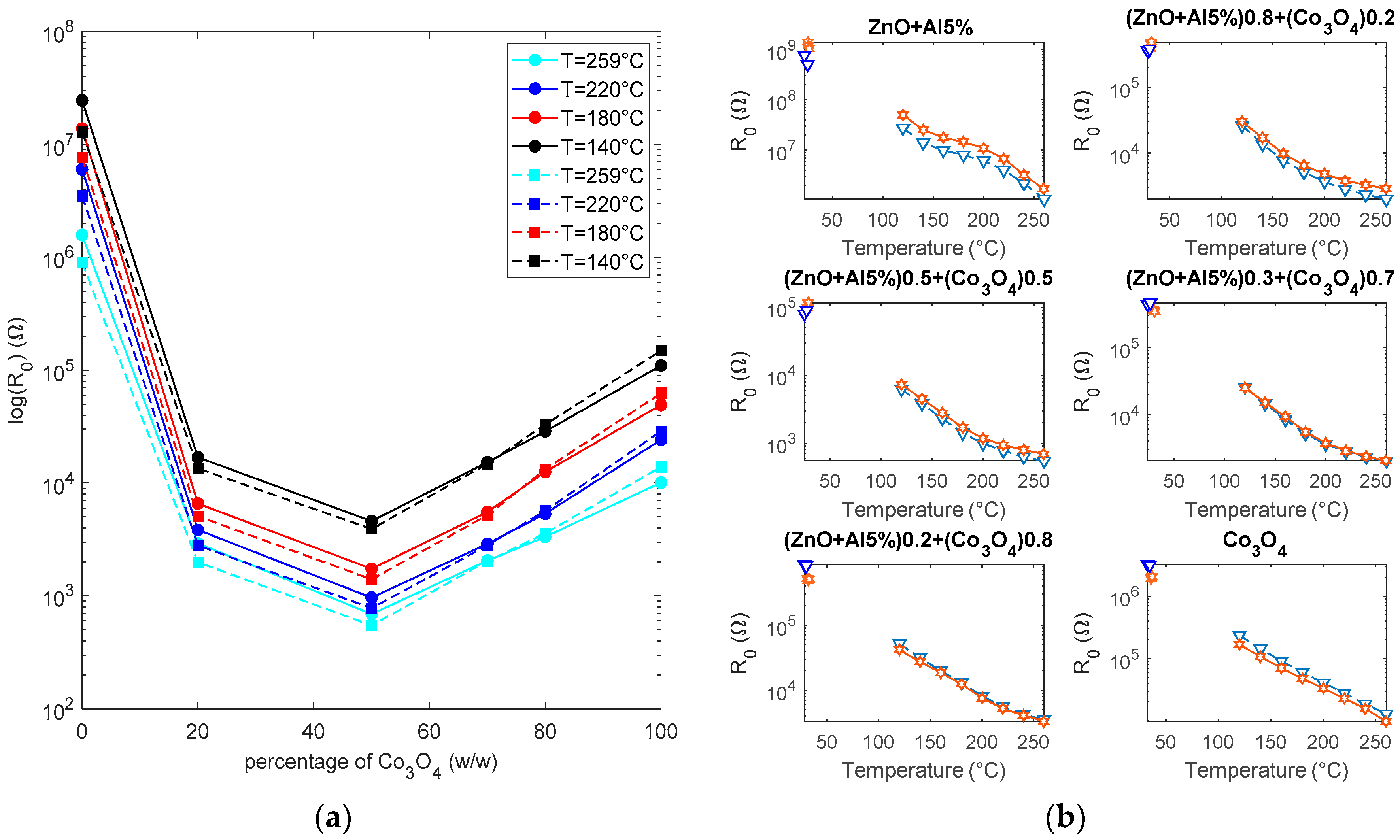
6.1. Resistance Baseline
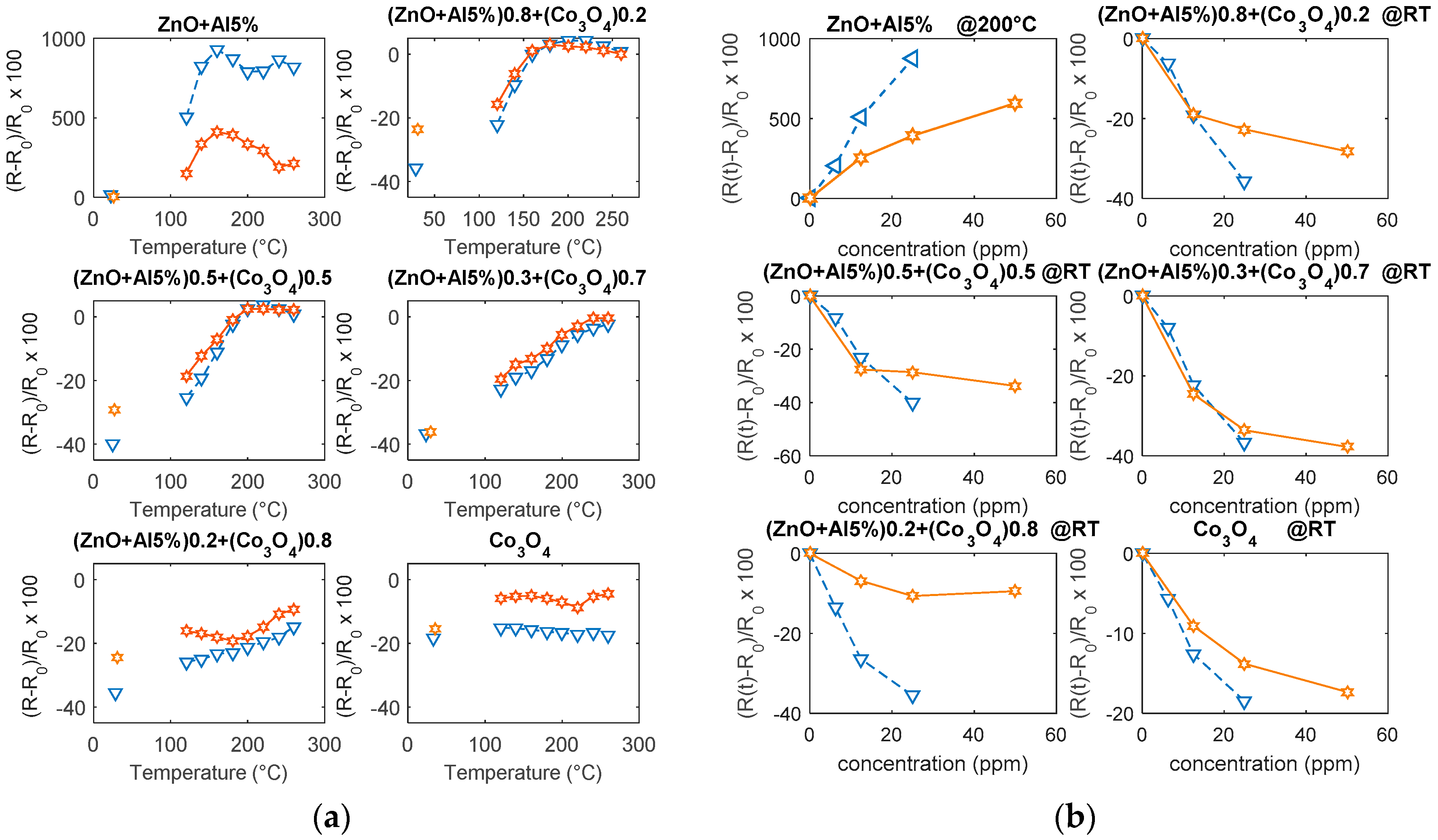
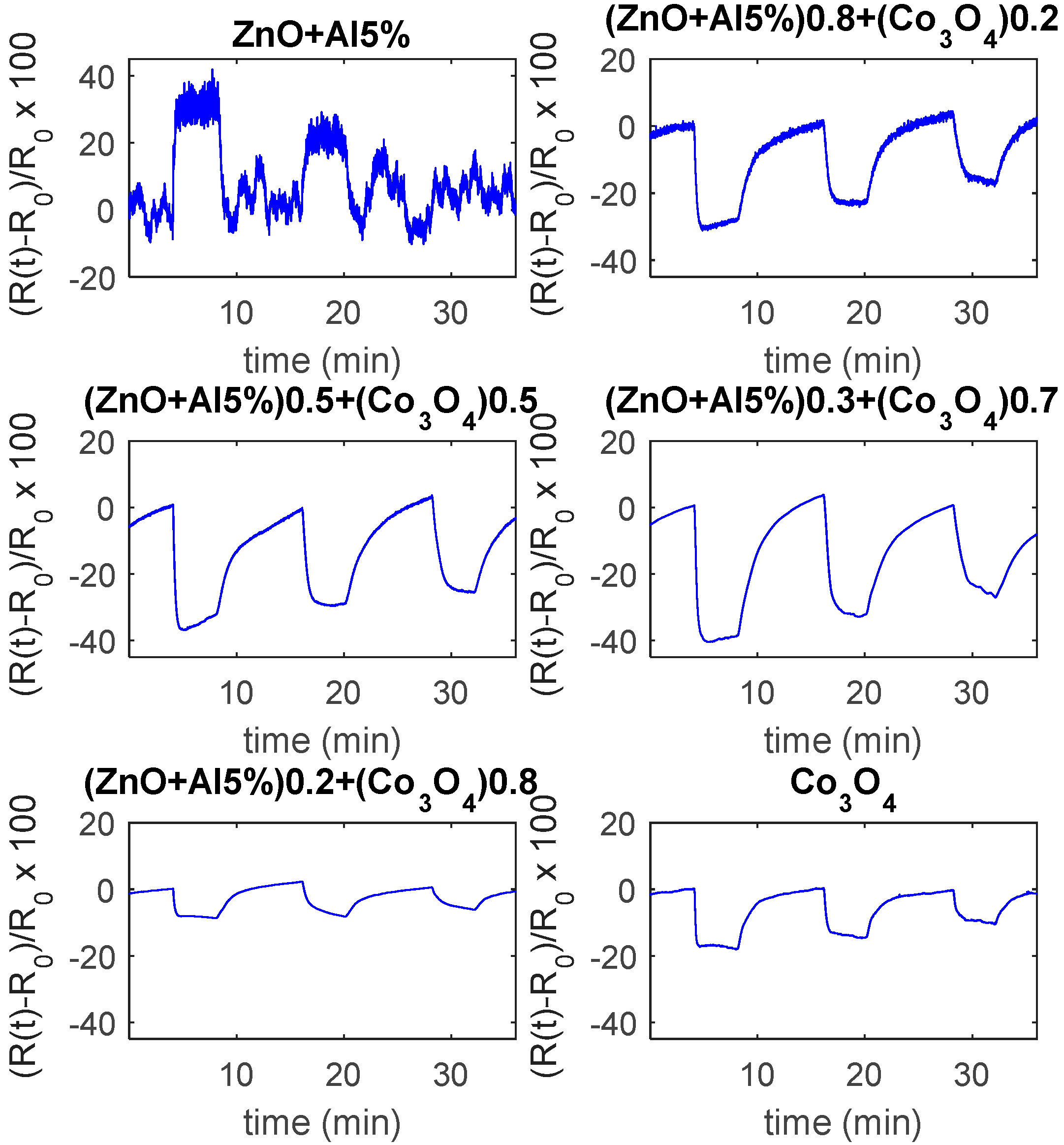
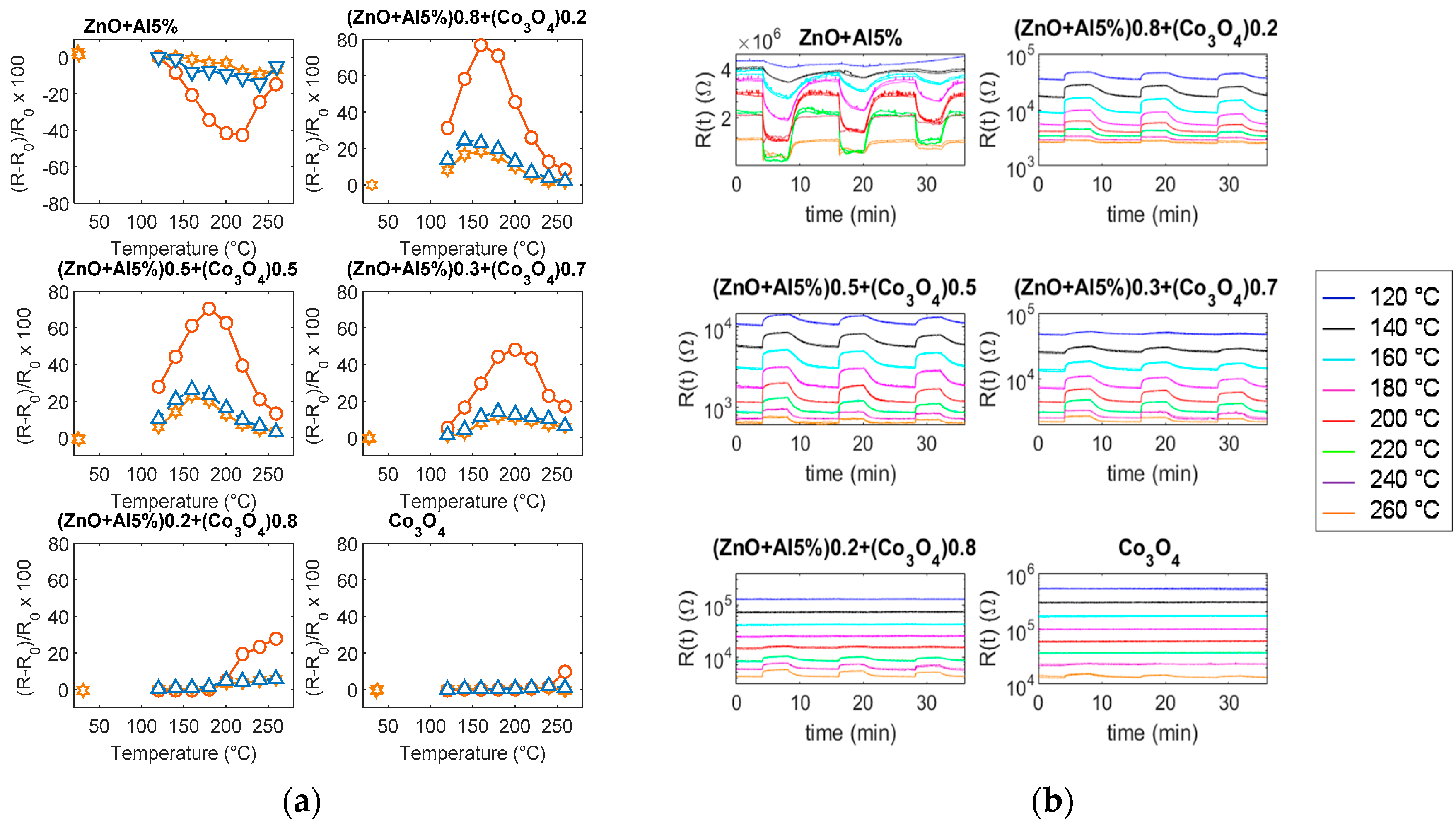
6.2. Response to NO2
6.3. Response to CO
7. Discussions and Conclusions
- even a small quantity of Co3O4, has a major influence on the behavior of the sensing layer (reduction of the resistance in the presence of oxidizing gases);
- 50%–50% mixtures are characterized by a resistance that is lower than the resistance of both pure Al-ZnO and pure Co3O4;
- the behavior of the sensors cannot only be attributed to the presence of Co3O4 grains or Co3O4 grain paths, as it can be seen from the value of the baseline resistance, the activation energy in air, and the response to the tested gases. Both the magnitude of the response, the optimum temperature and the speed cannot be justified by the superimposition of the responses of the two base materials; and,
- water mainly influences the response of the base materials, especially to NO2, and it causes an increase of the sensor responses; the tested composites show a reduced influence of water.
- (1)
- hetero-junctions resistance is low;
- (2)
- hetero-junctions respond to oxidizing gas (reducing gases) with a reduction of the activation energy and hence of the resistance (with an increase of the activation energy and hence of the resistance), therefore showing the same behavior as a p-type homojunction. Note that when in sensing layers there are hetero-junctions (between the n-type and p-type material) the behavior can’t be classified neither as p-type nor n-type.
- (3)
- The resistance magnitudes of the three possible junctions are different, namely: at Al-ZnO–Al-ZnO boundaries, the resistance is high, at Co3O4 - Co3O4 is low, at Co3O4 –Al-ZnO is very low. Therefore, channels of Co3O4 grains or Co3O4-ZnO grain sequences result in the most relevant contributions to conduction.
Author Contributions
Funding
Conflicts of Interest
References
- Addabbo, T.; Bertocci, F.; Fort, A.; Mugnaini, M.; Vignoli, V. WO3 nanograined chemosensor: A model of the sensing behavior. IEEE Trans. Nanotechnol. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Yang, D. Nanocomposite Films for Gas Sensing. Available online: https://www.intechopen.com/books/advances-in-nanocomposites-synthesis-characterization-and-industrial-applications/nanocomposite-films-for-gas-sensing/ (accessed on 12 February 2019).
- Ferroni, M.; Boscarino, D.; Comini, E.; Gnani, D.; Guidi, V.; Martinelli, G.; Nelli, P.; Rigato, V.; Sberveglieri, G. Nanosized thin films of tungsten–titanium mixed oxides as gas sensors. Sens. Actuators B 1999, 58, 289–294. [Google Scholar] [CrossRef]
- Comini, E.; Ferroni, M.; Guidi, V.; Faglia, G.; Martinelli, G.; Sberveglieri, G. Nanostructured mixed oxides compounds for gas sensing applications. Sens. Actuators B 2002, 84, 26–32. [Google Scholar] [CrossRef]
- Galatsis, K.; Li, Y.X.; Wlodarski, W.; Comini, E.; Sberveglieri, G.; Cantalini, C.; Santucci, S.; Passacantando, M. Comparison of single and binary oxide MoO3, TiO2 and WO3 sol–gel gas sensors. Sens. Actuators B 2002, 83, 276–280. [Google Scholar] [CrossRef]
- Sadek, Z.; Wlodarski, W.; Shin, K.; Kaner, R.B.; Kalantar-Zadeh, K. A layered surface acoustic wave gas sensor based on a polyaniline/In2O3 nanofibre composite. Nanotechnology 2006, 17, 4488–4492. [Google Scholar] [CrossRef]
- Rumyantseva, M.; Kovalenko, V.; Gaskov, A.; Makshina, E.; Yuschenko, V.; Ivanova, I.; Ponzoni, A.; Faglia, G.; Comini, E. Nanocomposites SnO2/Fe2O3: Sensor and catalytic properties. Sens. Actuators B 2006, 118, 208–214. [Google Scholar] [CrossRef]
- Li, C.; Thostenson, E.T.; Chou, T.-W. Sensors and actuators based on carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2008, 68, 1227–1249. [Google Scholar] [CrossRef]
- Sergiienko, S.A.; Kukla, O.L.; Yaremov, P.S.; Solomakha, V.N.; Shvets, O.V. The influence of preparation conditions and doping on the physicochemical and sensor properties of mesoporous tin oxide. Sens. Actuators B 2013, 177, 643–653. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide–based heterojunctions for gas sensing: A review. Sens. Actuators B 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Addabbo, T.; Bertocci, F.; Fort, A.; Gregorkiewitz, M.; Mugnaini, M.; Spinicci, R.; Vignoli, V. Gas sensing properties and modeling of YCoO3 based perovskite materials. Sens. Actuators B Chem. 2015, 221, 1137–1155. [Google Scholar] [CrossRef]
- Addabbo, T.; Bertocci, F.; Fort, A.; Gregorkiewitz, M.; Mugnaini, M.; Spinicci, R.; Vignoli, V. Gas sensing properties of YMnO3 based materials for the detection of NOx and CO. Sens. Actuators B Chem. 2017, 244, 1054–1070. [Google Scholar] [CrossRef]
- Gas’kov, A.M.; Rumyantseva, M.N. Nature of gas sensitivity in nanocrystalline metal oxides. Russ. J. Appl. Chem. 2001, 74, 440–444. [Google Scholar] [CrossRef]
- Yamazoe, N.; Kurokawa, Y.; Seiyama, T. Effects of additives on semiconductor gas sensors. Sens. Actuators 1983, 4, 283–289. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Korotcenkov, G. Practical aspects in design of one–electrode semiconductor gas sensors: Status report. Sens. Actuators B 2007, 121, 664–678. [Google Scholar] [CrossRef]
- Yamaura, H.; Moriya, K.; Miura, N.; Yamazoe, N. Mechanism of sensitivity promotion in CO sensors using indium oxide and cobalt oxide. Sens. Actuators B 2000, 65, 39–41. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. The role of grain size on the thermal instability of nanostructured metal oxides used in gas sensor applications and approaches for grain-size stabilization. Prog. Cryst. Growth 2012, 58, 167–208. [Google Scholar] [CrossRef]
- Gas’kov, A.; Rumyantseva, M. Metal oxide nanocomposites: Synthesis and characterization in relation with gas sensing phenomena. In Sensors for Environment, Health and Security; Baraton, M.I., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 3–29. [Google Scholar]
- Rumyantseva, M.N.; Safonova, O.V.; Boulova, M.N.; Ryabova, L.I.; Gas´kov, A.M. Dopants in nanocrystalline tin dioxide. Russ. Chem. Bull. Intern. Ed. 2003, 52, 1217–1238. [Google Scholar] [CrossRef]
- Ferro, R.; Rodriguez, J.A.; Jimenez, I.; Cirera, A.; Cerda, J.; Morante, J.R. Gas–sensing properties of sprayed films of (CdO)x(ZnO)1 − x mixed oxide. IEEE Sens. J. 2005, 5, 48–52. [Google Scholar] [CrossRef]
- Choi, S.-W.; Park, J.Y.; Kim, S.S. Synthesis of SnO2–ZnO core–shell nanofibers via a novel two–step process and their gas sensing properties. Nanotechnology 2009, 20, 465603. [Google Scholar] [CrossRef]
- Bai, S.; Li, D.; Han, D.; Luo, R.; Chen, A.; Chung, C.L. Preparation, characterization of WO3–SnO2 nanocomposites and their sensing properties for NO2. Sens. Actuators B 2010, 150, 749–755. [Google Scholar] [CrossRef]
- Trakhtenberg, L.I.; Gerasimov, G.N.; Gromov, V.F.; Belysheva, T.V.; Ilegbusi, O.J. Gas semiconducting sensors based on metal oxide nanocomposites. J. Mater. Sci. Res. 2012, 1, 56–68. [Google Scholar] [CrossRef]
- Trakhtenberg, L.I.; Gerasimov, G.N.; Gromov, V.F.; Belysheva, T.V.; Ilegbusi, O.J. Effect of composition on sensing properties of SnO2 + In2O3 mixed nanostructured films. Sens. Actuators B 2012, 169, 32–38. [Google Scholar] [CrossRef]
- Patil, S.J.; Patil, A.V.; Dighavkar, C.G.; Thakare, K.S.; Borase, R.Y.; Nandre, S.J.; Deshpande, N.G.; Ahire, R.R. Semiconductor metal oxide compounds-based gas sensors: A literature review. Front. Mater. Sci. 2015, 9, 14–37. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. Ordered mesoporous Ag-doped TiO2/SnO2 nanocomposite based highly sensitive and selective VOC sensors. J. Mater. Chem. A 2016, 4, 1033–1043. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Schadler, L.S.; Braun, P.V. Nanocomposite Science and Technology; Wiley–VCH: Weinheim, Germany, 2003. [Google Scholar]
- Viswanathan, V.; Laha, T.; Balani, K.; Agarwal, A.; Seal, S. Challenges and advances in nanocomposite processing techniques. Mater. Sci. Eng. R 2006, 54, 121–285. [Google Scholar] [CrossRef]
- Moya, J.S.; Lopez-Esteban, S.; Pecharroman, C. The challenge of ceramic/metal microcomposites and nanocomposites. Prog. Mater. Sci. 2007, 52, 1017–1090. [Google Scholar] [CrossRef]
- Asim, N. Nanocomposites: Preparation, Properties and Performance; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2008; pp. 241–251. [Google Scholar]
- Cury Camargo, P.H.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Korotcenkov, G. (Ed.) Chemical Sensors: Fundamentals of Gas Sensing Materials; Momentum Press: New York, NY, USA, 2010. [Google Scholar]
- Korotcenkov, G. Handbook of Gas Sensor Materials; Springer: New York, NY, USA, 2013. [Google Scholar]
- Tricoli, A.; Righettoni, M.; Teleki, A. Semiconductor gas sensors: Dry synthesis and application. Angew. Chem. Int. Ed. 2010, 49, 7632–7659. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K.; Gulina, L.B.; Tolstoy, V.P. Synthesis of metal oxide–based nanocomposites and multicomponent compounds using Layer–by–Layer method and prospects for their application. J. Teknologi (Sci. Eng.) 2015, 75, 16–26. [Google Scholar] [CrossRef]
- Bertocci, F.; Fort, A.; Mugnaini, M.; Vignoli, V. Stability evaluation of YCoO3 based perovskites used for NO2 detection. In Proceedings of the 2016 IEEE Sensors Applications Symposium (SAS), Catania, Italy, 20–22 April 2016. [Google Scholar]
- Kim, J.W.; Lee, S.J.; Biswas, P.; Lee, T.I.; Myoung, J.M. Solution-processed n-ZnO nanorod/p-Co3O4 nanoplate heterojunction light-emitting diode. Appl. Surface Sci. 2017, 406, 192–198. [Google Scholar] [CrossRef]
- Na, C.W.; Woo, H.S.; Kim, I.D.; Lee, J.H. Selective detection of NO2 and C2H5OH using Co3O4-decorated ZnO nanowire network sensor. Chem. Commun. 2011, 47, 5148–5150. [Google Scholar] [CrossRef] [PubMed]
- Bekermann, D.; Gasparotto, A.; Barreca, D.; Maccato, C.; Comini, E.; Sada, C.; Sberveglieri, G.; Devi, A.; Fischer, R.A. Co3O4/ZnO nanocomposites: From plasma synthesis to gas sensing applications. ACS Appl. Mater. Interfaces 2012, 4, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhu, G.X.; Chen, J.Z.; Xu, H.; Shen, X.P.; Yuan, A.H. Co3O4/ZnO nanocomposites for gas-sensing applications. Appl. Surf. Sci. 2013, 265, 379–384. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.Y.; Liu, J.Y.; Wang, J.; Sun, Y.B. Facile synthesis of mesoporous ZnO/Co3O4 microspheres with enhanced gas-sensing for ethanol. Sens. Actuator B Chem. 2015, 211, 1492–1498. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.Y.; Kheel, H.J.; Lee, C.M. Oxidizing gas sensing properties of the n-ZnO/p-Co3O4 composite nanoparticle network sensor. Sens. Actuator B Chem. 2016, 222, 1193–1200. [Google Scholar] [CrossRef]
- Bertocci, F.; Fort, A.; Vignoli, V.; Shahin, L.; Mugnaini, M.; Berni, R. Assessment and Optimization for Novel Gas Materials Through the Evaluation of Mixed Response Surface Models. IEEE Trans. Instrum. Meas. 2015, 64, 1084–1092. [Google Scholar] [CrossRef]
- Addabbo, T.; Bertocci, F.; Fort, A.; Mugnaini, M.; Vignoli, V.; Shahin, L. Versatile measurement system for the characterization of gas sensing materials. In Proceedings of the 2013 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Minneapolis, MN, USA, 6–9 May 2013. [Google Scholar]
- El Mir, L.; El Ghoul, J.; Alaya, S.; Ben Salem, M.; Barthou, C.; von Bardeleben, H.J. Synthesis and luminescence properties of vanadium-doped man-sized zinc oxide aerogel. Phys. B Condens. Matter 2008, 403, 1770–1774. [Google Scholar] [CrossRef]
- Nanto, H.; Sokooshi, H.; Kawai, T. Aluminum-doped ZnO thin film gas sensor capable of detecting freshness of sea foods. Sens. Actuators B 1993, 14, 715–717. [Google Scholar] [CrossRef]
- Hongxiao, J.; Xiaojian, G.; Bo, H.; Langsheng, L.; Chiya, W.; Dingfeng, J.; Xiaoling, P.; Xinqing, W.; Hongliang, G. Fabrication of mesoporous Co3O4 from LP-FDU-12 via nanocasting route and effect of wall/pore size on their magnetic properties. J. Phys. Chem. C 2012, 116, 13374–13381. [Google Scholar]
- Xu, J.M.; Cheng, J.P. The advances of Co3O4 as gas sensing materials: A review. J. Alloys Compd. 2016, 686, 753–768. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Xuan, L.; Zhu, Z.; Pan, Q.; Shi, K.; Zhang, G. Novel Co3O4 nanocrystalline chain material as a high performance gas sensor at room temperature. J. Alloys Compd. 2018, 768, 190–197. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Shan, F.K.; Yu, Y.S. Band gap energy of pure and Al-doped ZnO thin films. J. Eur. Ceram. Soc. 2004, 24, 1869–1872. [Google Scholar] [CrossRef]
- Fort, A.; Mugnaini, M.; Rocchi, S.; Serrano-Santos, M.B.; Spinicci, R.; Vignoli, V. Surface State Model for Conductance Responses During Thermal-Modulation of SnO2-Based Thick Film Sensors: Part II—Experimental Verification. IEEE Trans. Instrum. Meas. 2006, 55, 2107–2117. [Google Scholar] [CrossRef]
- Nagarajan, V.; Chandiramouli, R. A DFT study on adsorption behaviour of CO on Co3O4 nanostructures. Appl. Surface Sci. 2016, 385, 113–121. [Google Scholar] [CrossRef]
- Saputro, A.G.; Agusta, M.K.; Yuliarto, B.; Dipojono, H.K.; Rusydi, F.; Maezono, R. Selectivity of CO and NO adsorption on ZnO (0002) surfaces: A DFT investigation. Appl. Surface Sci. 2017, 410, 373–382. [Google Scholar]
- Bârsan, N.; Hübner, M.; Weimar, U. Conduction mechanisms in SnO2 based polycrystalline thick film gas sensors exposed to CO and H2 in different oxygen backgrounds. Sens. Actuators B Chem. 2011, 157, 510–517. [Google Scholar] [CrossRef]
- Fort, A.; Mugnaini, M.; Pasquini, I.; Rocchi, S.; Vignoli, V. Modeling of the influence of H2O on metal oxide sensor responses to CO. Sens. Actuators B Chem. 2011, 159, 82–91. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fort, A.; Panzardi, E.; Vignoli, V.; Hjiri, M.; Aida, M.S.; Mugnaini, M.; Addabbo, T. Co3O4/Al-ZnO Nano-composites: Gas Sensing Properties. Sensors 2019, 19, 760. https://doi.org/10.3390/s19040760
Fort A, Panzardi E, Vignoli V, Hjiri M, Aida MS, Mugnaini M, Addabbo T. Co3O4/Al-ZnO Nano-composites: Gas Sensing Properties. Sensors. 2019; 19(4):760. https://doi.org/10.3390/s19040760
Chicago/Turabian StyleFort, Ada, Enza Panzardi, Valerio Vignoli, Mokhtar Hjiri, Mohamed Salah Aida, Marco Mugnaini, and Tommaso Addabbo. 2019. "Co3O4/Al-ZnO Nano-composites: Gas Sensing Properties" Sensors 19, no. 4: 760. https://doi.org/10.3390/s19040760






