Investigation of Micro-volume Viscosity with Janus Microbeads Based on Rotational Brownian Motion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rotational Brownian Motion and Cross-Correlation Algorithm
2.2. Measurement Concept and Experimental Setup
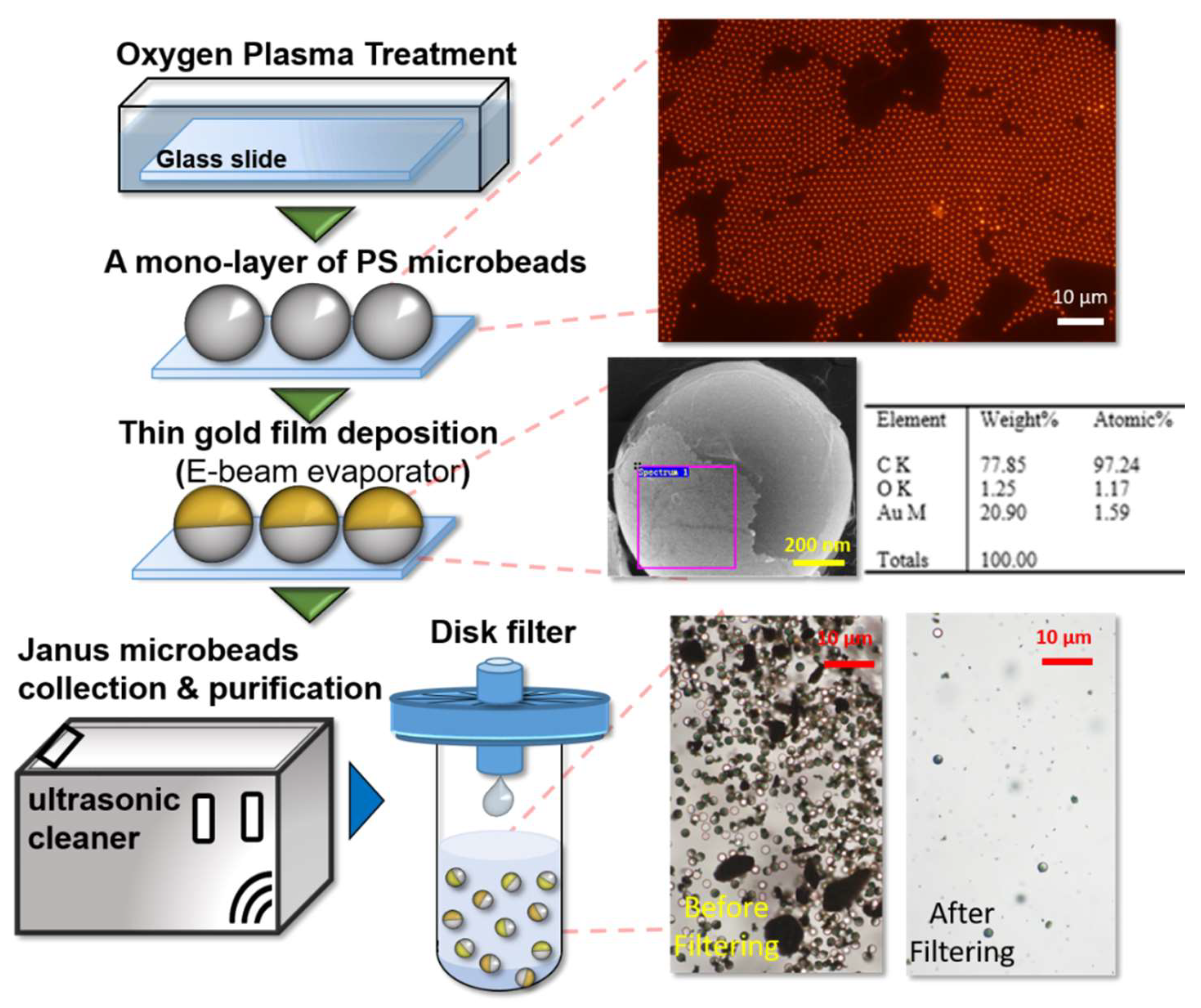
2.3. Fabrication of Janus Microbeads
3. Results and Discussion
3.1. Evaluation of Rotational Brownian Motion with Different Microbead Sizes
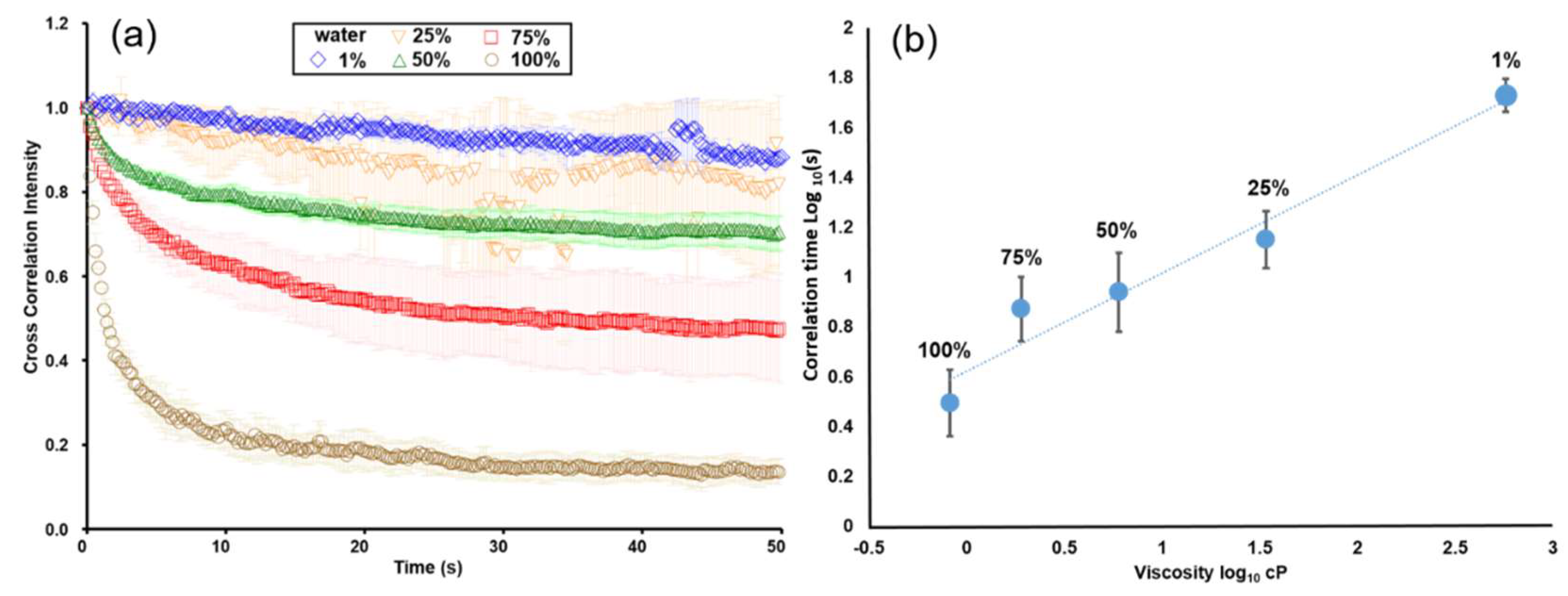
3.2. Calibration of the Viscosity of Glycerol–Water Mixtures
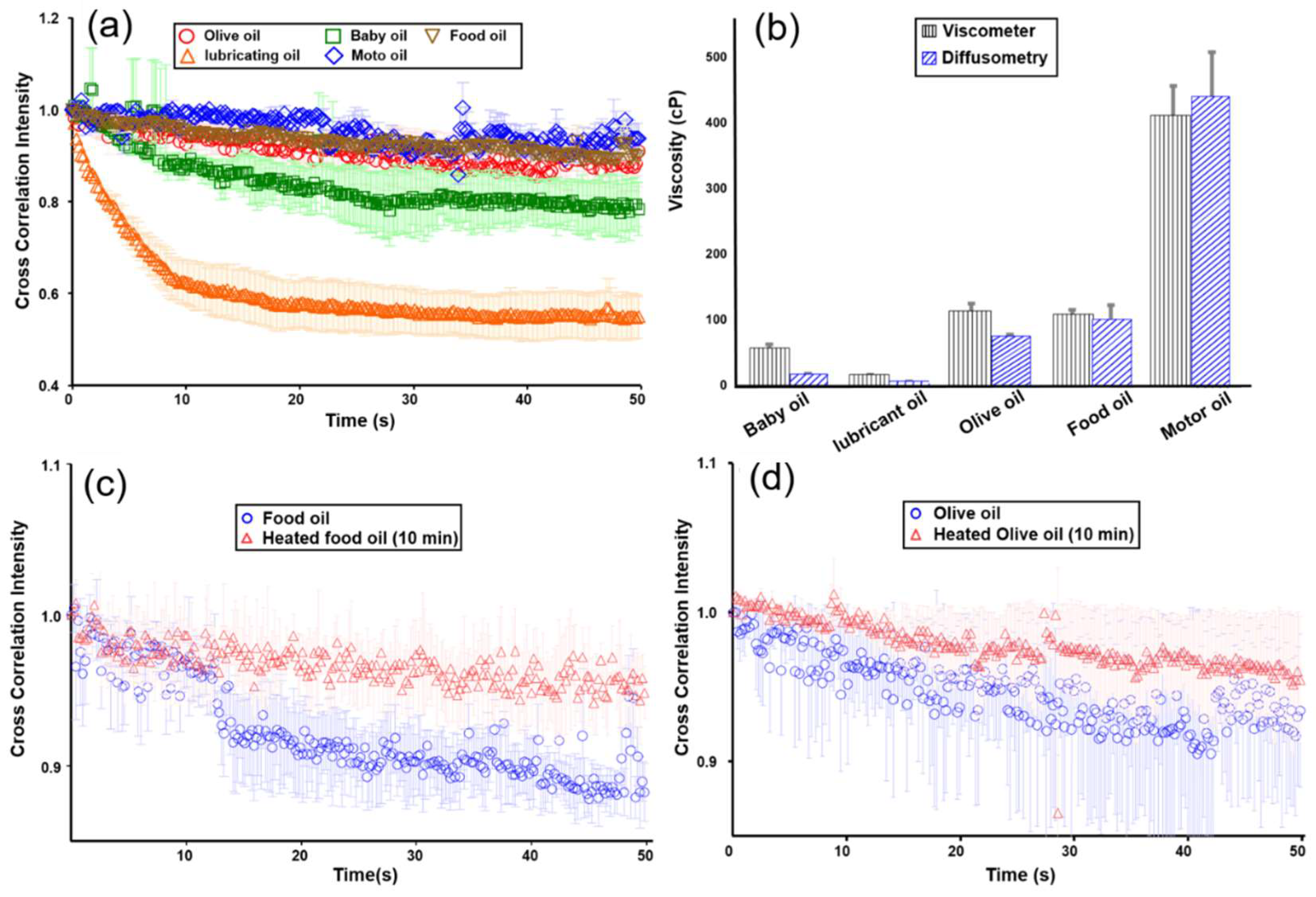
3.3. Viscosity Measurements of Oil Products and their Degradation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bercea, M.; Darie, R.N.; Nita, L.E.; Morariu, S. Temperature responsive gels based on pluronic F127 and poly(vinylalcohol). Ind. Eng. Chem. Res. 2011, 50, 4199–4206. [Google Scholar] [CrossRef]
- Kümmerli, R.; Griffin Ashleigh, S.; West Stuart, A.; Buckling, A.; Harrison, F. Viscous medium promotes cooperation in the pathogenic bacterium Pseudomonas aeruginosa. Proc. Roy. Soc. B Biol Sci. 2009, 276, 3531–3538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.C.O.; Santos, I.M.G.; Souza, A.G. Effect of heating and cooling on rheological parameters of edible vegetable oils. J. Food Eng. 2005, 67, 401–405. [Google Scholar] [CrossRef]
- Sánchez-Gimeno, A.C.; Negueruela, A.I.; Benito, M.; Vercet, A.; Oria, R. Some physical changes in Bajo Aragón extra virgin olive oil during the frying process. Food Chem. 2008, 110, 654–658. [Google Scholar] [CrossRef]
- Perumalla Venkata, R.; Subramanyam, R. Evaluation of the deleterious health effects of consumption of repeatedly heated vegetable oil. Toxicol. Rep. 2016, 3, 636–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, X.F.; Ng, C.Y.; Jaarin, K.; Mustafa, M.R. Effects of repeated heating of cooking oils on antioxidant content and endothelial function. Austin J. Pharmacol. Ther. 2015, 3, 1068. [Google Scholar]
- Chen, G.; Zhao, L.; Liu, Y.; Liao, F.; Han, D.; Zhou, H. Regulation of blood viscosity in disease prevention and treatment. Chin. Sci. Bull. 2012, 57, 1946–1952. [Google Scholar] [CrossRef] [Green Version]
- Naghedi-Baghdar, H.; Nazari, S.-M.; Taghipour, A.; Nematy, M.; Shokri, S.; Mehri, M.-R.; Molkara, T.; Javan, R. Effect of diet on blood viscosity in healthy humans: A systematic review. Electron. Physician 2018, 10, 6563–6570. [Google Scholar] [CrossRef] [PubMed]
- Keedy, A. An overview of intracranial aneurysms. McGill J. Med. 2006, 9, 141–146. [Google Scholar] [PubMed]
- Yeom, E.; Kang, Y.J.; Lee, S.-J. Changes in velocity profile according to blood viscosity in a microchannel. Biomicrofluidics 2014, 8, 034110. [Google Scholar] [CrossRef] [PubMed]
- Peisker, H.; Heepe, L.; Kovalev, A.E.; Gorb, S.N. Comparative study of the fluid viscosity in tarsal hairy attachment systems of flies and beetles. J. R. Soc. Interface 2014, 11, 20140752. [Google Scholar] [CrossRef] [PubMed]
- Sklubalová, Z.; Zatloukal, Z. A comparison of capillary and rotational viscometry of aqueous solutions of hypromellose. Pharmazie 2007, 62, 779–781. [Google Scholar] [PubMed]
- Shin, S.; Ku, Y.; Park, M.-S.; Suh, J.-S. Measurement of blood viscosity using a pressure-scanning capillary viscometer. Clin. Hemorheol. Microcirc. 2004, 30, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Schaschke, C.J. High pressure viscosity measurement with falling body type viscometers. Int. Rev. Chem. Eng. 2010, 2, 564–576. [Google Scholar]
- Mark, M.; Häusler, K.; Dual, J.; Reinhart, W.H. Oscillating viscometer—Evaluation of a new bedside test. Biorheology 2006, 43, 133–146. [Google Scholar] [PubMed]
- Prasad, V.S.K.; Balasubramaniam, K.; Kannan, E.; Geisinger, K.L. Viscosity measurements of melts at high temperatures using ultrasonic guided waves. J. Mater. Proc. Technol. 2008, 207, 315–320. [Google Scholar] [CrossRef]
- Martin, M.; Christian, M.; Jan, D.; Tobias, K. Viscosity quantification using multi-contrast magnetic particle imaging. New J. Phys. 2018, 20, 083001. [Google Scholar]
- Muhammad, Y.; Arifin, A. Design of oil viscosity sensor based on plastic optical fiber. J. Phys. Conf. Ser. 2018, 979, 012083. [Google Scholar]
- Zhang, Y.; Wu, X.; Wang, Y.; Zhu, S.; Gao, B.Z.; Yuan, X.C. Measurement of the microscopic viscosities of microfluids with a dynamic optical tweezers system. Laser Phys. 2014, 24, 065601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Nava, G.; Vitali, V.; Bragheri, F.; Osellame, R.; Bellini, T.; Cristiani, I.; Minzioni, P. Integrated optofluidic chip for low-volume fluid viscosity measurement. Micromachines 2017, 8, 65. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, S.Y.; Jee, S.; Atajanov, A.; Yang, S. Micro-viscometer for measuring shear-varying blood viscosity over a wide-ranging shear rate. Sensors 2017, 17, 1442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, Y.; Wang, T.; Ding, J.; Liu, X.; Zhao, Y.; Jiang, Z. A MEMS resonant sensor to measure fluid density and viscosity under flexural and torsional vibrating modes. Sensors 2016, 16, 830. [Google Scholar] [CrossRef] [PubMed]
- Paquet-Mercier, F.; Parvinzadeh Gashti, M.; Bellavance, J.; Taghavi, S.M.; Greener, J. Through thick and thin: A microfluidic approach for continuous measurements of biofilm viscosity and the effect of ionic strength. Lab Chip 2016, 16, 4710–4717. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Esquerra, R.M.; Meléndez, P.A.; Chandrasekaran, S.S.; Kliger, D.S. Microviscosity in E. coli cells from time-resolved linear dichroism measurements. J. Phys. Chem. B 2018, 122, 11381–11389. [Google Scholar] [CrossRef] [PubMed]
- Suhling, K.; Siegel, J.; Lanigan, P.M.P.; Lévêque-Fort, S.; Webb, S.E.D.; Phillips, D.; Davis, D.M.; French, P.M.W. Time-resolved fluorescence anisotropy imaging applied to live cells. Opt. Lett. 2004, 29, 584–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suhling, K.; French, P.M.W.; Phillips, D. Time-resolved fluorescence microscopy. Photochem. Photobiol. Sci. 2005, 4, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Diffusion in cells measured by fluorescence recovery after photobleaching. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2003; Volume 360, pp. 635–648. [Google Scholar]
- Chambers, J.E.; Kubánková, M.; Huber, R.G.; López-Duarte, I.; Avezov, E.; Bond, P.J.; Marciniak, S.J.; Kuimova, M.K. An optical technique for mapping microviscosity dynamics in cellular organelles. ACS Nano 2018, 12, 4398–4407. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Kuo, C.Y.; Wei, M.T.; Wu, K.; Su, P.T.; Huang, C.S.; Chiou, A. Intracellular viscoelasticity of HeLa cells during cell division studied by video particle-tracking microrheology. J. Biomed. Opt. 2014, 19, 011008. [Google Scholar] [CrossRef] [PubMed]
- Sie, Y.-S.; Chuang, H.-S. A micro-volume viscosity measurement technique based on μPIV diffusometry. Microfluid. Nanofluid. 2014, 16, 65–72. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Wang, J.-C.; Chuang, H.-S. Rapid bead-based antimicrobial susceptibility testing by optical diffusometry. PLoS ONE 2016, 11, e0148864. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-Y.; Wang, J.-C.; Chuang, H.-S. Simultaneous and quantitative monitoring of co-cultured Pseudomonas aeruginosa and Staphylococcus aureus with antibiotics on a diffusometric platform. Sci. Rep. 2017, 7, 46336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, H.-S.; Chen, Y.-J.; Cheng, H.-P. Enhanced diffusometric immunosensing with grafted gold nanoparticles for detection of diabetic retinopathy biomarker tumor necrosis factor-α. Biosens. Bioelectron. 2018, 101, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-C.; Chi, S.-W.; Shieh, D.-B.; Chuang, H.-S. Development of a self-driving bioassay based on diffusion for simple detection of microorganisms. Sens. Actuators B Chem. 2019, 278, 140–146. [Google Scholar] [CrossRef]
- Wang, J.-C.; Chi, S.-W.; Yang, T.-H.; Chuang, H.-S. Label-free monitoring of microorganisms and their responses to antibiotics based on self-powered microbead sensors. ACS Sens. 2018, 3, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Einstein, A.; Fürth, R. Investigations on the Theory of Brownian Movement; Dover Publications: New York, NY, USA, 1956. [Google Scholar]
- Debye, P. Polar molecules. J. Soc. Chem. Ind. 1929, 48, 1036–1037. [Google Scholar]
- Liang, M.; Harder, R.; Robinson, I.K. Brownian motion studies of viscoelastic colloidal gels by rotational single particle tracking. IUCrJ 2014, 1 Pt 3, 172–178. [Google Scholar] [CrossRef]
- Liu, J.; Gardel, M.L.; Kroy, K.; Frey, E.; Hoffman, B.D.; Crocker, J.C.; Bausch, A.R.; Weitz, D.A. Microrheology probes length scale dependent rheology. Phys. Rev. Lett. 2006, 96, 118104. [Google Scholar] [CrossRef] [PubMed]
- Lavalette, D.; Tétreau, C.; Tourbez, M.; Blouquit, Y. Microscopic viscosity and rotational diffusion of proteins in a macromolecular environment. Biophys. J. 1999, 76, 2744–2751. [Google Scholar] [CrossRef]
- Love, J.C.; Gates, B.D.; Wolfe, D.B.; Paul, K.E.; Whitesides, G.M. Fabrication and wetting properties of metallic half-shells with submicron diameters. Nano Lett. 2002, 2, 891–894. [Google Scholar] [CrossRef]
- Volk, A.; Kähler, C.J. Density model for aqueous glycerol solutions. Exp. Fluids 2018, 59, 75. [Google Scholar] [CrossRef] [Green Version]
- Cheng, N.-S. Formula for the viscosity of a glycerol−water mixture. Ind. Eng. Chem. Res. 2008, 47, 3285–3288. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-J.; Chen, W.-L.; Phong, P.H.; Chuang, H.-S. Investigation of Micro-volume Viscosity with Janus Microbeads Based on Rotational Brownian Motion. Sensors 2019, 19, 1217. https://doi.org/10.3390/s19051217
Chen C-J, Chen W-L, Phong PH, Chuang H-S. Investigation of Micro-volume Viscosity with Janus Microbeads Based on Rotational Brownian Motion. Sensors. 2019; 19(5):1217. https://doi.org/10.3390/s19051217
Chicago/Turabian StyleChen, Chun-Jui, Wei-Long Chen, Pham Hong Phong, and Han-Sheng Chuang. 2019. "Investigation of Micro-volume Viscosity with Janus Microbeads Based on Rotational Brownian Motion" Sensors 19, no. 5: 1217. https://doi.org/10.3390/s19051217
APA StyleChen, C.-J., Chen, W.-L., Phong, P. H., & Chuang, H.-S. (2019). Investigation of Micro-volume Viscosity with Janus Microbeads Based on Rotational Brownian Motion. Sensors, 19(5), 1217. https://doi.org/10.3390/s19051217





