Black Phosphorus-New Nanostructured Material for Humidity Sensors: Achievements and Limitations
Abstract
:1. Introduction
2. Black Phosphorus
2.1. Black Phosphorus and Its Crystal and Electronic Structures
2.2. Features of Black Phosphorus and Phosphorene Synthesis
2.2.1. Bulk Black Phosphorus
2.2.2. Phosphorene
3. BP-Based Humidity Sensors
3.1. Achievements
3.1.1. Resistive Humidity Sensors
3.1.2. Other Types of Humidity Sensors
3.2. Limitations of BP-Based Humidity Sensors
3.2.1. The Tunability of Black Phosphorus Properties
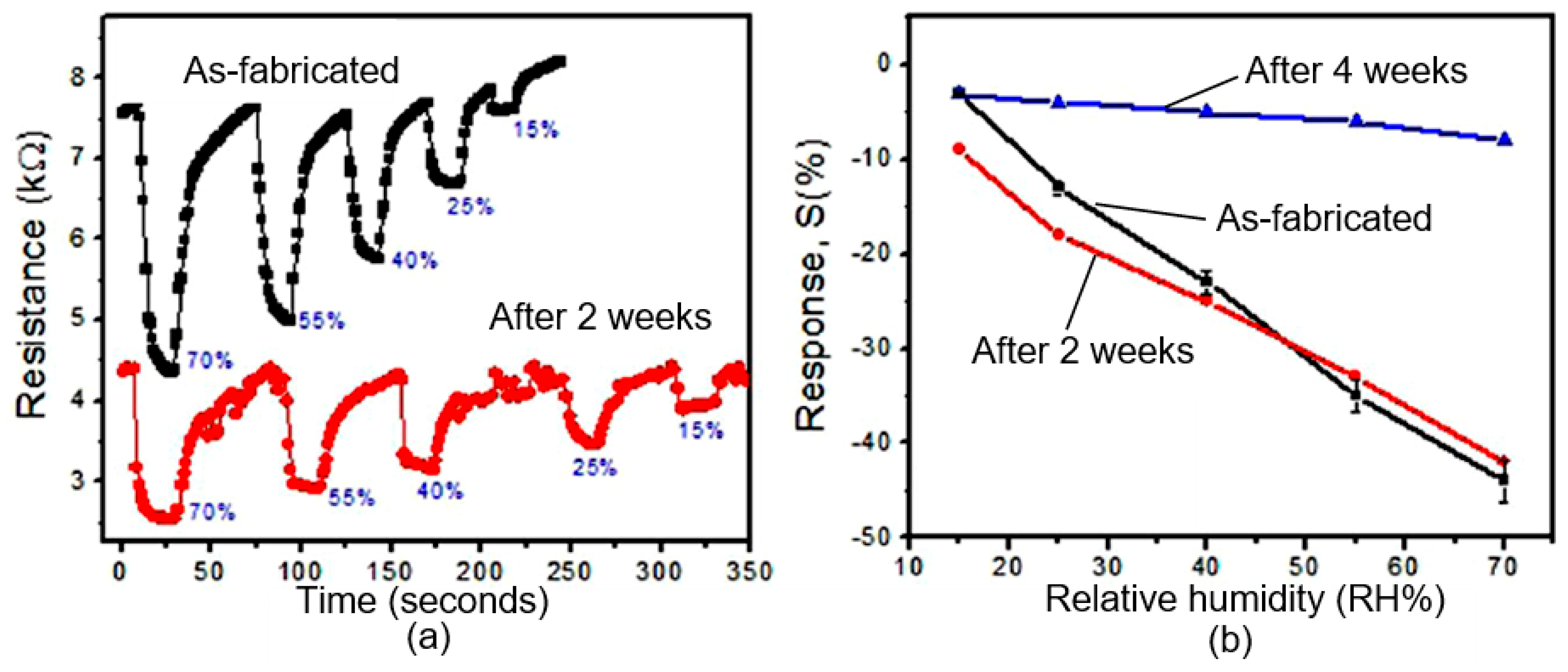
3.2.2. Instability of Black Phosphorus
4. Is It Possible to Improve the Stability and Reproducibility of BP Parameters?
5. Summary
Funding
Conflicts of Interest
References
- Davis, R.E.; McGregor, G.R.; Enfield, K.B. Humidity: A review and primer on atmospheric moisture and human health. Environ. Res. 2016, 144, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korotcenkov, G. Why do we need to control humidity? In Handbook of Humidity Measurement: Methods, Materials and Technologies. Vol. 1: Spectroscopic Methods of Humidity Measurement; CRC Press: Boca Raton, FL, USA, 2018; pp. 17–45. [Google Scholar]
- The Rotronic Humidity Handbook. Available online: http://docplayer.net/13211741-The-rotronic-humidity-handbook.html (accessed on 26 February 2019).
- Grange, R.I.; Hand, D.W. A review of the effects of atmospheric humidity on the growth of horticultural crops. J. Hortic. Sci. 1987, 62, 125–134. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of Humidity Measurement: Methods, Materials and Technologies. Vol. 1: Spectroscopic Methods of Humidity Measurement; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Traversa, E. Ceramic sensors for humidity detection: The state–of–the–art and future developments. Sens. Actuators B 1995, 23, 1335–1356. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Srivastava, R. Humidity sensor: An overview. Int. J. Green Nanotechnol. 2012, 4, 302–309. [Google Scholar] [CrossRef]
- Alwis, L.; Sun, T.; Grattan, K.T.V. Optical fibre-based sensor technology for humidity and moisture measurement: Review of recent progress. Measurement 2013, 46, 4052–4074. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle; mechanism; and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef] [PubMed]
- Blank, T.A.; Eksperiandova, L.P.; Beliko, K.N. Recent trends of ceramic humidity sensors development: A review. Sens. Actuators B 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of Humidity Measurement: Methods; Materials and Technologies. Vol. 2: Electronic and Electrical Humidity Sensors; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Korotcenkov, G. Handbook of Humidity Measurement: Methods, Materials and Technologies. Vol. 3: Sensing Materials and Technologies; CRC Press: Boca Raton, FL, USA, 2020; in press. [Google Scholar]
- Fletcher, G.F.; Galambos, J.T. Phosphorus poisoning in humans. Arch. Int. Med. 1963, 112, 846–852. [Google Scholar] [CrossRef]
- Island, J.O.; Steele, G.A.; van der Zant, H.S.; Castellanos-Gomez, A. Environmental instability of few-layer black phosphorus. 2D Mater. 2015, 2, 011002. [Google Scholar] [CrossRef] [Green Version]
- Park, C.M.; Sohn, H.J. Black phosphorus and its composite for lithium rechargeable batteries. Adv. Mater. 2007, 19, 2465–2468. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.Q.; Ou, X.D.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sa, B.; Li, Y.-L.; Qi, J.; Ahuja, R.; Sun, Z. Strain engineering for phosphorene: The potential application as a photocatalyst. J. Phys. Chem. C 2014, 118, 26560–26568. [Google Scholar] [CrossRef]
- Xia, F.; Wang, H.; Jia, Y. Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics. Nat. Commun. 2014, 5, 4458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, A.N.; Liu, B.; Chen, L.; Ma, Y.; Cong, S.; Aroonyadet, N.; Kopf, M.; Nilges, T.; Zhou, C. Black phosphorus gas sensors. ACS Nano 2015, 9, 5618–5624. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Sun, S.; Wang, W.; Liu, J.; Liu, Z.; Jimmy, C.Y. A black-red phosphorus heterostructure for efficient visible-light-driven photocatalysis. J. Mater. Chem. A 2015, 3, 3285–3288. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.; Zhuo, Z.; Kou, L.; Ma, W.; Shao, B.; Du, A.; Meng, S.; Frauenheim, T. Novel excitonic solar cells in phosphorrene-TiO2 heterostructures with extraordinary charge separation efficiency. J. Phys. Chem. Lett. 2016, 7, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kim, S.Y.; Jang, H.W. Black phosphorus: Critical review and potential for water splitting photocatalyst. Nanomaterials 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, R.; Sofer, Z.; Pumera, M. Black phosphorus rediscovered: From bulk material to monolayers. Angew. Chem. Int. Ed. Engl. 2017, 56, 8052–8072. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Narayan, R.; Sasikala, S.P.; Lee, K.E.; Jung, H.J.; Kim, S.O. Phosphorene for energy and catalytic application—Filling the gap between graphene and 2D metal chalcogenides. 2D Mater. 2017, 4, 042006. [Google Scholar] [CrossRef]
- Lin, S.; Chui, Y.; Li, Y.; Lau, S.P. Liquid-phase exfoliation of black phosphorus and its applications. FlatChem 2017, 2, 15–37. [Google Scholar] [CrossRef]
- Yi, Y.; Yu, X.-F.; Zhou, W.; Wang, J.; Chu, P.K. Two-dimensional black phosphorus: Synthesis; modification; properties; and applications. Mater. Sci. Eng. R 2017, 120, 1–33. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilgha, A.; Lin, Y.; Xu, J.; Lu, X. Black phosphorus and its biomedical applications. Theranostics 2018, 8, 1005–1026. [Google Scholar] [CrossRef] [PubMed]
- Irshad, R.; Tahir, K.; Li, B.; Sher, Z.; Ali, J.; Nazir, S. A revival of 2D materials; phosphorene: Its application as sensors. J. Ind. Eng. Chem. 2018, 64, 60–69. [Google Scholar] [CrossRef]
- Wang, W.; Xie, G.; Luo, J. Black phosphorus as a new lubricant. Friction 2018, 6, 116–142. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Hui, K.S.; Hui, K.N. 2D black phosphorus: From preparation to applications for electrochemical energy storage. Adv. Sci. 2018, 5, 1700491. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Wang, D.; Wang, X.; Zhang, D.; Koratkar, N.; Rong, M. Recent advances in phosphorene as a sensing material. Nano Today 2018, 20, 13–32. [Google Scholar] [CrossRef]
- Khandelwal, A.; Mani, K.; Karigerasi, M.H.; Lahiri, I. Phosphorene—The two-dimensional black phosphorous: Properties; synthesis and applications. Mater. Sci. Eng. B 2017, 221, 17–34. [Google Scholar] [CrossRef]
- Du, Y.; Ouyang, C.; Shi, S.; Lei, M. Ab initio studies on atomic and electronic structures of black phosphorus. J. Appl. Phys. 2010, 107, 093718. [Google Scholar] [CrossRef]
- Wei, Q.; Peng, X. Superior mechanical flexibility of phosphorene and few-layer black phosphorus. Appl. Phys. Lett. 2014, 104, 251915. [Google Scholar] [CrossRef] [Green Version]
- Appalakondaiah, S.; Vaitheeswaran, G.; Lebegue, S.; Christensen, N.E.; Svane, A. Effect of van der Waals interactions on the structural and elastic properties of black phosphorus. Phys. Rev. B 2012, 86, 035105. [Google Scholar] [CrossRef]
- Aldave, S.H.; Yogeesh, M.N.; Zhu, W.N.; Kim, J.; Sonde, S.S.; Nayak, A.P.; Akinwande, D. Characterization and sonochemical synthesis of black phosphorus from red phosphorus. 2D Mater. 2016, 3, 014007. [Google Scholar] [CrossRef]
- Zhang, C.; Lian, J.; Yi, W.; Jiang, Y.; Liu, L.; Hu, H.; Xiao, W.D.; Du, S.X.; Sun, L.L.; Gao, H.J. Surface structures of black phosphorus investigated with scanning tunneling microscopy. J. Phys. Chem. C 2009, 113, 18823–18826. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, G.Y.; Lee, H.W.; Liu, N.; Wang, H.T.; Yao, H.B.; Yang, W.; Cui, Y. Formation of stable phosphorus–carbon bond for enhanced performance in black phosphorus nanoparticle–graphite composite battery anodes. Nano Lett. 2014, 14, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, Y.C.; Deng, Y.X.; Ye, P.D. Semiconducting black phosphorus: Synthesis; transport properties and electronic applications. Chem. Soc. Rev. 2015, 44, 2732–2743. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Kikegawa, T.; Fujimura, T.; Endo, S.; Akahama, Y.; Akai, T.; Shimomura, O.; Yamaoka, S.; Yagi, T.; Akimoto, S.; et al. Synchrotron radiation diffraction study of phase transitions in phosphorus at high pressures and temperatures. Phys. B + C 1986, 139, 301–304. [Google Scholar] [CrossRef]
- Ahuja, R. Calculated high pressure crystal structure transformations for phosphorus. Phys. Status Solidi B 2003, 235, 282–287. [Google Scholar] [CrossRef]
- Morita, A. Semiconducting black phosphorus. Appl. Phys. A Mater. Sci. Process. 1986, 39, 227–242. [Google Scholar] [CrossRef]
- Takao, Y.; Morita, A. Electronic structure of black phosphorus: Tight binding approach. Phys. B + C 1981, 105, 93–98. [Google Scholar] [CrossRef]
- Asahina, H.; Morita, A. Band structure and optical properties of black phosphorus. J. Phys. C Solid State Phys. 1984, 17, 1839–1852. [Google Scholar] [CrossRef]
- Takahashi, T.; Tokailin, H.; Suzuki, S.; Sagawa, T.; Shirotani, I. Electronic band structure of black phosphorus studied by angle-resolved ultraviolet photoelectron spectroscopy. J. Phys. C: Solid State Phys. 1985, 18, 825. [Google Scholar] [CrossRef]
- Han, C.; Yao, M.; Bai, X.; Miao, L.; Zhu, F.; Guan, D.; Wang, S.; Gao, C.L.; Liu, C.; Qian, D.; et al. Electronic structure of black phosphorus studied by angle-resolved photoemission spectroscopy. Phys. Rev. B 2014, 90, 085101. [Google Scholar] [CrossRef]
- Rudenko, A.N.; Katsnelson, M.I. Quasiparticle band structure and tight-binding model for single-and bilayer black phosphorus. Phys. Rev. B 2014, 89, 201408. [Google Scholar] [CrossRef]
- Wu, R.J.; Topsakal, M.; Low, T.; Robbins, M.C.; Haratipour, N.; Jeong, J.S.; Wang, S.; Gao, C.L.; Liu, C.; Qian, D.; et al. Atomic and electronic structure of exfoliated black phosphorus. J. Vac. Sci. Technol. A 2015, 33, 060604. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, J.; Zeng, X.C. Electron-transport properties of few-layer black phosphorus. J. Phys. Chem. Lett. 2015, 6, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Wang, M.; Zhu, X.; Rodin, A.S.; Su, H.; Neto, A.H.C. Phosphorene: From theory to applications. Nat. Rev. Mater. 2016, 1, 16061. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Kwong, C.W.; Davey, K.; Qiao, S.Z. 2D phosphorene as a water splitting photocatalyst: Fundamentals to applications. Energy Environ. Sci. 2016, 9, 709–728. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, G.; Zhang, Y.-W. Layer-dependent band alignment and work function of few-layer phosphorene. Sci. Rep. 2014, 4, 6677. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.F.; Tománek, D.; Ye, P.D. Phosphorene: An unexplored 2D semiconductor with a high hole mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, R.T.; Robinson, J.A.; Schaak, R.E.; Sun, D.; Sun, Y.F.; Mallouk, T.E.; Terrones, M. Transition metal dichalcogenides and beyond: Synthesis; properties; and applications of single and few-layer nanosheets. Acc. Chem. Res. 2015, 48, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, R.; Pei, J.; Myint, Y.W.; Wang, F.; Wang, Z.; Zhang, S.; Yu, Z.; Lu, Y. Unambiguous identification of monolayer phosphorene by phase-shifting interferometry. arXiv, 2014; arXiv:1412.6701. [Google Scholar]
- Zhang, S.; Yang, J.; Xu, R.; Wang, F.; Li, W.; Ghufran, M.; Zhang, Y.-W.; Yu, Z.; Zhang, G.; Qin, Q.; et al. Extraordinary photoluminescence and strong temperature/angle-dependent Raman responses in few-layer phosphorene. ACS Nano 2014, 8, 9590–9596. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, D.; Backes, C.; Doherty, E.; Cucinotta, C.S.; Berner, N.C.; Boland, C.; Lee, K.; Harvey, A.; Lynch, P.; Gholamyand, Z.; et al. Liquid exfoliation of solvent-stabilised black phosphorus: Applications beyond electronics. Nat. Commun. 2015, 6, 8563. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Kong, X.; Hu, Z.-X.; Yang, F.; Ji, W. High-mobility transport anisotropy and linear dichroism in few-layer black phosphorus. Nat. Commun. 2014, 5, 4475. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.P.; Doganov, R.A.; Seixas, L.; Carvalho, A.; Tan, J.Y.; Taniguchi, T.; Yakovlev, N.; Castro Neto, A.H.; Ozyilmaz, B. Electron doping of ultrathin black phosphorus with Cu adatoms. Nano Lett. 2016, 16, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, S.; Zeng, H.; Wang, Q.J. Lateral black phosphorene p-n junctions formed via chemical doping for high performance near-infrared photodetector. Nano Energy 2016, 25, 34–41. [Google Scholar] [CrossRef]
- Das, S.; Demarteau, M.; Roelofs, A. Ambipolar phosphorene field effect transistor. ACS Nano 2014, 8, 11730–11738. [Google Scholar] [CrossRef] [PubMed]
- Schwierz, F. Graphene transistors. Nat. Nanotechnol. 2010, 5, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Sevik, C.; Sevinçli, H. Promising thermoelectric properties of phosphorenes. Nanotechnology 2016, 27, 355705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Cai, Y.; Zhang, G.; Zhang, Y.-W. Thermoelectric properties of phosphorene at the nanoscale. J. Mater. Sci. 2016, 31, 3179–3186. [Google Scholar] [CrossRef]
- Dai, J.; Zeng, X.C. Bilayer phosphorene: Effect of stacking order on bandgap and its potential applications in thin-film solar cells. J. Phys. Chem. Lett. 2014, 5, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.R. First-principles study of MoS2, phosphorene and graphene based single electron transistor for gas sensing applications. Sens. Actuators B 2016, 222, 492–498. [Google Scholar] [CrossRef]
- Donarelli, M.; Ottaviano, L. 2D materials for gas sensing applications: A review on graphene oxide, MoS2, WS2 and phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.Y.; Lu, W.J.; Shao, D.F.; Sun, Y.P. Enhanced thermoelectric performance of phosphorene by strain-induced band convergence. Phys. Rev. B 2014, 90, 085433. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-B.; Guo, P.; Cao, T.-F.; Liu, H.; Lau, W.-M.; Liu, L.-M. Structures, stabilities, and electronic properties of defects in monolayer black phosphorus. Sci. Rep. 2016, 5, 10848. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Anderson, G.; Zhao, R.; Alruqi, A.; Mroczkowska, J.E.; Sumanasekera, G.; Jasinski, J.B. Recent advances in synthesis; properties; and applications of phosphorene. NpJ 2D Mater. Appl. 2017, 1, 5. [Google Scholar] [CrossRef]
- Dhanabalan, S.C.; Ponraj, J.S.; Guo, Z.; Li, S.; Bao, Q.; Zhang, H. Emerging trends in phosphorene fabrication towards next generation devices. Adv. Sci. 2017, 4, 1600305. [Google Scholar] [CrossRef] [PubMed]
- Bridgman, P.W. Two new modifications of phosphorus. J. Am. Chem. Soc. 1914, 36, 1344–1363. [Google Scholar] [CrossRef]
- Maruyama, Y.; Suzuki, S.; Kobayashi, K.; Tanuma, S. Synthesis and some properties of black phosphorus single crystals. Phys. B + C 1981, 105, 99–102. [Google Scholar] [CrossRef]
- Shirotani, I. Growth of large single crystals of black phosphorus at high pressures and temperatures; and its electrical properties. Mol. Cryst. Liq. Cryst. 1982, 86, 203–211. [Google Scholar] [CrossRef]
- Endo, S.; Akahama, Y.; Terada, S.; Narita, S. Growth of large single crystals of black phosphorus under high pressure. Jpn. J. Appl. Phys. 1982, 21, L482–L484. [Google Scholar] [CrossRef]
- Krebs, H.; Schultze-Gebhardt, F. Über die Struktur und Eigenschaften der Halbmetalle. VII. Neubestimmung der Struktur des glasigen Selens nach verbesserten röntgenographischen Methoden. Acta Crystallogr. 1955, 8, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Lange, S.; Schmidt, P.; Nilges, T. Au3SnP7@black phosphorus: An easy access to black phosphorus. Inorg. Chem. 2007, 46, 4028–4035. [Google Scholar] [CrossRef] [PubMed]
- Nilges, T.; Kersting, M.; Pfeifer, T. A fast low-pressure transport route to large black phosphorus single crystals. J. Solid State Chem. 2008, 181, 1707–1711. [Google Scholar] [CrossRef]
- Köpf, M.; Eckstein, N.; Pfister, D.; Grotz, C.; Krüger, I.; Greiwe, M.; Hansen, T.; Kohlmann, H.; Nilges, T. Access and in situ growth of phosphorene-precursor black phosphorus. J. Cryst. Growth 2014, 405, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.L.; Nan, H.Y.; Hong, J.H.; Chen, Y.M.; Zhu, C.; Liang, Z.; Ma, X.; Ni, Z.; Jin, C.; Zhang, Z. Plasma-assisted fabrication of monolayer phosphorene and its Raman characterization. Nano Res. 2014, 7, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Jang, S.K.; Lai, S.; Xu, J.; Choi, Y.J.; Park, J.H.; Lee, S. Plasma-treated thickness-controlled two-dimensional black phosphorus and its electronic transport properties. ACS Nano 2015, 9, 8729–8736. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Lee, J.Y.; Lee, G.H.; Kim, J. Tuning the thickness of black phosphorus via ion bombardment-free plasma etching for device performance improvement. J. Mater. Chem. C 2016, 4, 6234–6239. [Google Scholar] [CrossRef]
- Andres, C.-G.; Vicarelli, L.; Prada, E.; Island, J.O.; Narasimha-Acharya, K.L.; Blanter, S.I.; Groenendijk, D.J.; Buscema, M.; Steele, G.A.; Alvarez, J.V.; et al. Isolation and characterization of fewlayer black phosphorus. 2D Mater. 2014, 1, 025001. [Google Scholar]
- Zhao, G.; Wang, T.; Shao, Y.; Wu, Y.; Huang, B.; Hao, X. A novel mild phase-transition to prepare black phosphorus nanosheets with excellent energy applications. Small 2017, 13, 1602243. [Google Scholar] [CrossRef] [PubMed]
- Erande, M.B.; Pawar, M.S.; Late, D.J. Humidity sensing and photodetection behavior of electrochemically exfoliated atomically thin-layered black phosphorus nanosheets. ACS Appl. Mater. Interfaces 2016, 8, 11548–11556. [Google Scholar] [CrossRef] [PubMed]
- Joshua, B.S.; Daniel, H.; Hai-Feng, J. Growth of 2D black phosphorus film from chemical vapor deposition. Nanotechnology 2016, 27, 215602. [Google Scholar]
- Yang, Z.; Hao, J.; Yuan, S.; Lin, S.; Yau, H.M.; Dai, J.; Lau, S.P. Field-effect transistors based on amorphous black phosphorus ultrathin films by pulsed laser deposition. Adv. Mater. 2015, 27, 3748–3754. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Pu, H.; Wells, S.A.; Wen, Z.; Mao, S.; Chang, J.; Hersam, M.C.; Chen, J. Ultrahigh sensitivity and layer-dependent sensing performance of phosphorene-based gas sensors. Nat. Commun. 2015, 6, 8632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, C.; Yan, Z.; Song, X.; Zeng, H. 2D materials via liquid exfoliation: A review on fabrication and applications. Sci. Bull. 2015, 60, 1994–2008. [Google Scholar] [CrossRef]
- Brent, J.R.; Savjani, N.; Lewis, E.A.; Haigh, S.J.; Lewis, D.J.; O’Brien, P. Production of fewlayer phosphorene by liquid exfoliation of black phosphorus. Chem. Commun. 2014, 50, 13338–13341. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, H.; Lu, S.; Wang, Z.; Tang, S.; Sha, J.; Sun, Z.; Xie, H.; Wang, H.; Yu, X.-F. From black phosphorus to phosphorene: Basic solvent exfoliation; evolution of Raman scattering; and applications to ultrafast photonics. Adv. Funct. Mater. 2015, 25, 6996–7002. [Google Scholar] [CrossRef]
- Yasaei, P.; Kumar, B.; Foroozan, T.; Wang, C.; Asadi, M.; Tuschel, D.; Indacochea, J.E.; Klie, R.F.; Salehi-Khojin, A. High-quality black phosphorus atomic layers by liquid-phase exfoliation. Adv. Mater. 2015, 27, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Yasaei, P.; Behranginia, A.; Foroozan, T.; Asadi, M.; Kim, K.; Khalili-Araghi, F.; Salehi-Khojin, A. Stable and selective humidity sensing using stacked black phosphorus flakes. ACS Nano 2015, 9, 9898–9905. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.A.; Brent, J.R.; Derby, B.; Haigh, S.J.; Lewis, D.J. Solution processing of two-dimensional black phosphorus. Chem. Commun. 2017, 53, 1445–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef]
- Ang, P.K.; Wang, S.; Bao, Q.; Thong, J.T.L.; Loh, K.P. High-throughput synthesis of graphene by intercalation-exfoliation of graphite oxide and study of ionic screening in graphene transistor. ACS Nano 2009, 3, 3587–3594. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.M.; Yan, H.J.; Ouyang, C.Y. First principles investigation of dynamic performance in the process of lithium intercalation into black phosphorus. Acta Phys. Sin. Chin. Ed. 2012, 61, 247101. [Google Scholar]
- Sun, J.; Lee, H.-W.; Pasta, M.; Yuan, H.; Zheng, G.; Sun, Y.; Li, Y.; Cui, Y. A phosphorene–graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat. Nanotechnol. 2015, 10, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wood, J.D.; Wells, S.A.; Lee, J.-H.; Liu, X.; Chen, K.-S.; Hersam, M.C. Solvent exfoliation of electronic-grade, two-dimensional black phosphorus. ACS Nano 2015, 9, 3596–3604. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Wells, S.A.; Jariwala, D.; Chen, K.-S.; Cho, E.; Sangwan, V.K.; Liu, X.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Effective passivation of exfoliated black phosphorus transistors against ambient degradation. Nano Lett. 2014, 14, 6964–6970. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wells, S.A.; Wood, J.D.; Lee, J.-H.; Liu, X.; Ryder, C.R.; Zhu, J.; Guest, J.R.; Husko, C.A.; Hersam, M.C. Stable aqueous dispersions of optically and electronically active phosphorene. Proc. Natl. Acad. Sci. USA 2016, 113, 11688–11693. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-Y.; Gao, L.-F.; Hu, C.-X.; Zhu, Z.-Y.; Zhao, M.; Wang, Q.; Zhang, H.-L. Preparation of large size, few-layer black phosphorus nanosheets via phytic acid-assisted liquid exfoliation. Chem. Commun. 2016, 52, 8107–8110. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Brent, J.R.; Shorie, M.; Kaur, H.; Chadha, G.; Thomas, A.G.; Lewis, E.A.; Rooney, A.; Nguyen, L.; Zhong, X.L.; et al. Nanostructured aptamer-functionalized black phosphorus sensing platform for label-free detection of myoglobin, a cardiovascular disease biomarker. ACS Appl. Mater. Interfaces 2016, 8, 22860–22868. [Google Scholar] [CrossRef] [PubMed]
- Brent, J.R.; Ganguli, A.K.; Kumar, V.; Lewis, D.J.; McNaughter, P.D.; O’Brien, P.; Sabherwal, P.; Tedstone, A.A. On the stability of surfactant-stabilised few-layer black phosphorus in aqueous media. RSC Adv. 2016, 6, 86955–86958. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yang, X.; Shao, W.; Chen, S.; Xie, J.; Zhang, X.; Wang, J.; Xie, Y. Ultrathin black phosphorus nanosheets for efficient singlet oxygen generation. J. Am. Chem. Soc. 2015, 137, 11376–11382. [Google Scholar] [CrossRef] [PubMed]
- Erande, M.B.; Suryawanshi, S.R.; More, M.A.; Late, D.J. Electrochemically exfoliated black phosphorus nanosheets—Prospective field emitters. Eur. J. Inorg. Chem. 2015, 2015, 3102–3107. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Latiff, N.M.; Eng, A.Y.S.; Sofer, Z.; Pumera, M. Black phosphorus nanoparticle labels for immunoassays via hydrogen evolution reaction mediation. Anal. Chem. 2016, 88, 10074–10079. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Kim, M.; Jeong, S.; Han, J.; Cheon, J. Chemical synthetic strategy for single-layer transition-metal chalcogenides. J. Am. Chem. Soc. 2014, 136, 14670–14673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rui, X.; Tang, Y.; Liu, Y.; Wei, J.; Chen, S.; Li, W.; Liu, Y.; Deng, J.; Ma, B. Wet-chemical processing of phosphorus composite nanosheets for high-rate and high-capacity Lithium-ion batteries. Adv. Energy Mater. 2016, 6, 1502409. [Google Scholar] [CrossRef]
- Bagher, S.; Mansouri, N.; Aghaie, E. Phosphorene: A new competitor for graphene. Int. J. Hydrogen Energy 2016, 41, 4085–4095. [Google Scholar] [CrossRef]
- Chen, X.; Dobson, J.F.; Raston, C.L. Vortex fluidic exfoliation of graphite and boron nitride. Chem. Commun. 2012, 48, 3703–3705. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ge, B.; Chen, J.; Nathan, A.; Xin, L.L.; Ma, H.; Min, H.; Zhu, C.; Xia, W.; Li, Z.; et al. Scalable shear-exfoliation of high-quality phosphorene nanoflakes with reliable electrochemical cycleability in nano batteries. 2D Mater. 2016, 3, 025005. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, H.; Sun, J.; Ma, W.; Li, L.; Li, W.; Du, J. Novel QCM humidity sensors using stacked black phosphorus nanosheets as sensing film. Sens. Actuators B 2017, 244, 259–264. [Google Scholar] [CrossRef]
- Late, D.J. Liquid exfoliation of black phosphorus nanosheets and its application as humidity sensor. Microporous Mesoporous Mater. 2016, 225, 494–503. [Google Scholar] [CrossRef]
- Zhu, C.; Feng, X.; Zhang, L.; Li, M.; Chen, J.; Xu, S.; Huang, G.; Chen, W.; Sun, L. Ultrafast preparation of black phosphorus quantum dots for efficient humidity sensing. Chem. Eur. J. 2016, 22, 7357–7362. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.-T.; Park, I.; Park, A.-R.; Park, C.-M.; Jeon, K.-J. Black P/graphene hybrid: A fast response humidity sensor with good reversibility and stability. Sci. Rep. 2017, 7, 10561. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Xu, J.; Yao, Y. SIW resonator humidity sensor based on layered black phosphorus. Electron. Lett. 2017, 53, 249–251. [Google Scholar] [CrossRef]
- Chen, C.-M.; Xu, J. A miniaturized evanescent mode HMSIW humidity sensor. Int. J. Microw. Wirel. Technol. 2018, 10, 87–91. [Google Scholar] [CrossRef]
- Walia, S.; Sabri, Y.; Ahmed, T.; Field, M.R.; Ramanathan, R.; Arash, A.; Bhargava, S.K.; Sriram, S.; Bhaskaran, M.; Bansal, V.; et al. Defining the role of humidity in the ambient degradation of few-layer black phosphorus. 2D Mater. 2017, 4, 015025. [Google Scholar] [CrossRef]
- He, P.; Brent, J.R.; Ding, H.; Yang, J.; Lewis, D.J.; Brien, P.O.; Derby, B. Fully printed high performance humidity sensors based on two-dimensional materials. Nanoscale 2018, 10, 5599–5606. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Kim, S.; Jung, S.; Jang, S.; Kim, J. Suspended black phosphorus nanosheet gas sensors. Sens. Actuators B 2017, 250, 569–573. [Google Scholar] [CrossRef]
- Donarelli, M.; Ottaviano, L.; Giancaterini, L.; Fioravanti, G.; Perrozzi, F.; Cantalini, C. Exfoliated black phosphorus gas sensing properties at room temperature. 2D Mater. 2016, 3, 025002. [Google Scholar] [CrossRef]
- Miao, J.; Cai, L.; Zhang, S.; Nah, J.; Yeom, J.; Wang, C. Air-stable humidity sensor using few-layer black phosphorus. ACS Appl. Mater. Interfaces 2017, 9, 10019. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Q.; Zhang, G.; Zhang, Y.W. Electronic properties of phosphorene/graphene and phosphorene/hexagonal boron nitride heterostructures. J. Phys. Chem. C 2015, 119, 13929–13936. [Google Scholar] [CrossRef]
- Das, S.; Zhang, W.; Demarteau, M.; Hoffmann, A.; Dubey, M.; Roelofs, A. Tunable transport gap in phosphorene. Nano Lett. 2014, 14, 5733–5739. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Baik, S.S.; Ryu, S.H.; Sohn, Y.; Park, S.; Park, B.-G.; Denlinger, J.; Yi, Y.; Choi, H.J.; Kim, K.S. Observation of tunable band gap and anisotropic dirac semimetal state in black phosphorus. Science 2015, 349, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Soklaski, R.; Liang, Y.; Yang, L. Layer-controlled band gap and anisotropic excitons in few-layer black phosphorus. Phys. Rev. B 2014, 89, 235319. [Google Scholar] [CrossRef]
- Conley, H.J.; Wang, B.; Ziegler, J.I.; Haglund, R.F., Jr.; Pantelides, S.T.; Bolotin, K.I. Bandgap engineering of strained monolayer and bilayer MoS2. Nano Lett. 2013, 13, 3626–3630. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, Q.; Tong, Y.; Wang, J. Light-induced ambient degradation of few-layer black phosphorus: Mechanism and protection. Angew. Chem. Int. Ed. 2016, 55, 11437–11441. [Google Scholar] [CrossRef] [PubMed]
- Rodin, A.S.; Carvalho, A.; Castro Neto, A.H. Strain-induced gap modification in black phosphorus. Phys. Rev. Let. 2014, 112, 176801. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Pandey, R.; Karna, S.P. Phosphorene oxide: Stability and electronic properties of a novel two-dimensional material. Nanoscale 2015, 7, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lu, W.; Chen, B.; Zhong, D.; Huang, L.; Dong, L.; Jin, C.; Zhang, Z. Performance change of few layer black phosphorus transistors in ambient. AIP Adv. 2015, 5, 107112. [Google Scholar] [CrossRef] [Green Version]
- Ling, X.; Wang, H.; Huang, S.X.; Xia, F.N.; Dresselhaus, M.S. The renaissance of black phosphorus. Proc. Natl. Acad. Sci. USA 2015, 112, 4523–4530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favron, A.; Gaufrès, E.; Fossard, F.; Phaneuf-L’Heureux, A.-L.; Tang, N.Y.; Lévesque, P.L.; Lioseau, A.; Leonelli, R.; Francoeur, S.; Martel, R. Photooxidation and quantum confinement effects in exfoliated black phosphorus. Nat. Mater. 2015, 14, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qiao, J.; He, K.; Bliznakov, S.; Sutter, E.; Chen, X.; Luo, D.; Meng, F.; Su, D.; Decker, J.; et al. Degradation of black phosphorus: The role of oxygen and water. Chem. Mater. 2016, 28, 8330–8339. [Google Scholar] [CrossRef]
- Wang, G.; Slough, W.J.; Pandey, R.; Karna, S.P. Degradation of phosphorene in air: Understanding at atomic level. 2D Mater. 2016, 3, 025011. [Google Scholar] [CrossRef]
- Ziletti, A.; Carvalho, A.; Campbell, D.K.; Coker, D.F.; Neto, A.C. Oxygen defects in phosphorene. Phys. Rev. Lett. 2015, 114, 046801. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M.T.; Tadich, A.; Carvalho, A.; Ziletti, A.; Donnell, K.M.O.; Koenig, S.P.; Coker, D.F.; Ozyilmaz, B.; Castro Neto, A.H.; Fuhrer, M.S. Creating a stable oxide at the surface of black phosphorus. ACS Appl. Mater. Interfaces 2015, 7, 14557–14562. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wood, J.D.; Chen, K.-S.; Cho, E.; Hersam, M.C. In situ thermal decomposition of exfoliated two-dimensional black phosphorus. J. Phys. Chem. Lett. 2015, 6, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, D.; Liu, J.; Chang, H.; Sun, Y.; Yin, N. Air-stable black phosphorus devices for ion sensing. ACS Appl. Mater Interfaces 2015, 7, 24396. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.P.; Doganov, R.A.; Schmidt, H.; Neto, A.H.C.; Ozyilmaz, B. Electric field effect in ultrathin black phosphorus. Appl. Phys. Lett. 2014, 104, 103106. [Google Scholar] [CrossRef] [Green Version]
- Wan, B.S.; Yang, B.C.; Wang, Y.; Zhang, J.Y.; Zeng, Z.M.; Liu, Z.Y.; Wang, W. Enhanced stability of black phosphorus field-effect transistors with SiO2 passivation. Nanotechnology 2015, 26, 453702. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Kozawa, D.; Liu, A.T.; Koman, V.B.; Wang, Q.H.; Strano, M.S. A study of bilayer phosphorene stability under MoS2-passivation. 2D Mater. 2017, 4, 025091. [Google Scholar] [CrossRef]
- Xing, C.; Jing, G.; Liang, X.; Qiu, M.; Li, Z.; Cao, R.; Li, X.; Fan, D.; Zhang, H. Graphene oxide/black phosphorus nanoflake aerogels with robust thermo-stability and significantly enhanced photothermal properties in air. Nanoscale 2017, 9, 8096–8101. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.J.R.; Abdelwahab, I.; Chu, L.; Poh, S.M.; Liu, Y.; Lu, J.; Lu, J.; Chen, W.; Loh, K.P. Quasi-monolayer black phosphorus with high mobility and air stability. Adv. Mater. 2018, 30, 1704619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, N.; Tao, H.; Yan, C.; Huang, J.; Liu, T.; Robertson, A.W.; Texter, J.; Wang, J.; Sun, Z. Exfoliation of Stable 2D black phosphorus for device fabrication. Chem. Mater. 2017, 29, 6445–6456. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef] [PubMed]
- Ziletti, A.; Carvalho, A.; Trevisanutto, P.E.; Campbell, D.K.; Coker, D.F.; Castro Neto, A.H. Phosphorene oxides: Bandgap engineering of phosphorene by oxidation. Phys. Rev. B 2015, 91, 085407–085416. [Google Scholar] [CrossRef]

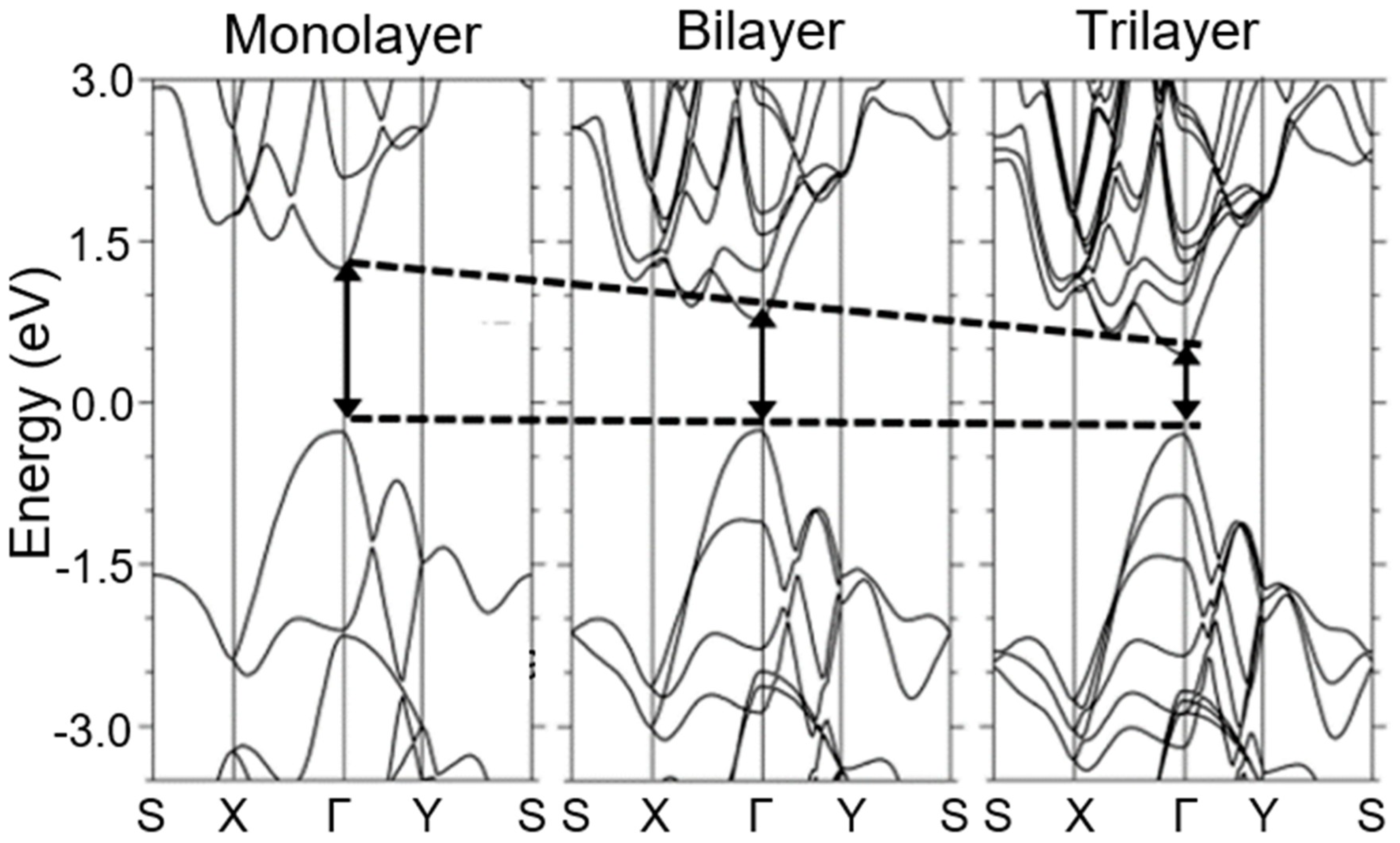










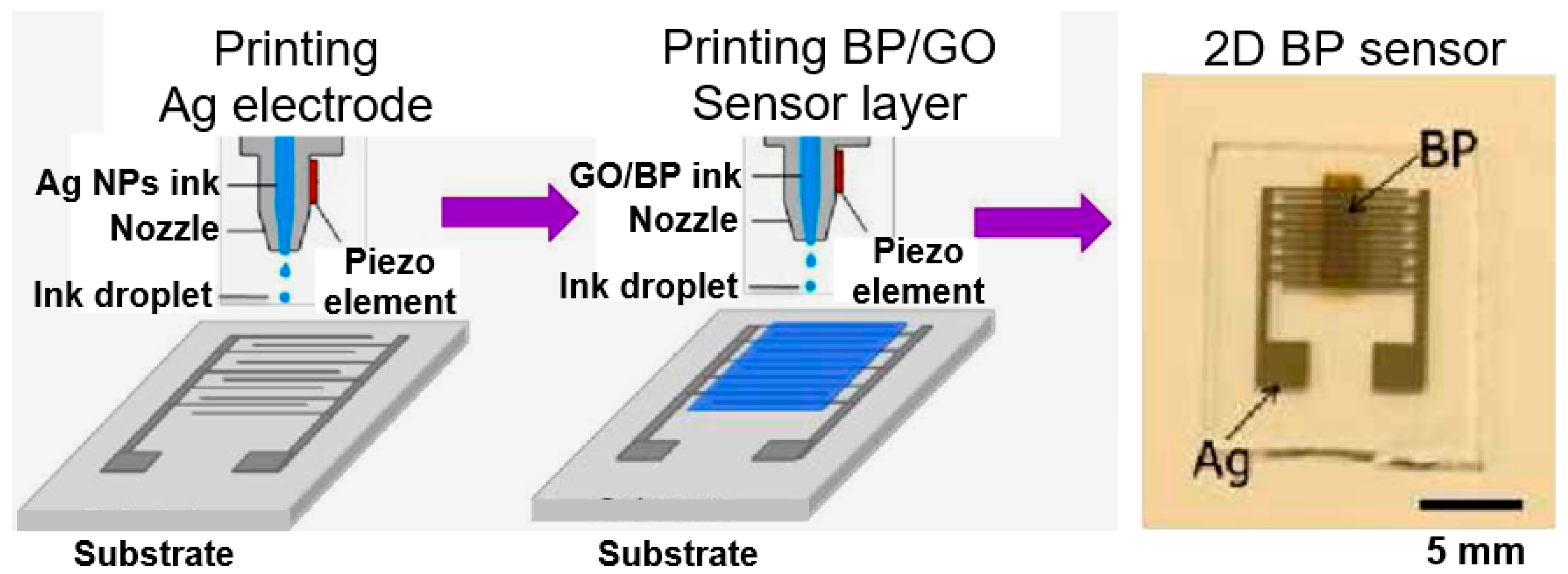
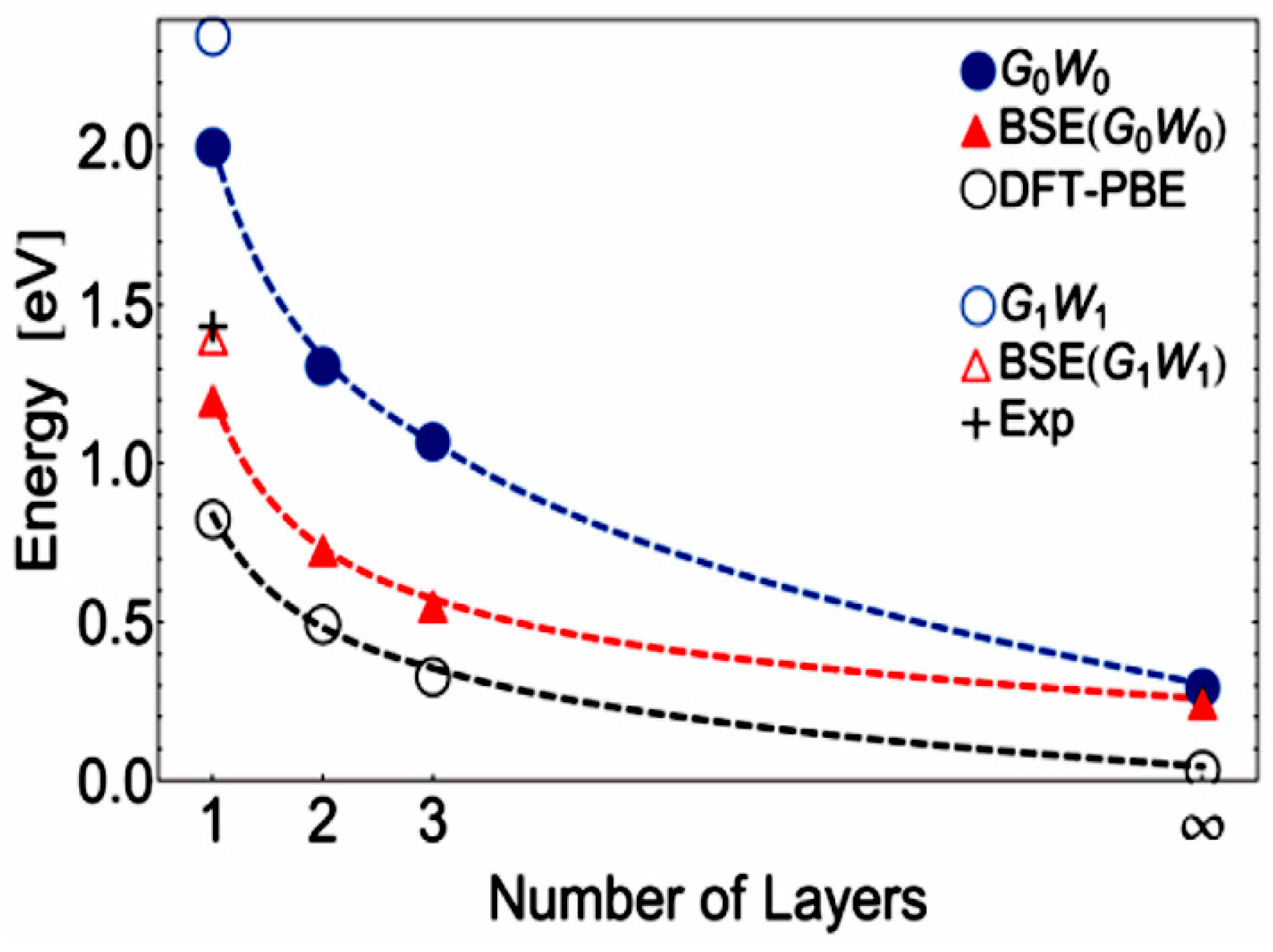
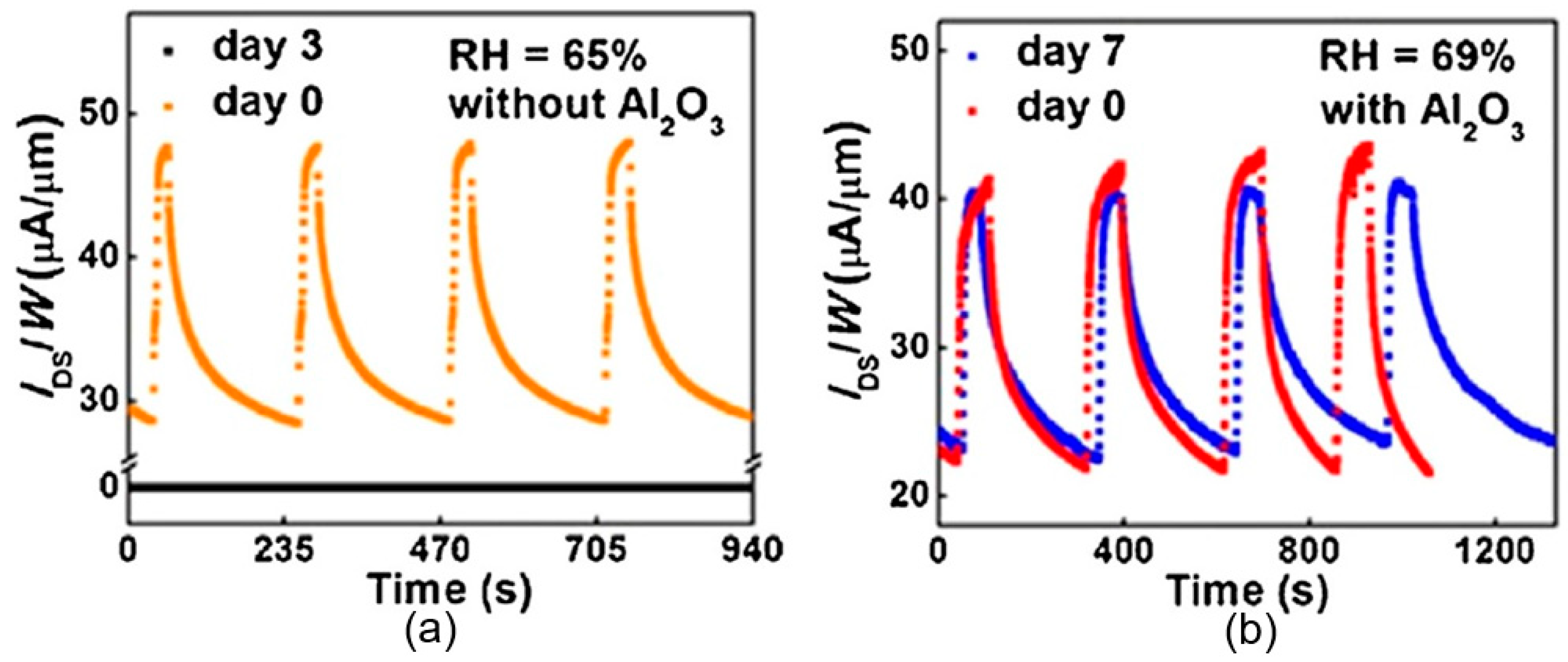







| Material Type | Phosphorene | Graphene | MoS2 | h-BN |
|---|---|---|---|---|
| Conduction type | Semiconductor, ambipolar | Semimetal | Semiconductor, n-type | Insulator |
| Band gap (eV) | 0.3–2.0 | 0 | 1.2–1.8 | 5.9 |
| Carrier mobility (cm2/V·s) | 600–1000 | 200,000 | 200 | - |
| On/off ratio | 103–105 | 5.5–44 | 106–108 | - |
| Thermal conductance (W/m·K) | 10–36 | 2000–5000 | 34.5–52 | 250–360 |
| Thermoelectric figure of merit, ZT | 0.2–2.2 | 0 | 0.4 | - |
| Strain to failure (%) | 24–32 | 19.4–38 | 19.5–36 | 24 |
| Young’s modulus (GPa) | 35–166 | 1000 | 270 ± 100 | 220–880 |
| Process | Precursor | Treatment | Thickness, nm | Ref. |
|---|---|---|---|---|
| Mechanical exfoliation | BP | Scotch tape on SiO2/Si | 0.7–6 | [73] |
| ME-PDMS | BP | Scotch tape on SiO2/Si, curved PDMS | 1.6–2.8 | [85] |
| Hydrothermal | RP, NH4F | Teflon lined autoclave, 200 °C | 3 | [86] |
| Electrochemical exfoliation | BP, Pt, Na2SO4 | Voltage of +7 V was applied across electrode for 90 min | 1.4–10 | [87] |
| Plasma assisted exfoliation | BP | Ar+ plasma at 30 W, the pressure of 30 Pa, 20 s | 2–10 | [82] |
| LPE | BP, organic solvent/water/ionic liquids | Bath sonication for 24–48 h/tip sonication for 2–4 h, centrifugation at 2000–1000 rpm for 30 min | 0.7–6 | [73] |
| CVD | BP thin film over Si (substrate) SnI4, Sn | Tube furnace, 950 °C | 3.4 | [88] |
| Pulsed layer deposition | BP | KrF (λ: 248 nm, ν: 5 Hz), 150 °C, vacuum chamber | N/A | [89] |
| Materials | Transduction Methods | Sensor Response | Response Time (s) | Recovery Time (s) | Test Range (%RH) | Ref. |
|---|---|---|---|---|---|---|
| BP flakes | Resistive | ∼4 orders from 10%RH to 85%RH | <1 | ~1–2 | 10–85 | [95] |
| BP flakes | Resistive | 521% at 97%RH | 101 | 26 | 11–97 | [87] |
| BP flakes | Resistive | 99.17% at 97.3%RH | 255 | 10 | 11–97 | [116] |
| BP quantum dots | Resistive | ∼4 orders from 10%RH to 90%RH | - | - | 10–90 | [117] |
| BP/graphene | Resistive | 43.4% at 70%RH | 9 | 30 | 15–70 | [118] |
| BP flakes | Substrate-integrated waveguide (SIW) resonator | 197.67 kHz/%RH | - | - | 11–97 | [119] |
| 5.82 MHz/%RH at RH >84% | - | - | 11–97 | [120] | ||
| BP flakes | Quartz crystal microbalance (QCM) | 82.7 Hz/pg at 90%RH | 14 | 10 | 10–90 | [115,121] |
| BP flakes | Capacitive | 3–4 orders from 11%RH to 97%RH (∆C/C) | 4.7 | 3 | 11–97 | [122] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korotcenkov, G. Black Phosphorus-New Nanostructured Material for Humidity Sensors: Achievements and Limitations. Sensors 2019, 19, 1010. https://doi.org/10.3390/s19051010
Korotcenkov G. Black Phosphorus-New Nanostructured Material for Humidity Sensors: Achievements and Limitations. Sensors. 2019; 19(5):1010. https://doi.org/10.3390/s19051010
Chicago/Turabian StyleKorotcenkov, Ghenadii. 2019. "Black Phosphorus-New Nanostructured Material for Humidity Sensors: Achievements and Limitations" Sensors 19, no. 5: 1010. https://doi.org/10.3390/s19051010





