A Room Temperature Gas Sensor Based on Sulfonated SWCNTs for the Detection of NO and NO2
Abstract
:1. Introduction
2. Experimental Approach
2.1. Materials and Methods
2.2. Gas Sensing Procedure
3. Results and Discussions
3.1. Characterization of Functionalized/Sulfonated CNTs
3.2. Structural Characterization
3.3. Gas Sensing Characterization
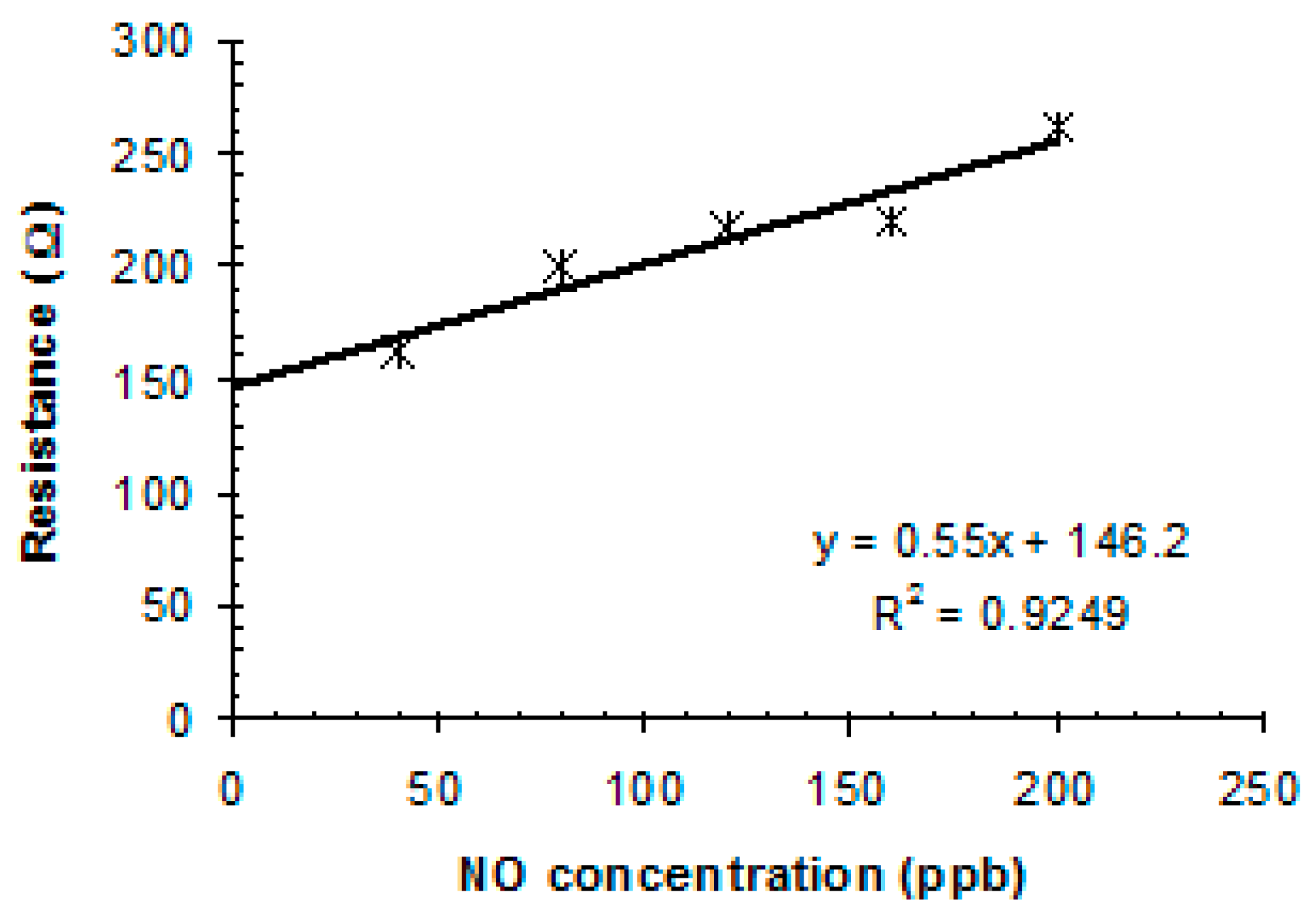
3.3.1. Performance of sulfonated-SWCNTs nanocomposite in detecting NO in N2
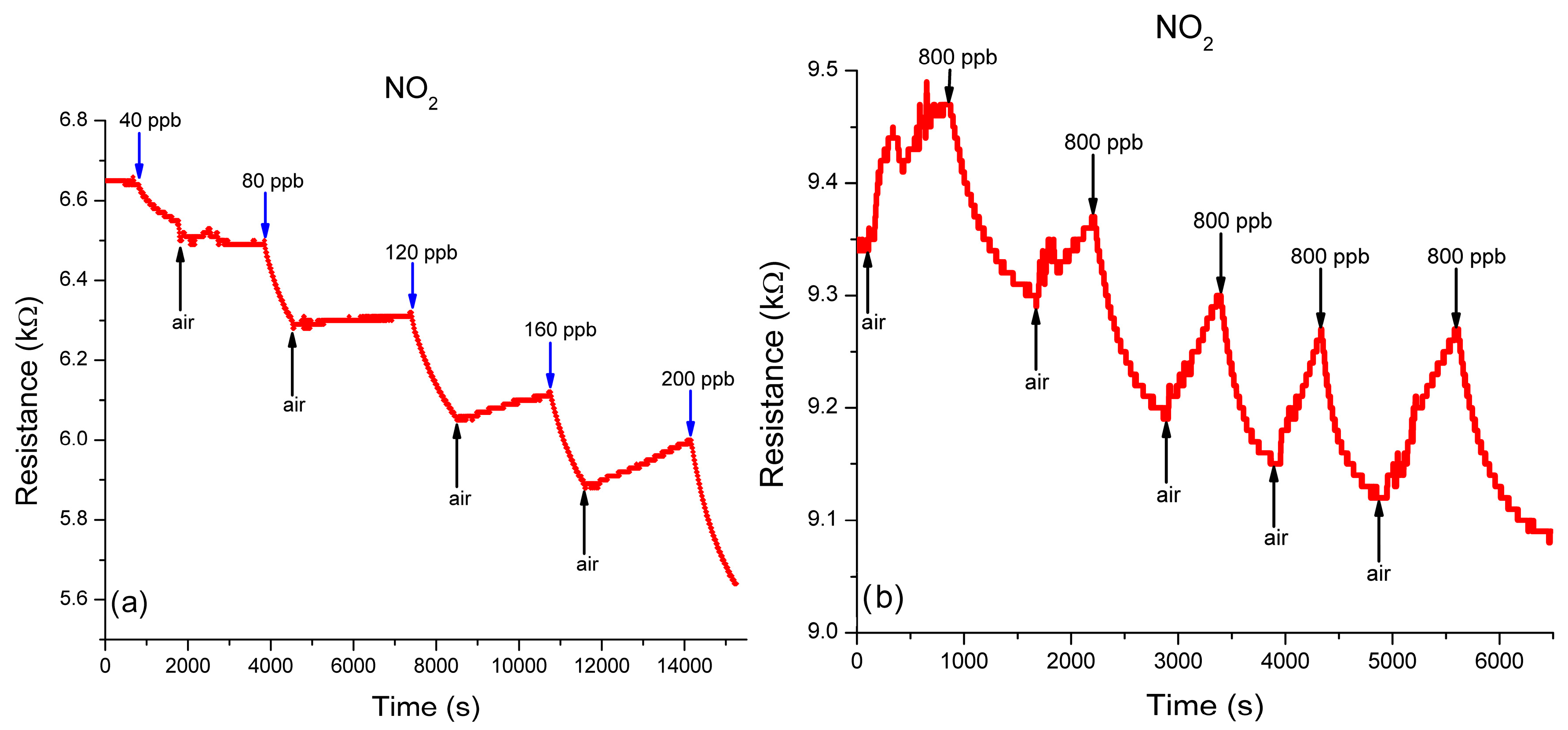
3.3.2. Performance of Sulfonated-SWCNTs Nanocomposite in Detecting NO2 in N2
3.3.3. Recovery after exposure to NO and NO2
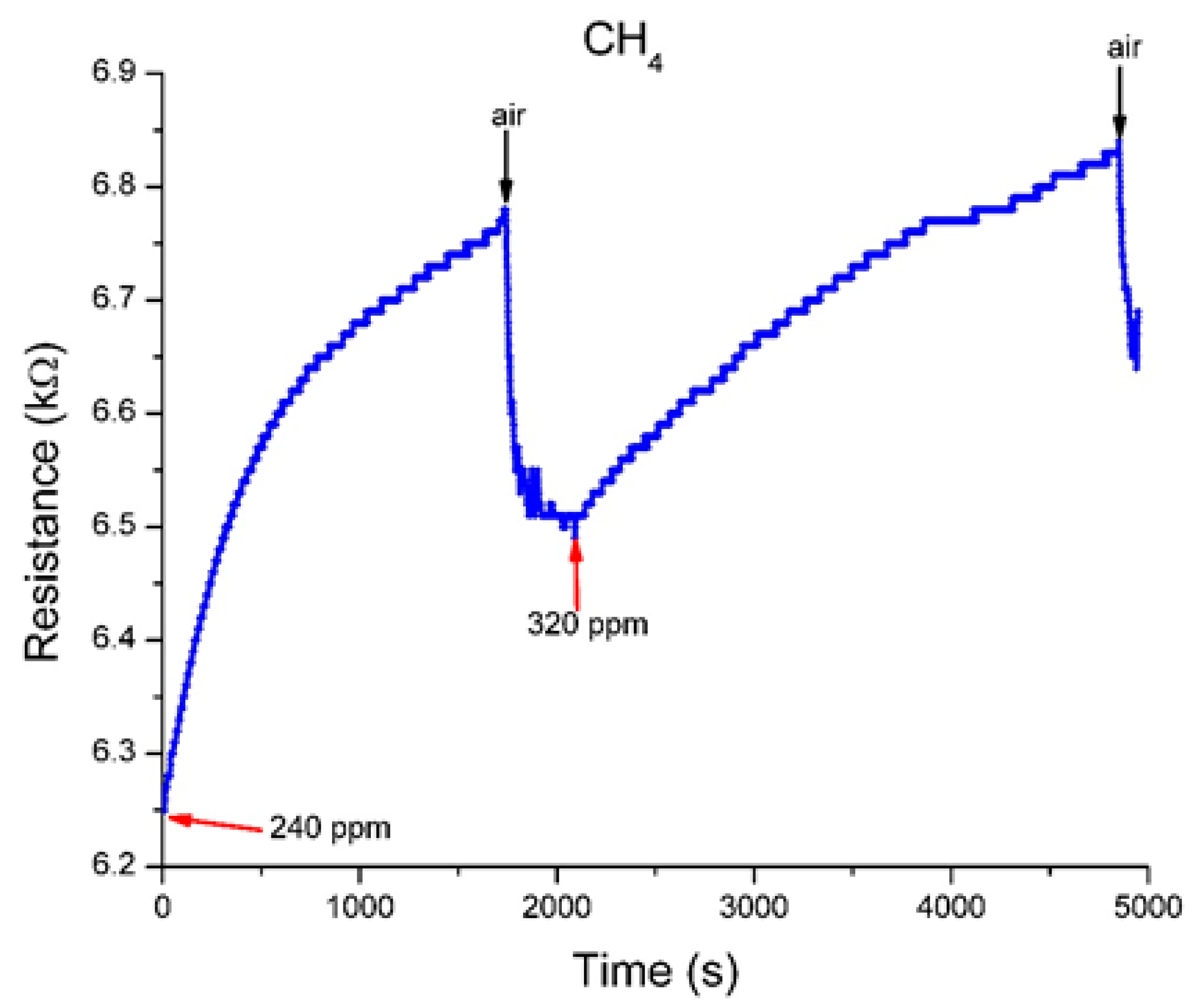
3.3.4. Exposure to H2 and CH4
3.4. Mechanism of Detection
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meyyappan, M. Carbon Nanotubes: Science and Applications; NASA Ames Research Center: Mofett Field, CA, USA, 2005.
- Bakhoum, E.G. Micro-and Nano-Scale Sensors and Transducers; Taylor and Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Battie, Y.; Ducloux, O. Coated and Functionalized Single-Walled Carbon Nanotubes (SWCNTs) as Gas Sensors; Woodhead Publishing Limited: Sawston, UK, 2013; pp. 356–385. [Google Scholar]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef] [Green Version]
- Ueda, T.; Bhuiyan, M.M.H.; Norimatsu, H.; Katsuki, S.; Ikegami, T.; Mitsugi, F. Development of carbon nanotube-based gas sensors for NOx gas detection working at low temperature. Phys. E 2008, 40, 2272–2277. [Google Scholar] [CrossRef]
- Qazi, M.; Koley, G. NO2 Detection Using Microcantilever Based Potentiometry. Sensors 2008, 8, 7144–7156. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, J.; Zhou, G.; Imakiire, H.; Ding, W.; Hara, M. Controlled fabrication of carbon nanotube NO2 gas sensor using dielectrophoretic impedance measurement. Sens. Actuators B 2005, 108, 398–403. [Google Scholar] [CrossRef]
- Rani, B.; Singh, U.; Chuhan, A.K.; Sharma, D.; Maheshwari, R. Photochemical smog pollution and its mitigation measures. J. Adv. Sci. Res. 2011, 2, 28–33. [Google Scholar]
- Lundberg, J.N.O.; Weitzberg, E.; Lundberg, J.M.; Alving, K. Nitric oxide in exhaled air. Eur. Respir. J. 1996, 9, 2671–2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Hu, J.; Lv, Y.; Hou, X. Recent progress in chemiluminescence for gas analysis. Appl. Spectrosc. Rev. 2010, 45, 474–489. [Google Scholar] [CrossRef]
- Kong, J.; Chapline, M.G.; Dai, H. Functionalized Carbon Nanotubes for Molecular Hydrogen Sensors. Adv. Mater. 2001, 13, 1384–1386. [Google Scholar] [CrossRef]
- Ong, K.G.; Zeng, K.; Grimes, C.A. A wireless, passive carbon nanotube-based gas sensor. IEEE Sens. J. 2002, 2, 82–88. [Google Scholar]
- Chopra, S.; McGuire, K.; Gothard, N.; Rao, A.M.; Pham, A. Selective gas detection using a carbon nanotube sensor. Appl. Phys. Lett. 2003, 83, 2280–2281. [Google Scholar] [CrossRef]
- Valentini, L.; Armentano, I.; Kenny, J.M.; Cantalini, C.; Lozzi, L.; Santucci, S. Sensors for sub-ppm NO2 gas detection based on carbon nanotube thin films. Appl. Phys. Lett. 2003, 82, 961. [Google Scholar] [CrossRef]
- Rouxinol, F.P.; Gelamo, R.V.; Moshkalev, S.A. Gas sensors based on decorated carbon nanotubes. In Carbon Nanotubes; InTech: Rijeka, Croatia, 2010; pp. 357–374. [Google Scholar]
- Goldoni, A.; Petaccia, L.; Lizzit, S.; Larciprete, R. Sensing gases with carbon nanotubes: A review of the actual situation. J. Phys. Condens. Matter 2010, 22, 013001. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, M.; Rigoni, F.; Paderno, M.; Borghetti, P.; Gagliotti, G.; Bertoni, M.; Ballarin Denti, A.; Schiavina, L.; Goldoni, A.; Sangaletti, L. Development of low-cost ammonia gas sensors and data analysis algorithms to implement a monitoring grid of urban environmental pollutants. J. Environ. Monit. 2012, 14, 1565. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Osorio, A.G.; Silveira, I.C.L.; Bueno, V.L.; Bergmann, C.P. H2SO4/HNO3/HCl—Functionalization and its effect on dispersion of carbon nanotubes in aqueous media. Appl. Surf. Sci. 2008, 255, 2485–2489. [Google Scholar] [CrossRef]
- Fareghi-Alamdari, R.; Golestanzadeh, M.; Agend, F.; Zekri, N. Application of highly sulfonated single-walled carbon nanotubes: An efficient heterogeneous catalyst for the one-pot synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes under solvent-free conditions. C. R. Chim. 2013, 16, 878–887. [Google Scholar] [CrossRef]
- Mansor, N.A.; Tessonnier, J.P.; Rinaldi, A.; Reiche, S.; Kutty, M.G. Chemically modified multi-walled carbon nanotubes (MWCNTs) with anchored acidic groups. Sains Malays. 2012, 41, 603–609. [Google Scholar]
- Rositani, F.; Antonucci, P.L.; Minutoli, M.; Giordano, N.; Villari, A. Infrared analysis of carbon blacks. Carbon 1987, 25, 325–332. [Google Scholar] [CrossRef]
- Dogan, H.; Yildiz, E.; Kaya, M.; Yinan, T. Sulfonated carbon black-based composite membranes for fuel cell applications. Bull. Mater. Sci. 2013, 36, 563–573. [Google Scholar] [CrossRef]
- Ionete, E.I.; Spiridon, S.I.; Monea, B.F.; Ebrasu-Ion, D.; Vaseashta, A. SWCNT-Pt-P2O5-Based Sensor for Humidity Measurements. IEEE Sens. J. 2016, 16, 7593–7599. [Google Scholar] [CrossRef]
- Hannon, A.; Lu, Y.; Li, J.; Meyyappan, M. Room temperature carbon nanotube based sensor for carbon monoxide detection. J. Sens. Sens. Syst. 2014, 3, 349–354. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Chang, H.; Lee, J.D.; Lee, S.M.; Lee, Y.H. Adsorption of NH3 and NO2 molecules on carbon nanotubes. Appl. Phys. Lett. 2001, 79, 3863–3865. [Google Scholar] [CrossRef]
- Yim, W.L.; Gong, X.G.; Liu, Z.F. Chemisorption of NO2 on carbon nanotubes. J. Phys. Chem. B 2003, 107, 9363–9369. [Google Scholar] [CrossRef]
- Ellison, M.D.; Crotty, M.J.; Koh, D.; Spray, R.L.; Tate, K.E. Adsorption of NO3 and NO2 on single-walled carbon nanotubes. J. Phys. Chem. B 2004, 108, 7939–7942. [Google Scholar] [CrossRef]
- Ricca, A.; Bauschlicher, C.W. The adsorption of NO2 on (9,0) and (10,0) carbon nanotubes. Chem. Phys. 2006, 323, 511–518. [Google Scholar] [CrossRef]
- Sayago, I.; Santos, H.; Horrillo, M.C.; Aleixandre, M.; Fernandez, M.J.; Terrado, E.; Tacchini, I.; Aroz, R.; Maser, W.K.; Benito, A.M.; et al. Carbon nanotube networks as gas sensors for NO2 detection. Talanta 2008, 77, 758–764. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Z.; Zhang, J.; Pu, J.; Lin, Y.; Xu, S.; Shen, L.; Chen, Q.; Shi, W. Fully printed, rapid-response sensors based on chemically modified graphene foe detecting NO2 at room temperature. ACS Appl. Mater. Interface 2014, 6, 7426–7433. [Google Scholar] [CrossRef] [PubMed]










| Gas Process | H2 (ppm) | CH4 (ppm) | |||||
|---|---|---|---|---|---|---|---|
| 25 | 100 | 125 | 150 | 200 | 240 | 320 | |
| Response | 2579 | 2347 | 1480 | 5233 | 3897 | 1694 | 2693 |
| Regeneration | 889 | 822 | 521 | 1352 | 2483 | 352 | 489 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionete, E.I.; Spiridon, S.I.; Monea, B.F.; Stratulat, E. A Room Temperature Gas Sensor Based on Sulfonated SWCNTs for the Detection of NO and NO2. Sensors 2019, 19, 1116. https://doi.org/10.3390/s19051116
Ionete EI, Spiridon SI, Monea BF, Stratulat E. A Room Temperature Gas Sensor Based on Sulfonated SWCNTs for the Detection of NO and NO2. Sensors. 2019; 19(5):1116. https://doi.org/10.3390/s19051116
Chicago/Turabian StyleIonete, Eusebiu Ilarian, Stefan Ionut Spiridon, Bogdan Florian Monea, and Elena Stratulat. 2019. "A Room Temperature Gas Sensor Based on Sulfonated SWCNTs for the Detection of NO and NO2" Sensors 19, no. 5: 1116. https://doi.org/10.3390/s19051116





