Enhanced Humidity Sensing Response of SnO2/Silicon Nanopillar Array by UV Irradiation
Abstract
:1. Introduction
2. Experiment
2.1. Preparation of the Large-Area and Ordered Polystyrene Spheres Array
2.2. Preparation of Ordered Silicon Nanopillar Array
2.3. Preparation of SnO2/Silicon Nanopillar Array Sensor
2.4. Measurement of Humidity Sensing Properties
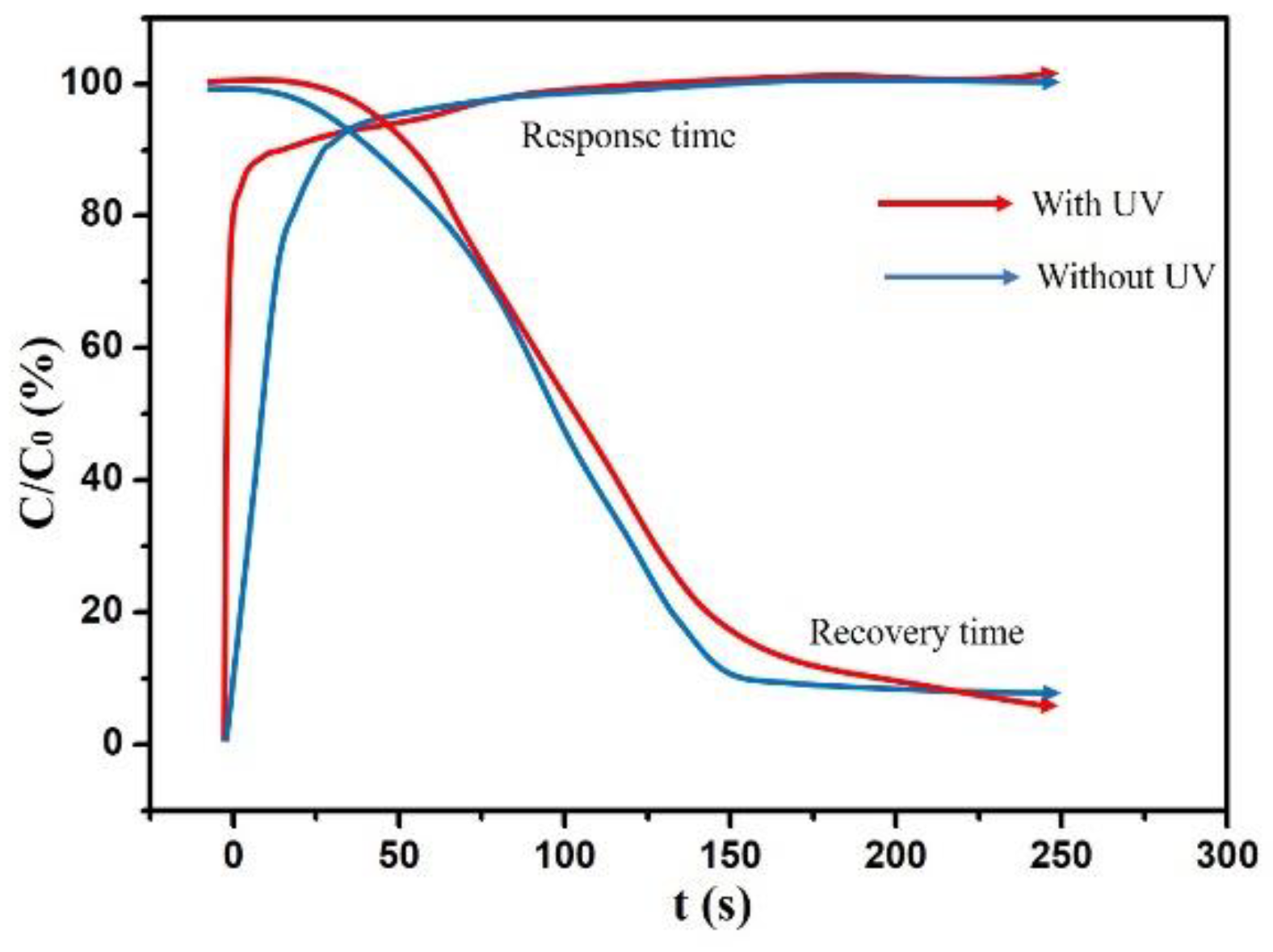
3. Discussion and Results
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Song, X.; Qi, Q.; Zhang, T.; Wang, C. A humidity sensor based on KCl-doped SnO2 nanofibers. Sens. Actuators B Chem. 2009, 138, 368–373. [Google Scholar] [CrossRef]
- Tricoli, A.; Righettoni, M.; Teleki, A. Semiconductor gas sensors: Dry synthesis and application. Angew. Chem. Int. Ed. 2010, 49, 7632–7659. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Jung, D.; Kim, J.; Lee, G.S. Enhanced humidity-sensing response of metal oxide coated carbon nanotube. Sens. Actuators A Phys. 2015, 223, 11–17. [Google Scholar] [CrossRef]
- Tian, H.L.; Fan, H.Q.; Li, M.M.; Ma, L. Zeolitic Imidazolate Framework Coated ZnO Nanorods as Molecular Sieving to Improve Selectivity of Formaldehyde Gas Sensor. ACS Sens. 2016, 1, 243–250. [Google Scholar] [CrossRef]
- Staerz, A.; Berthold, C.; Russ, T. The oxidizing effect of humidity on WO3 based sensors. Sens. Actuators B Chem. 2016, 237, 54–58. [Google Scholar] [CrossRef]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. A comparative study of RGO-SnO2 and MWCNT-SnO2 nanocomposites based SO2 gas sensors. Sens. Actuators B Chem. 2017, 248, 980–986. [Google Scholar] [CrossRef]
- Abbasi, A.; Sardroodi, J.J. Investigation of the adsorption of ozone molecules on TiO2/WSe2 nanocomposites by DFT computations: Applications to gas sensor devices. Appl. Surf. Sci. 2018, 436, 27–41. [Google Scholar] [CrossRef]
- Liu, X.; Chen, N.; Han, B.Q.; Xiao, X.; Chen, G.; Djerdj, I.; Wang, Y. Nanoparticle cluster gas sensor: Pt activated SnO2 nanoparticles for NH3 detection with ultrahigh sensitivity. Nanoscale 2015, 7, 14872–14880. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.; Chao, D.; Bai, G.; Xu, J.; Ren, Q.; Li, J. Fabrication of Ordered SnO2 Nanostructures with Enhanced Humidity Sensing Performance. Sensors 2017, 17, 2392. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ji, F.; Yin, M.L.; Ren, X.; Ma, Q.; Yan, J.; Liu, S.F. Ag Nanoparticle-Sensitized WO3 Hollow Nanosphere for Localized Surface Plasmon Enhanced Gas Sensors. ACS Appl. Mater. Interfaces 2016, 8, 18165–18172. [Google Scholar]
- Tomer, V.K.; Duhan, S. Highly sensitive and stable relative humidity sensors based on WO3 modified mesoporous silica. Appl. Phys. Lett. 2015, 106, 063105. [Google Scholar] [CrossRef]
- Hsu, C.L.; Su, I.L.; Hsueh, T.J. Tunable Schottky contact humidity sensor based on S-doped ZnO nanowires on flexible PET substrate with piezotronic effect. J. Alloys Compd. 2017, 705, 722–733. [Google Scholar] [CrossRef]
- Mahjoub, M.A.; Monier, G.; Robert-Goumet, C. Synthesis and Study of Stable and Size-Controlled ZnO-SiO2 Quantum Dots: Application as a Humidity Sensor. J. Phys. Chem. C 2016, 120, 11652–11662. [Google Scholar] [CrossRef]
- Li, X.G.; Zhao, Y.Y.; Wang, X.Y.; Wang, J.; Gaskov, A.M.; Akbar, S.A. Reduced graphene oxide (rGO) decorated TiO2 microspheres for selective room-temperature gas sensors. Sens. Actuators B Chem. 2016, 230, 330–336. [Google Scholar] [CrossRef]
- Nikfarjam, A.; Salehifar, N. Improvement in gas-sensing properties of TiO2 nanofiber sensor by UV irradiation. Sens. Actuators B Chem. 2015, 211, 146–156. [Google Scholar] [CrossRef]
- De Lacy Costello, B.P.J.; Ewen, R.J.; Ratciffe, N.M.; Richards, M. Highly sensitive room temperature sensors based on the UV-LED activation of zinc oxide nanoparticles. Sens. Actuators B Chem. 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Zhang, Y.; Kolmakov, A.; Lilach, Y.; Moskovits, M. Electronic control of chemistry and catalysis at the surface of an individual Tin oxide nanowire. J. Phys. Chem. B 2005, 109, 1923–1929. [Google Scholar] [CrossRef]
- Tian, Z.R.; Voigt, J.A.; Liu, J.; Mckenzie, B.; Mcdermott, M.J.; Rodriguez, M.A.; Konishi, H.; Xu, H. Complex and oriented ZnO nanostructures. Nat. Mater. 2003, 2, 821–826. [Google Scholar] [CrossRef]
- Arena, A.; Donato, N.; Saitta, G.; Bonavita, A.; Rizzo, G.; Neri, G. Flexible ethanol sensors on glossy paper substrates operating at room temperature. Sens. Actuators B Chem. 2010, 145, 488–494. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. In-situ synthesis of SnO2/SBA-15 hybrid nanocomposite as highly efficient humidity sensor. Sens. Actuators B Chem. 2015, 212, 517–525. [Google Scholar] [CrossRef]
- Georgieva, B.; Podolesheva, I.; Spasov, G.; Pirov, J. Nanosized thin SnO2 layers doped with Te and TeO2 as room temperature humidity sensors. Sensors 2014, 14, 8950–8960. [Google Scholar] [CrossRef]
- Feng, H.L.; Li, C.; Li, T.; Diao, F.; Xin, T.; Liu, B.; Wang, Y. Three-dimensional hierarchical SnO2 dodecahedral nanocrystals with enhanced humidity sensing properties. Sens. Actuators B Chem. 2017, 243, 704–714. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Yuan, H.; Xu, J.Q.; Xu, P.C.; Pan, Q.Y. Highly stable and sensitive humidity sensors based on quartz crystal microbalance coated with hexagonal lamelliform monodisperse mesoporous silica SBA-15 thin film. Sens. Actuators B Chem. 2010, 144, 164–169. [Google Scholar] [CrossRef]
- Tu, J.C.; Wang, R.; Geng, W.C.; Lai, X.Y.; Zhang, T.; Li, N.; Yue, N.L.; Li, X.T. Humidity sensitive property of Li-doped 3D periodic mesoporous silica SBA-16. Sens. Actuators B Chem. 2009, 136, 392–398. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Ozone measuring: What can limit application of SnO2-based conductometric gas sensors. Sens. Actuators B Chem. 2012, 161, 28–44. [Google Scholar] [CrossRef]
- Park, S.; An, S.; Mun, Y.; Lee, C. UV-enhanced NO2 gas sensing properties of SnO2-core/ZnO-shell nanowires at room temperature. ACS Appl. Mater. Interfaces 2013, 5, 4285–4292. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Nguyen, D.Q. NO gas sensing kinetics at room temperature under UV light irradiation of In2O3 nanostructures. Sci. Rep. 2016, 6, 35066. [Google Scholar]
- Li, W.; Hu, M.Y.; Ge, P.P.; Wang, J.; Guo, Y. Humidity sensing properties of morphology-controlled ordered silicon nanopillar. Appl. Surf. Sci. 2014, 317, 970–973. [Google Scholar] [CrossRef]
- Li, W.; Dai, E.; Bai, G.; Xu, J. Depth-dependent humidity sensing properties of silicon nanopillar array. Sens. Actuators B-Chem. 2016, 237, 526–533. [Google Scholar] [CrossRef]
- Li, W.; Ding, C.; Cai, Y.; Liu, J.; Wang, L.; Ren, Q.; Xu, J. Enhanced humidity sensitivity with silicon nanopillar array by UV light. Sensors 2018, 18, 660. [Google Scholar] [CrossRef]
- Sauerbrey, G. The use of quartz oscillators for weighing thin layers and for micro-weighing. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Traversa, E. Ceramic sensors for humidity detection: The state-of-the-art and future developments. Sens. Actuators B Chem. 1995, 23, 135–156. [Google Scholar] [CrossRef]
- Morrison, S.R. The Chemical Physics of Surface; Plenum Press: New York, NJ, USA, 1977. [Google Scholar]
- Shukla, S.K.; Rastogi, R.P.; Singh, N.B. Nanosize SnO2 through nitrate eutectic mixture for humidity sensors. Emerg. Mater. Res. 2015, 4, 12–17. [Google Scholar] [CrossRef]
- Sundaram, R. Comparative study on micromorphology and humidity sensitive properties of thick film and disc humidity sensors based on semiconducting SnWO4–SnO2 composites. Sens. Actuators B Chem. 2007, 124, 429–436. [Google Scholar] [CrossRef]
- Liu, Y.J.; Hao, L.Z.; Gao, W.; Liu, Y.M.; Li, G.X.; Xue, Q.Z.; Guo, W.Y.; Wu, Z.P.; Liu, X.H.; Zeng, H.Z.; et al. Growth and humidity-dependent electrical properties of bulk-like MoS2 thin films on Si. RSC Adv. 2015, 5, 74329–74335. [Google Scholar] [CrossRef]
- Ghosh, A.; Late, D.J.; Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. NO2 and humidity sensing characteristics of few-layer graphenes. J. Exp. Nanosci. 2009, 4, 313–322. [Google Scholar] [CrossRef]



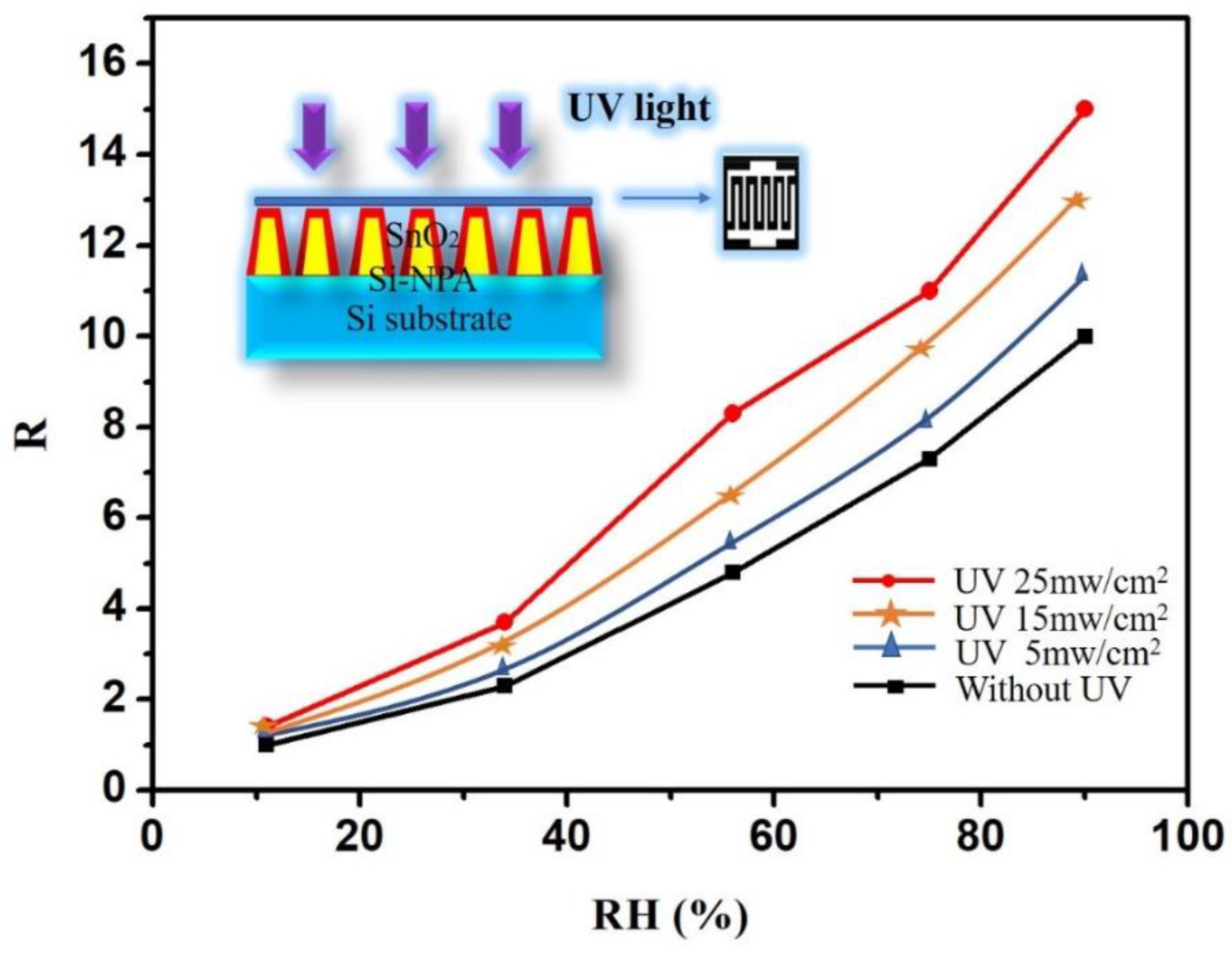



| 11% RH | 34% RH | 56% RH | 75% RH | 90% RH | |
|---|---|---|---|---|---|
| Without UV Response | 1 | 2.3 | 4.8 | 7.3 | 10.1 |
| With UV Response | 1.2 | 3.5 | 8.6 | 10.8 | 15.4 |
| Response Increase | 20% | 52% | 58% | 48% | 52% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, L.; Cai, Y.; Pan, P.; Li, J.; Ren, Q.; Xu, J. Enhanced Humidity Sensing Response of SnO2/Silicon Nanopillar Array by UV Irradiation. Sensors 2019, 19, 2141. https://doi.org/10.3390/s19092141
Li W, Wang L, Cai Y, Pan P, Li J, Ren Q, Xu J. Enhanced Humidity Sensing Response of SnO2/Silicon Nanopillar Array by UV Irradiation. Sensors. 2019; 19(9):2141. https://doi.org/10.3390/s19092141
Chicago/Turabian StyleLi, Wei, Linlin Wang, Yun Cai, Peifeng Pan, Jinze Li, Qingying Ren, and Jie Xu. 2019. "Enhanced Humidity Sensing Response of SnO2/Silicon Nanopillar Array by UV Irradiation" Sensors 19, no. 9: 2141. https://doi.org/10.3390/s19092141




