Studies on the Electrochemical Behaviour of Hydroquinone at L-cysteine Self-Assembled Monolayers Modified Gold Electrode
Abstract
:Introduction
Experimental
Apparatus
Reagents
Preparation of L-Cys/Au SAMs
Results and Discussion
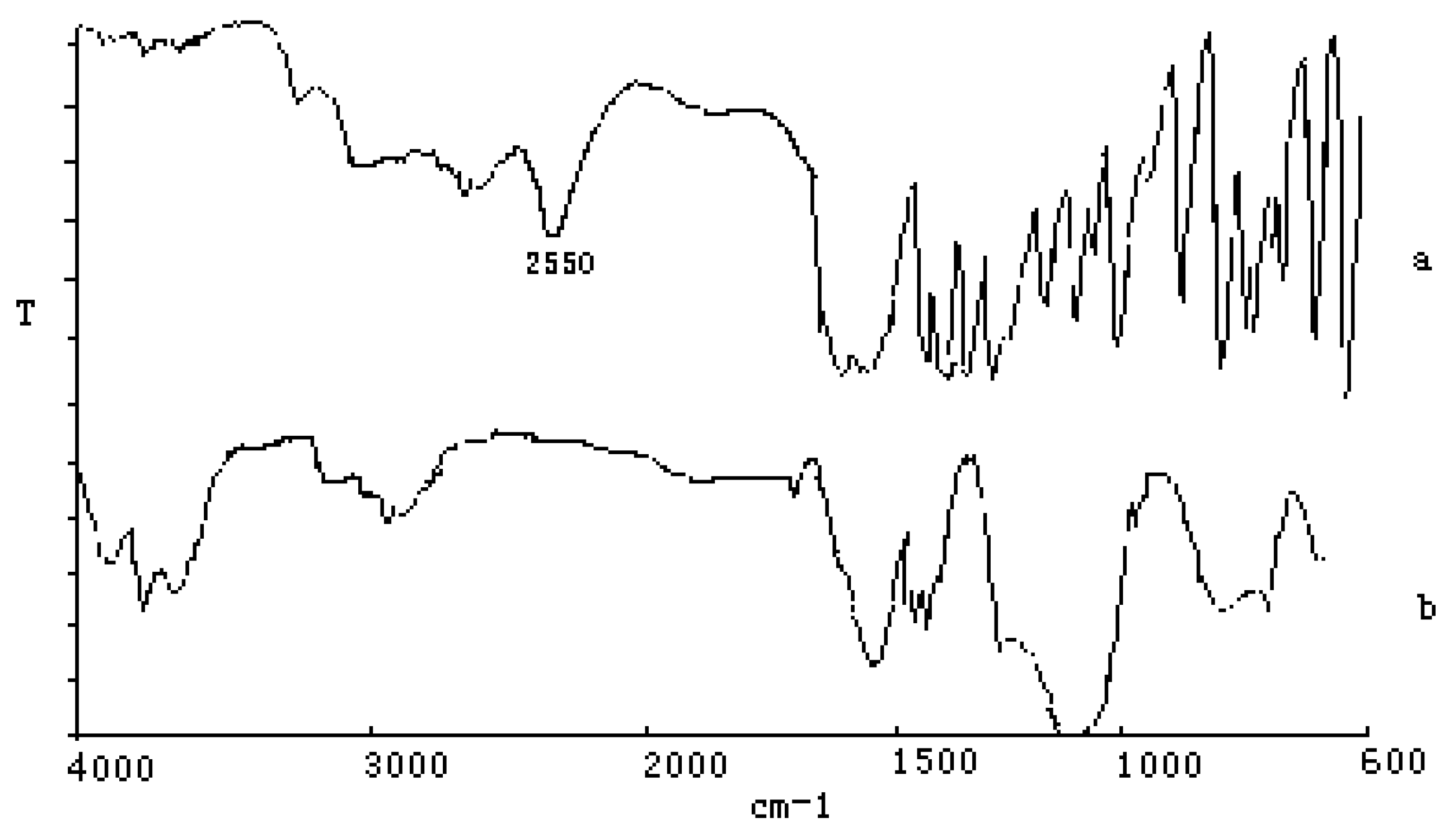
FTIR of L-Cys/Au SAMs
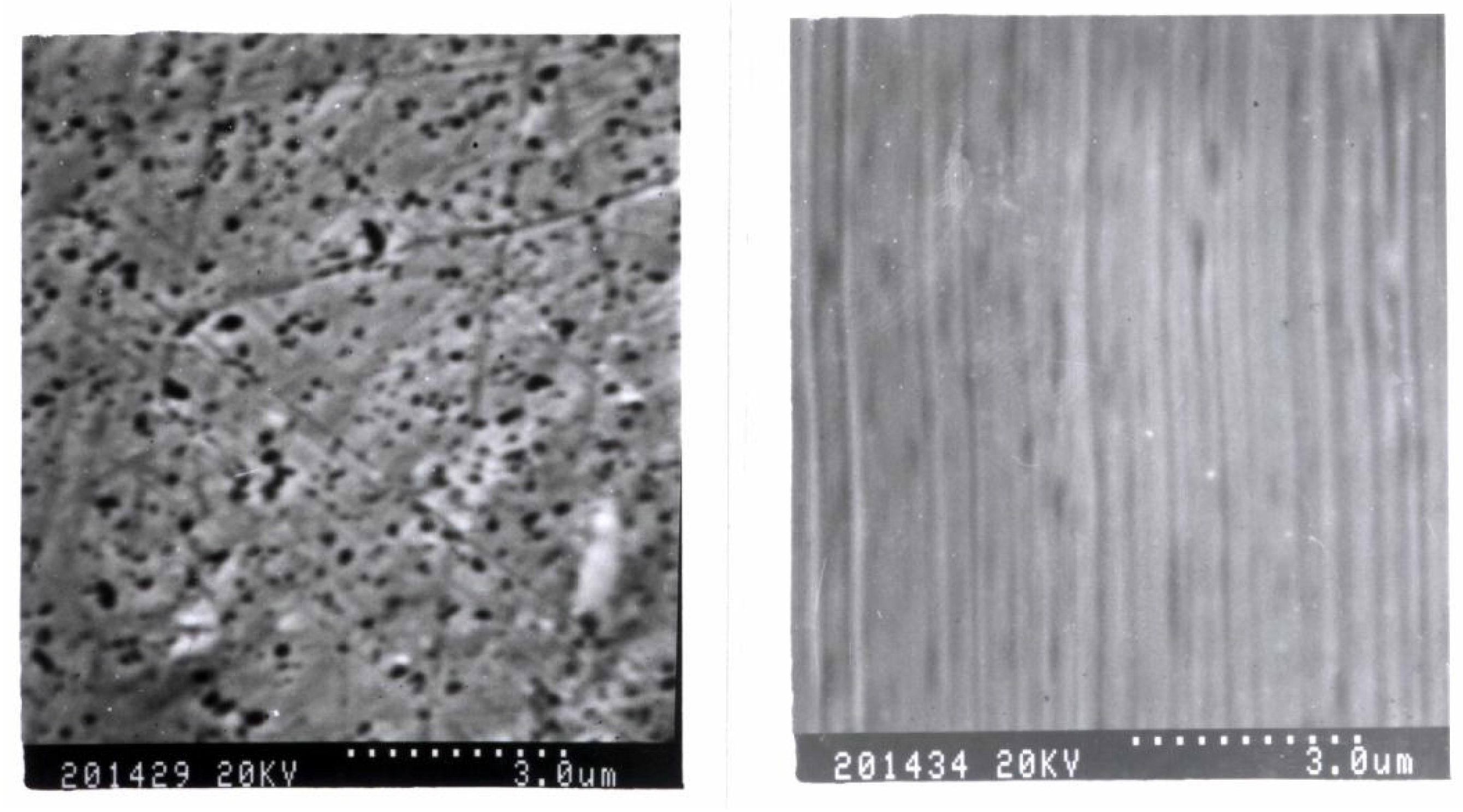
SEM of L-Cys/Au SAMs
Electrochemical behavior of L-Cys/Au SAMs
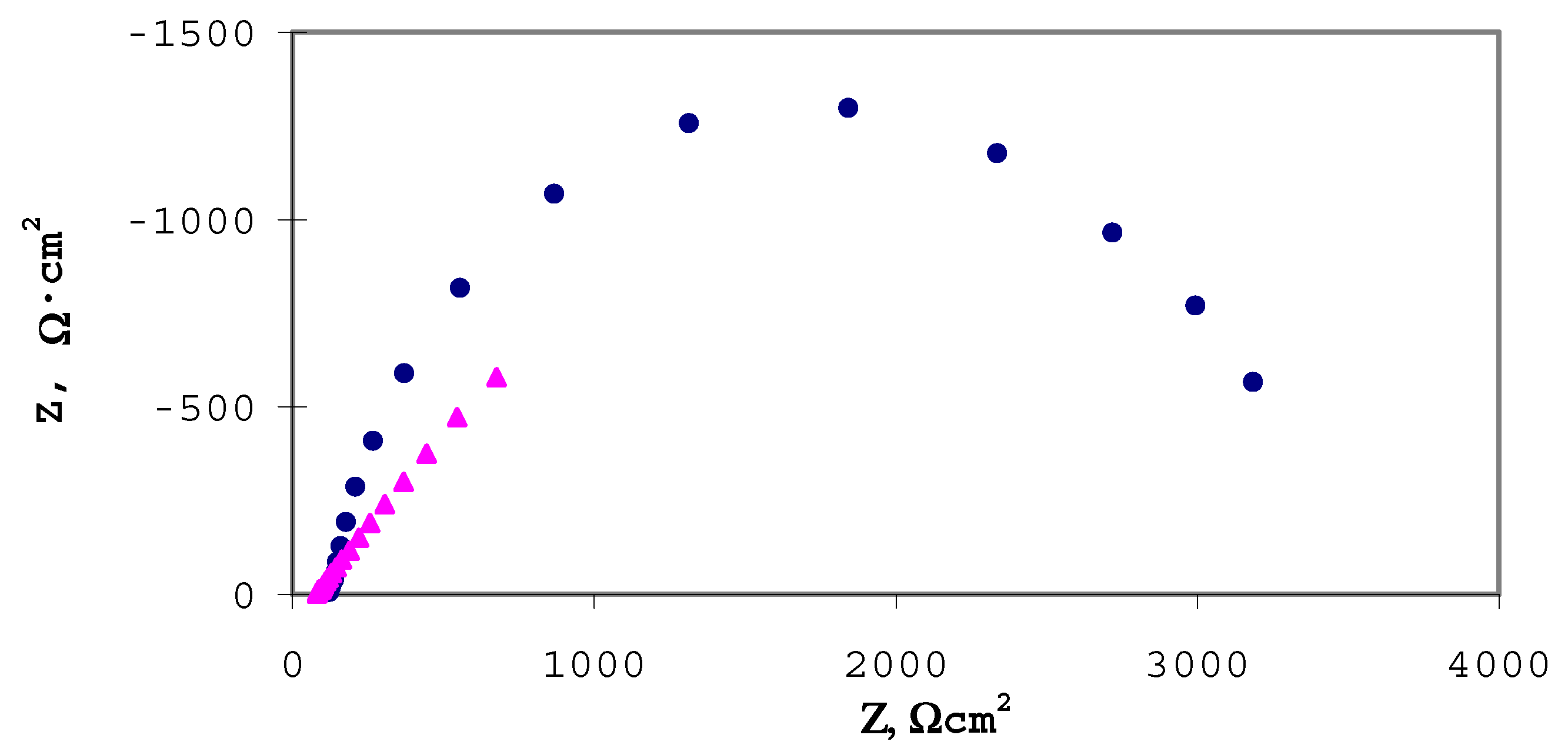
Impedance analysis in the presence of Fe(CN)63-/4-
Electrochemical properties of hydroquinone on L-Cys/Au SAMs

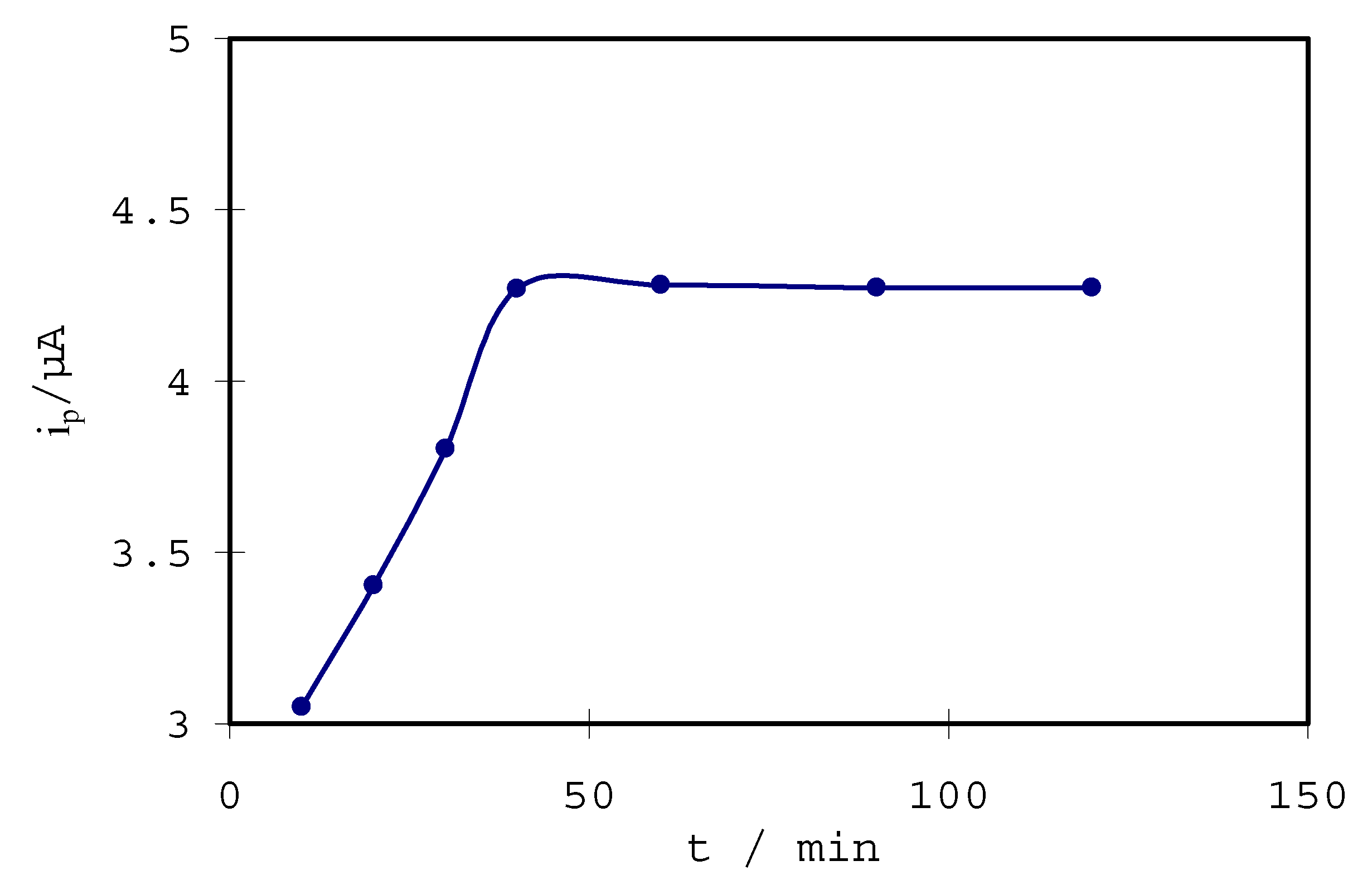
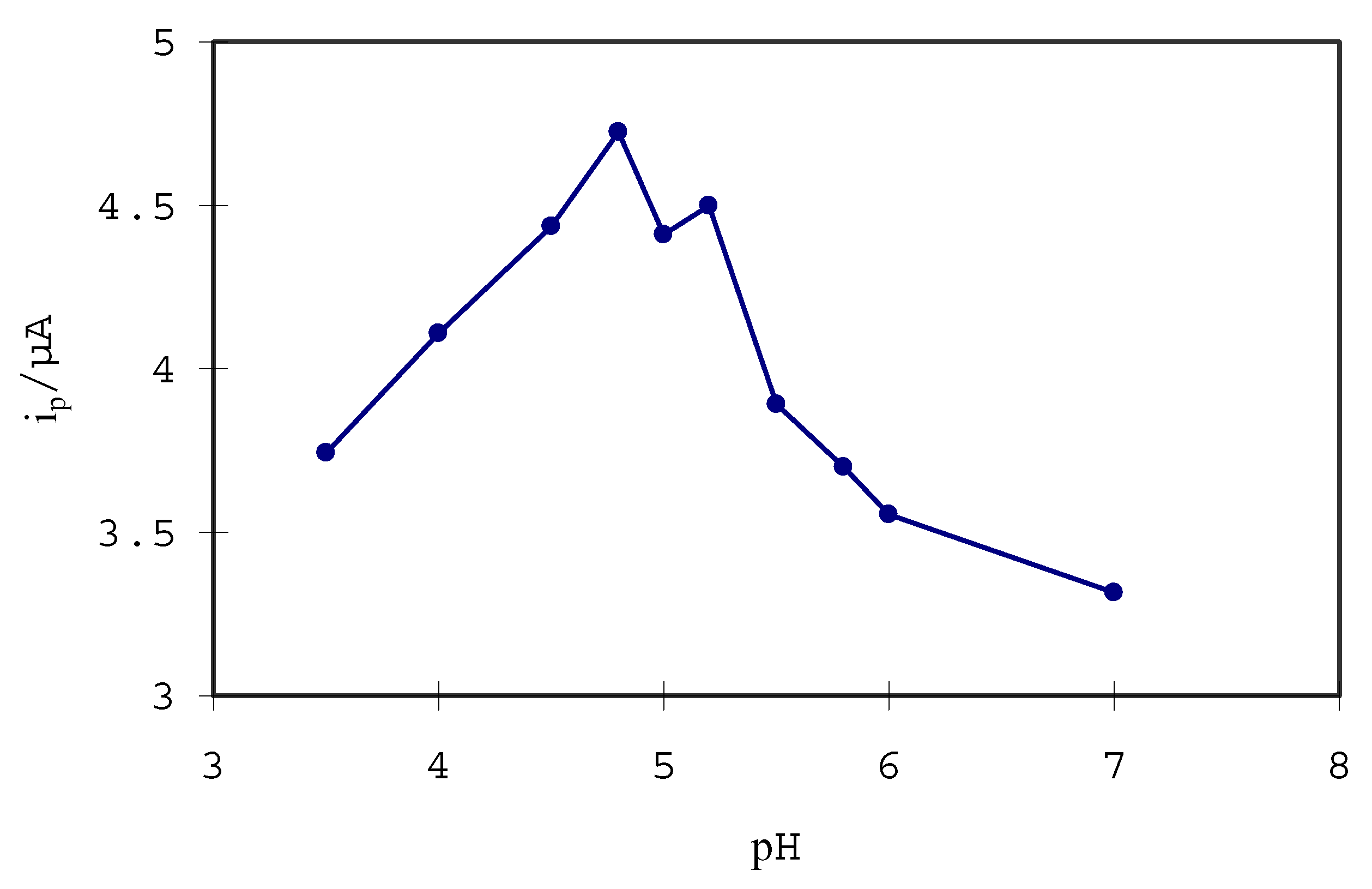
Optimization of L-Cys/Au SAMs for the determination of hydroquinone
Mechanism for the electrocatalytical process
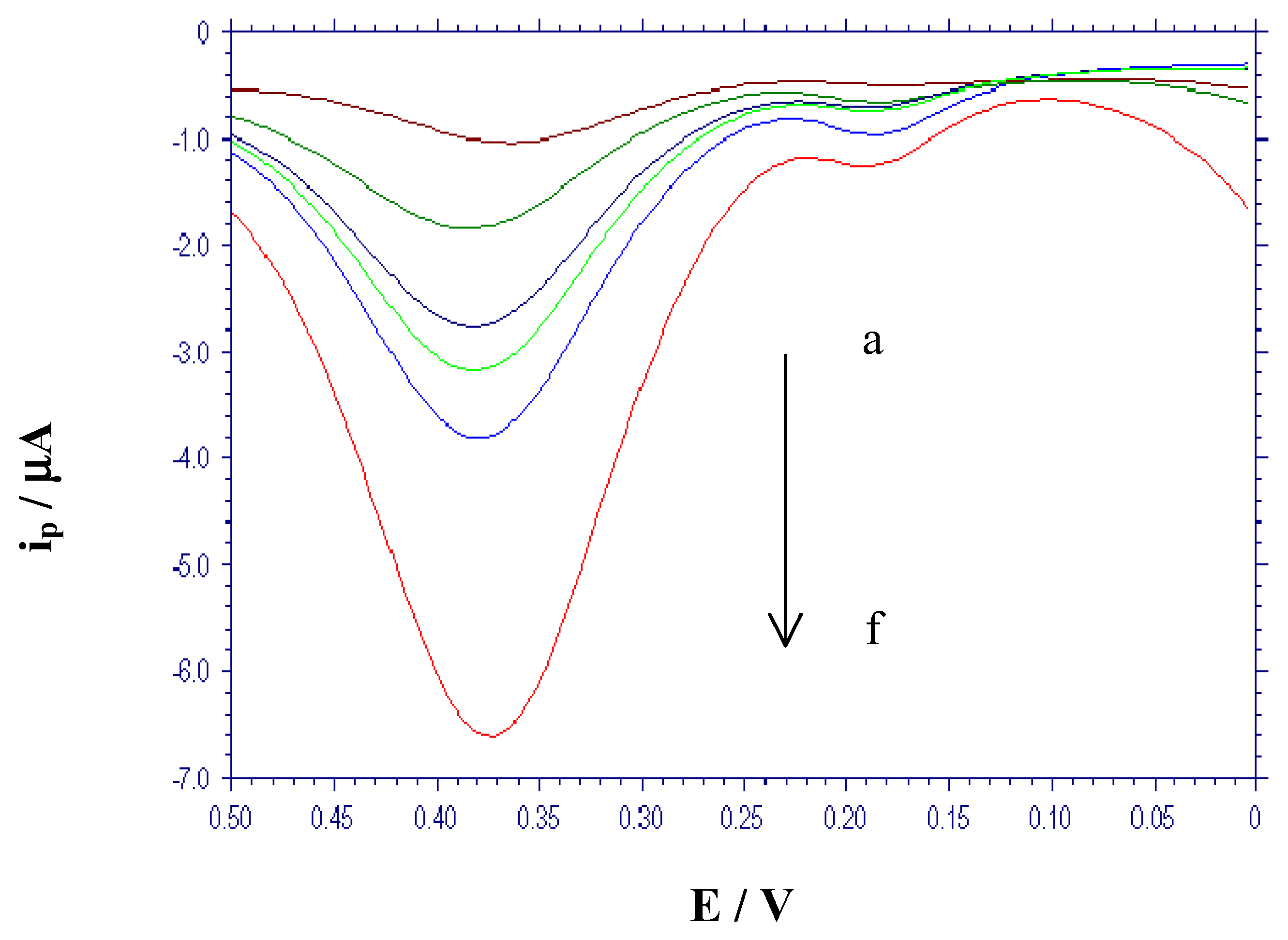
Calibration and quantitative determination to hydroquinone
| Sample(M) | Added(M) | Founded(M) | Recovery(%) |
|---|---|---|---|
| 1.00×10-5 | 3.00×10-5 | 3.99×10-5 | 99.67 |
| 1.00×10-5 | 3.50×10-5 | 4.47×10-5 | 99.14 |
| 1.00×10-5 | 6.00×10-5 | 6.87×10-5 | 97.83 |
| 1.00×10-5 | 9.00×10-5 | 1.01×10-4 | 101.11 |
| 1.00×10-5 | 1.00×10-4 | 1.11×10-4 | 102.00 |
References
- Ulman, A. Chem. Rev. 1996, 96, 1533. [PubMed]
- Daniel, M.; Iva, T. Electroanalysis 1996, 8, 207.
- Wink, Th.; Zuilen, S.J.; Bult, A.; Bennekom, W.P. Analyst 1997, 122, 43R. [PubMed]
- Widrig, C.A.; Alves, C.A.; Porter, M.D. J. Am. Chem. Soc. 1991, 113, 8550.
- Sun, L.; Crooks, R.M. J. Electrochem. Soc. 1991, 138, 23.
- Samant, M.G.; Brown, C.A.; Gordon, J.G. Langmuir 1991, 7, 437.
- Rubinstein, I.; Steinberg, S.; Tor, Y.; Shanzer, A. J. Sagiv. Nature 1988, 332, 426.
- Steinberg, S.; Tor, Y.; Sabatani, E.; Rubinstein, I. J. Am. Chem. Soc. 1991, 113, 5176.
- Steinberg, S.; Rubinstein, I. Langmuir 1992, 8, 1183.
- Turyan, I.; Mandler, D. Anal. Chem. 1994, 66, 56.
- Turyan, I.; Mandler, D. Anal.Chem. 1997, 69, 894. [PubMed]
- Berchmans, S.; Yegnaraman, V.; Sandhyarani, N. J. Electroanal. Chem. 1999, 468, 170.
- He, X.C.; Li, P.; Mo, J.Y. FENXI CESHI XUE BAO (Chinese) 2000, 19(1), 19.
- Willner, I.; Riklin, A. Anal. Chem. 1994, 66, 1535.
- Creager, S.E.; Olsen, K.G. Anal. Chim. Acta. 1995, 307, 277.
- Jung, S.K.; Wilson, G.S. Anal.Chem. 1996, 68, 591. [PubMed]
- Uvdal, K.; Bodo, P.; Liedberg, B. J. Colloid Interface Sci. 1992, 149, 162.
- Ihs, A.; Liedbeg, B. J. Colloid Interface Sci. 1991, 144, 282.
- Schlereth, D.D.; Katz, E.; Schmidt, H.L. Electroanalysis 1995, 7, 46. 20.
- Liu, A.C.; Chen, D.C.; Lin, C.C. Anal. Chem. 1999, 71, 1549.
- Gu, K.; Zhu, J.J.; Chen, H.Y. Chinese J. Anal. Chem. 1999, 27(10), 1172.
- Zhang, H.M.; Zhu, Z.W.; Li, N.Q. The progress of Electroanal. Chem. (Chinese); Xi’an: Xi’an Map Publishing House.
- Wu, X.Q.; Wang, Z.S.; Meng, X.Y.; Zhang, Z.R. Electrochemistry (Chinese) 2000, 6(2), 163.
- Uvdal, K.; Vikinge, T.P. Langmuir 2001, 17(6), 2008.
- Sluyters-Rehhach, M.; Sluyters, J.H. Electroanalytical Chemistry; Bard, A.J., Ed.; 1970; Vol.4, Marcel Dekker: New York. [Google Scholar]
- Amatore, C.; Saveant, J.M.; Tessier, D. J. Electroanal. Chem. 1983, 147, 39.
- Sabatani, E.; Rubinstain, I.; Maoz, I. J. Sagiv, J. Electroanal. Chem. 1987, 219, 365.
- Sample Availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Wang, S.; Du, D. Studies on the Electrochemical Behaviour of Hydroquinone at L-cysteine Self-Assembled Monolayers Modified Gold Electrode. Sensors 2002, 2, 41-49. https://doi.org/10.3390/s20200041
Wang S, Du D. Studies on the Electrochemical Behaviour of Hydroquinone at L-cysteine Self-Assembled Monolayers Modified Gold Electrode. Sensors. 2002; 2(2):41-49. https://doi.org/10.3390/s20200041
Chicago/Turabian StyleWang, Shengfu, and Dan Du. 2002. "Studies on the Electrochemical Behaviour of Hydroquinone at L-cysteine Self-Assembled Monolayers Modified Gold Electrode" Sensors 2, no. 2: 41-49. https://doi.org/10.3390/s20200041
APA StyleWang, S., & Du, D. (2002). Studies on the Electrochemical Behaviour of Hydroquinone at L-cysteine Self-Assembled Monolayers Modified Gold Electrode. Sensors, 2(2), 41-49. https://doi.org/10.3390/s20200041




