The Preparation of ZnGa2O4 Nano Crystals by Spray Coprecipitation and Its Gas Sensitive Characteristics
Abstract
:Introduction
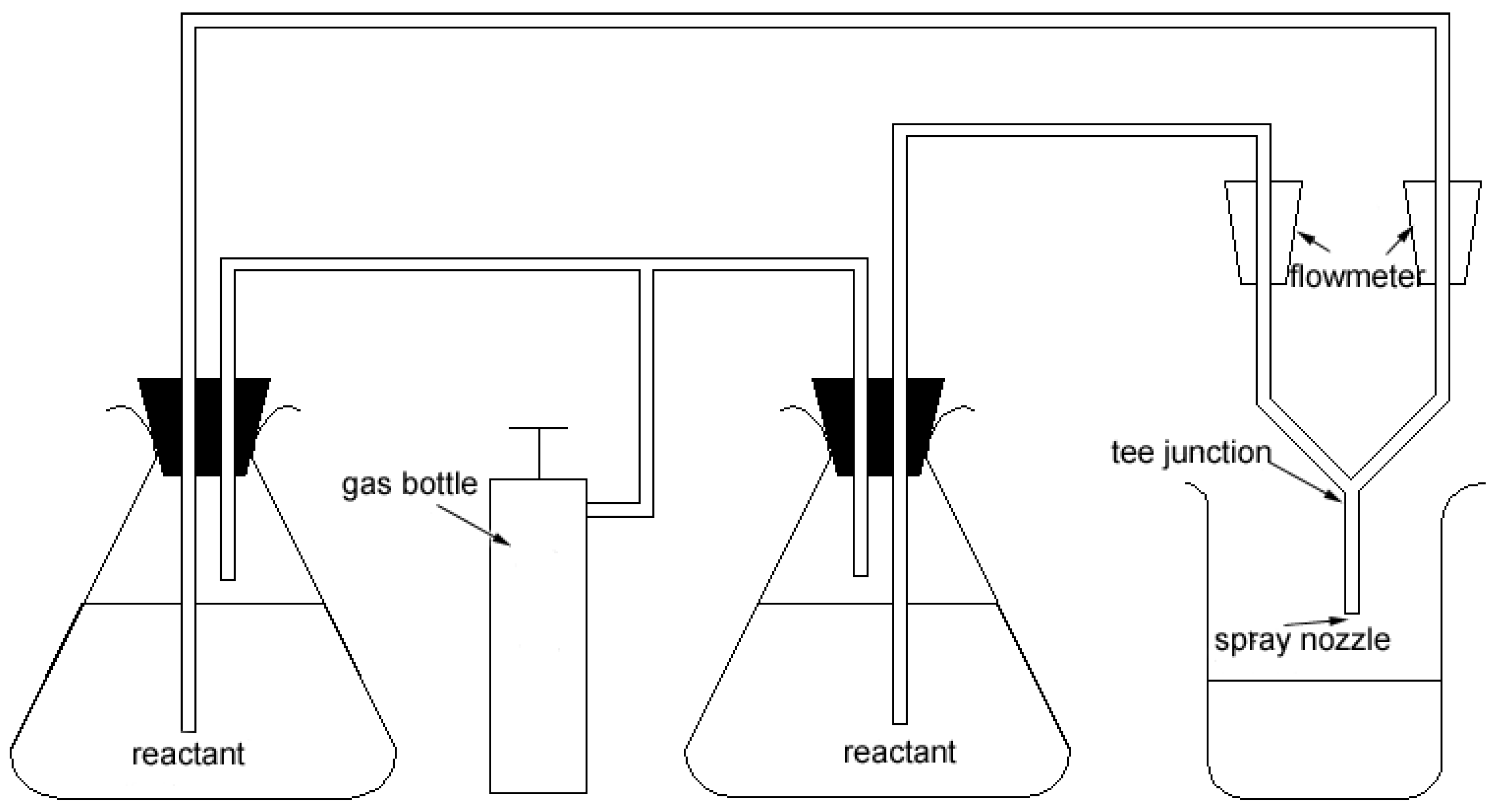
Experimental
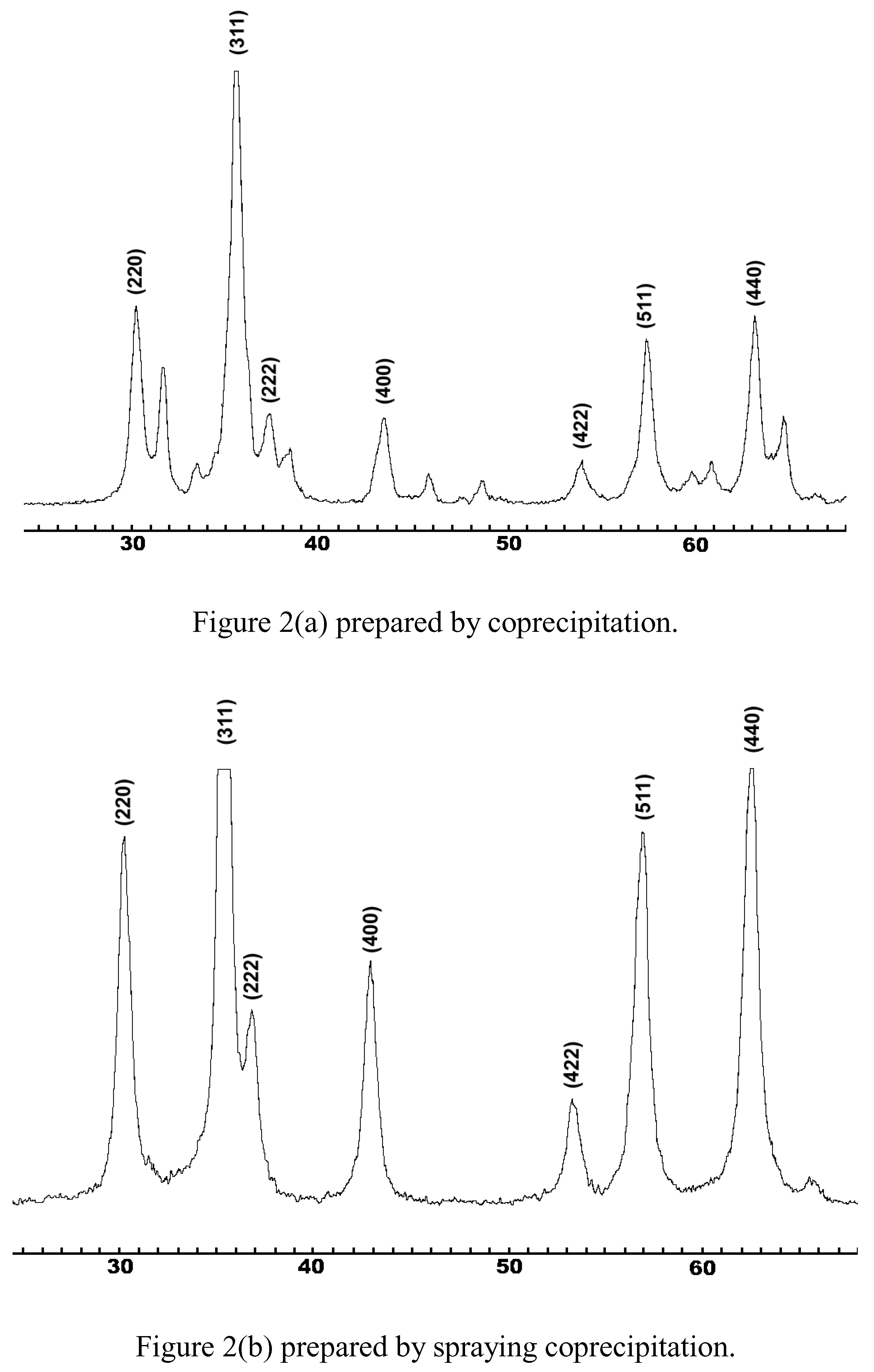
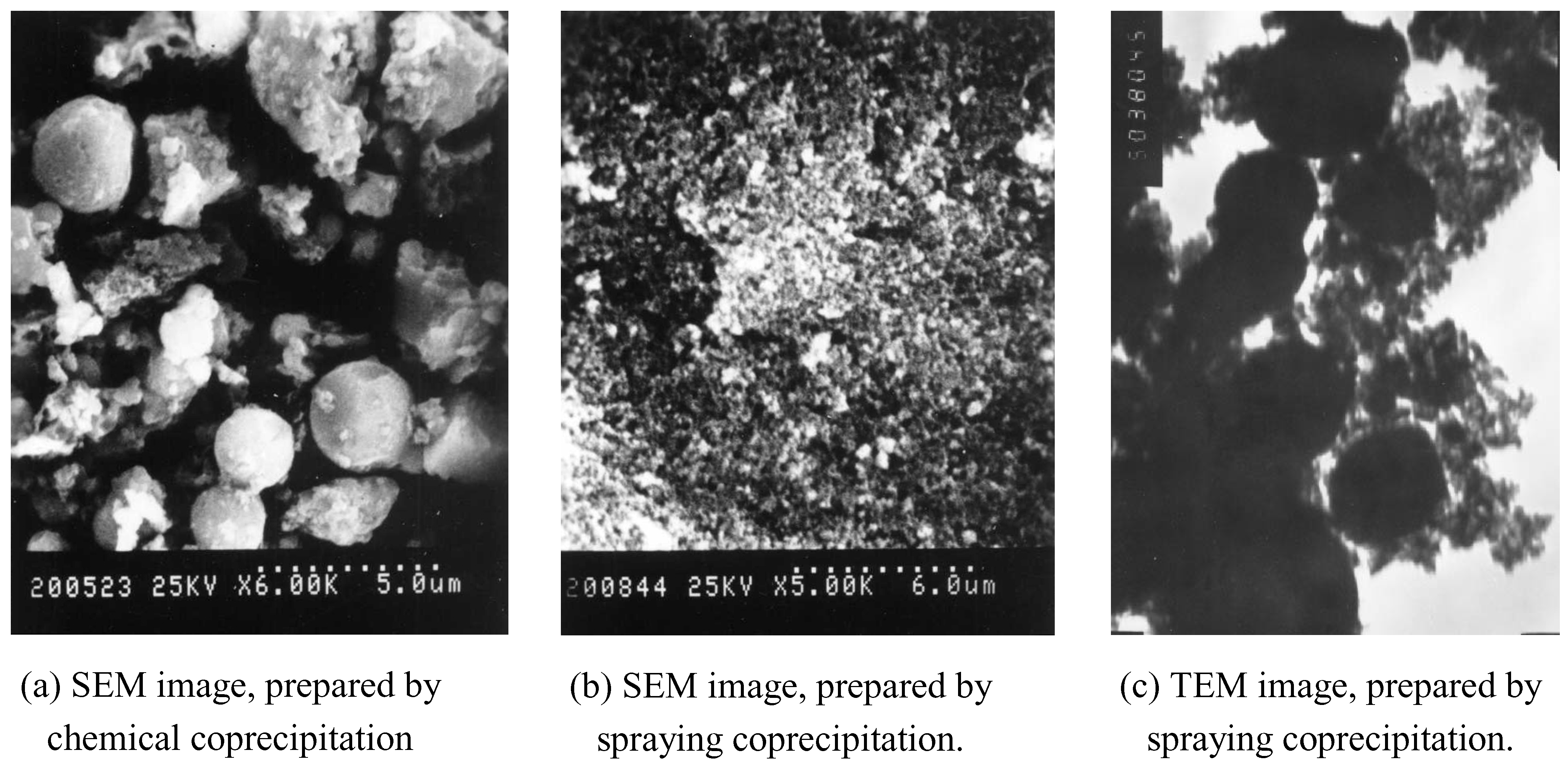
Results and Discussion

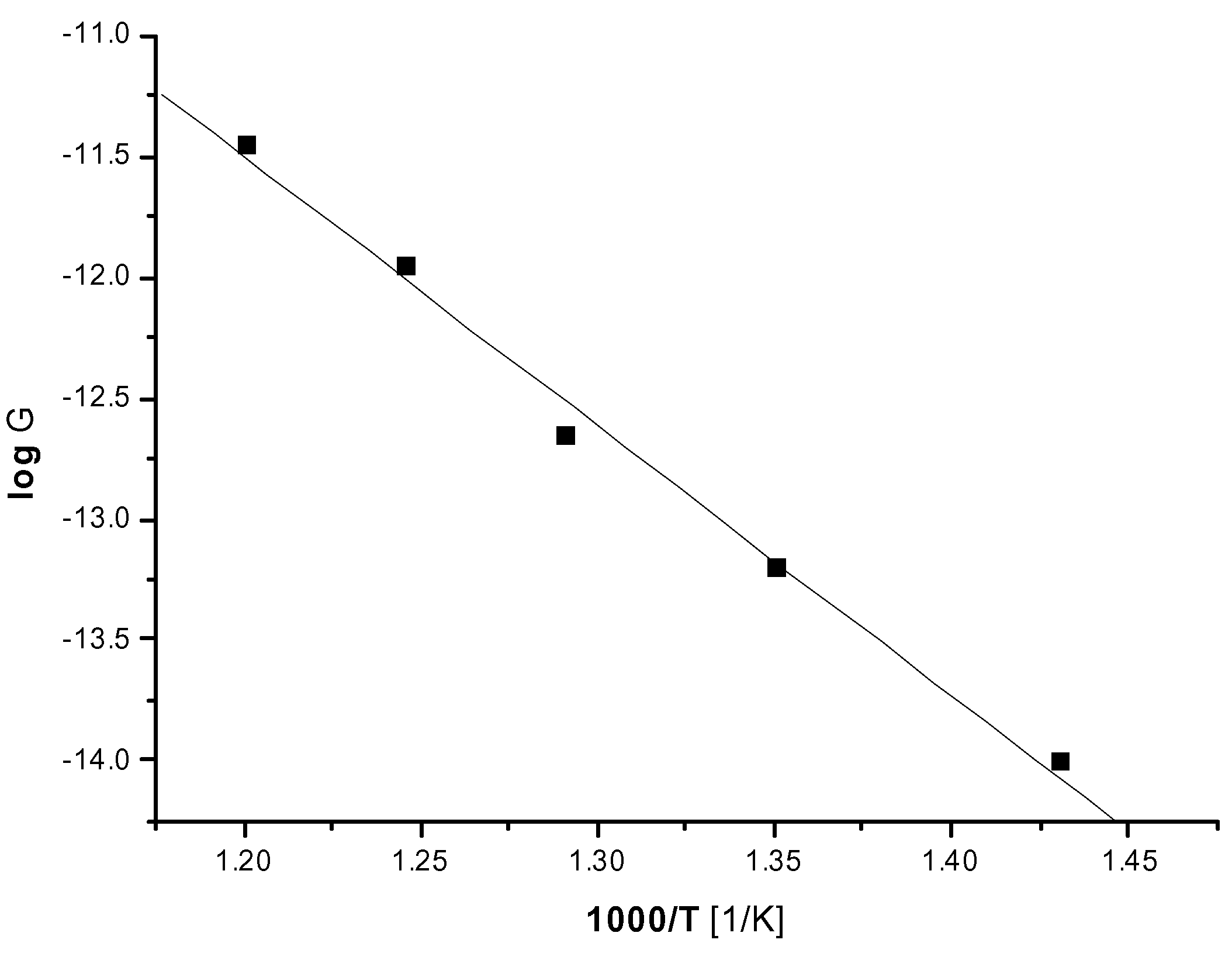
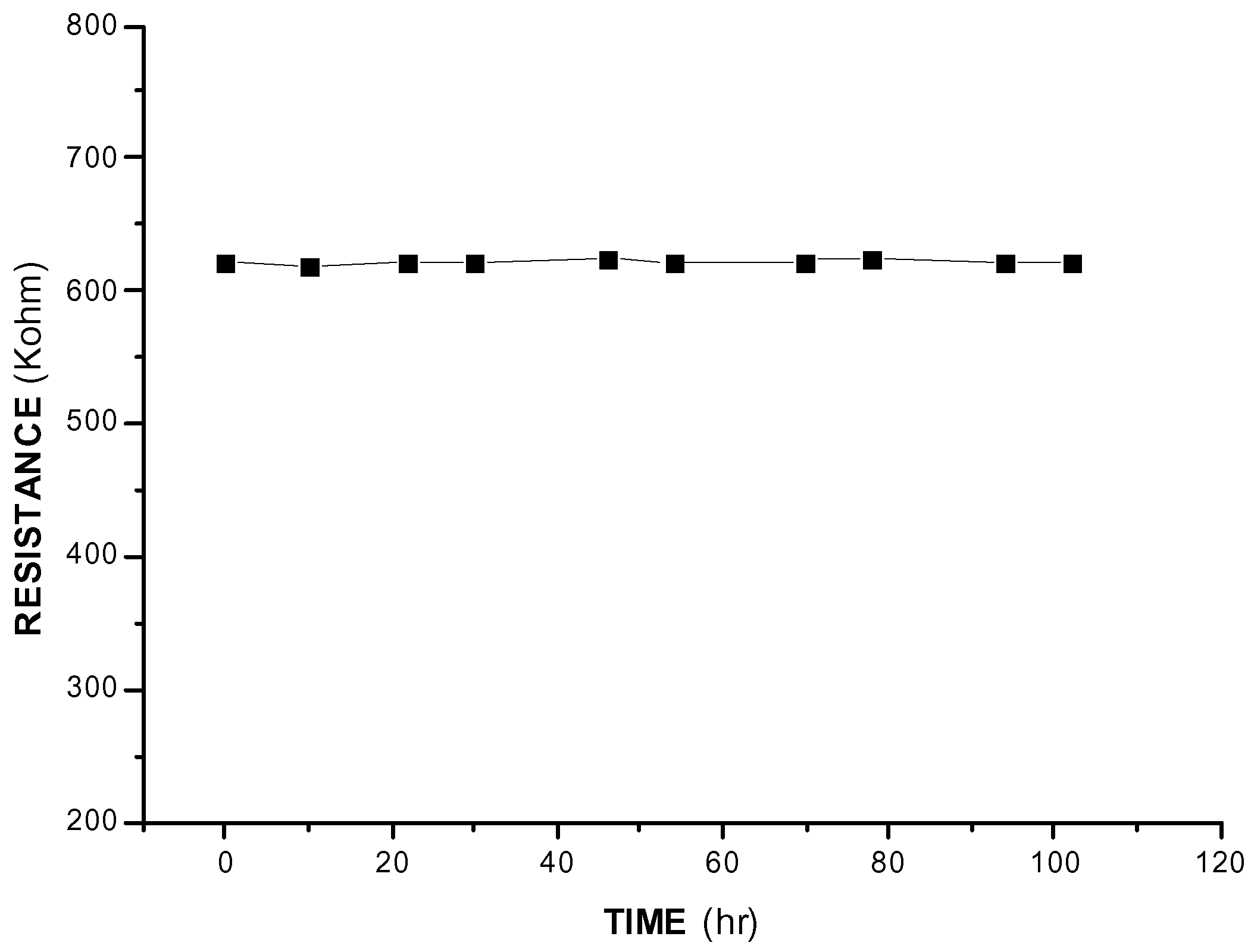
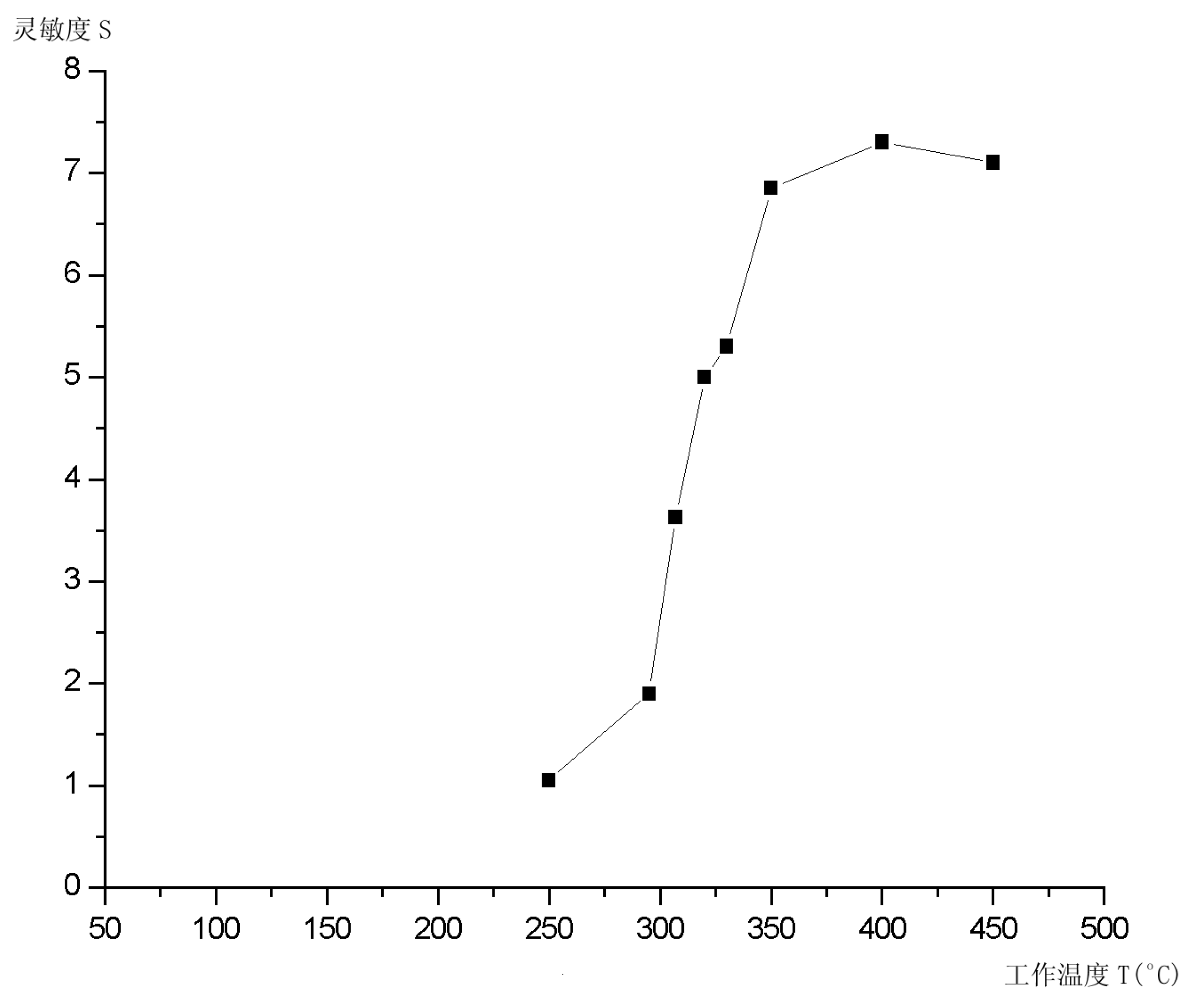
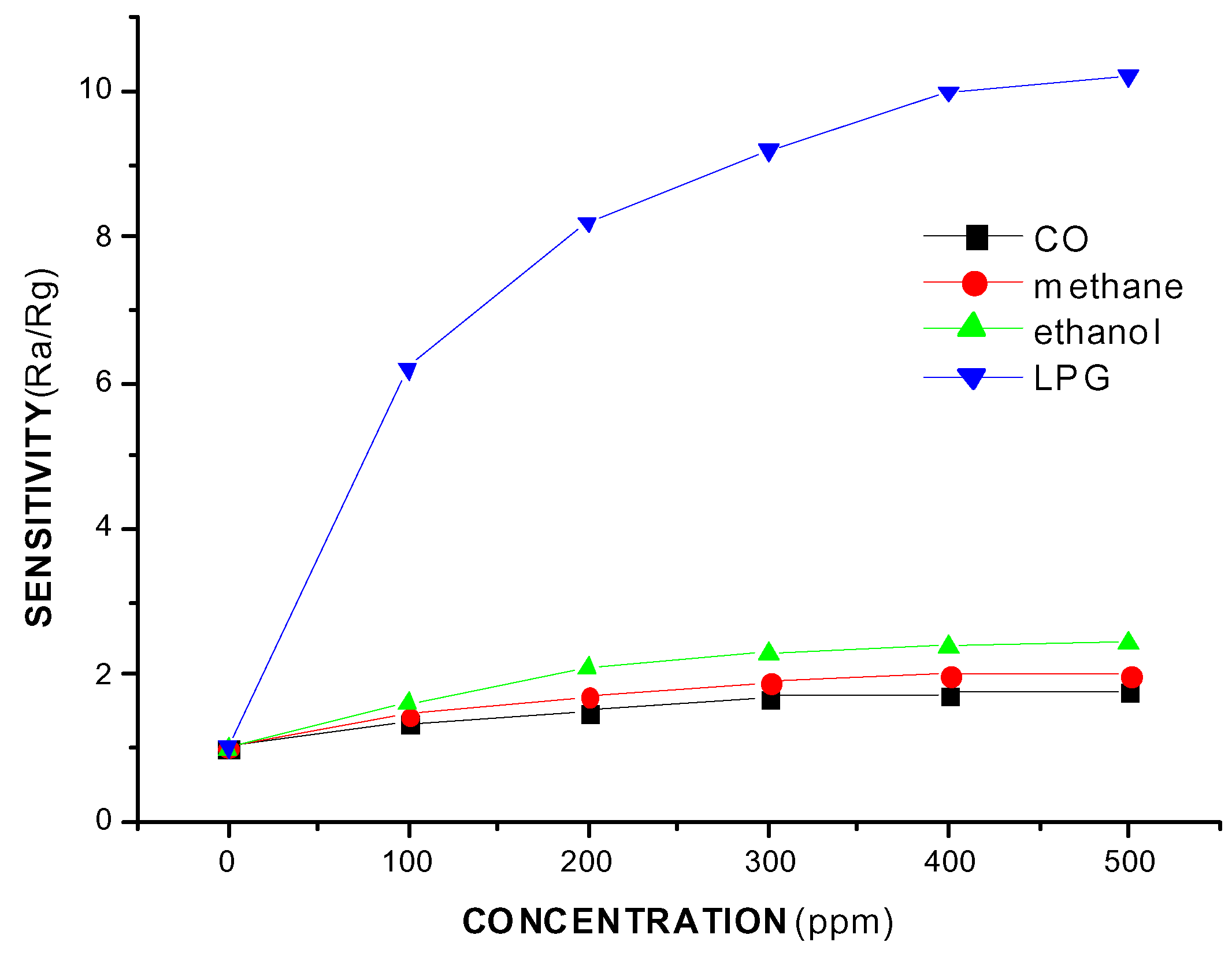
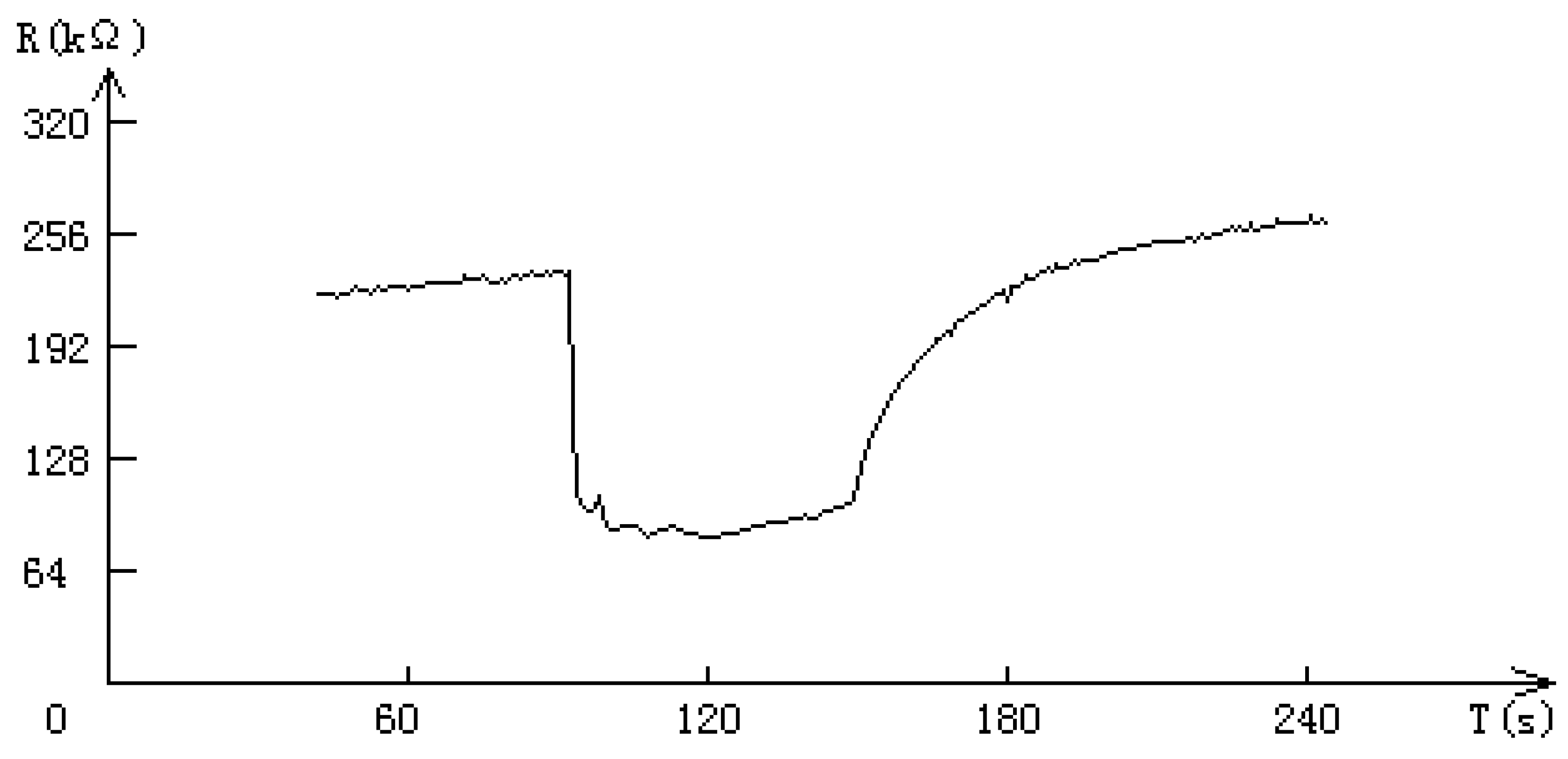
Conclusions
Acknowledgements
References
- Stambolova, I.J. Spray Pyrolysis Preparation and Humidity Sensing Characteristics of Spinel Zinc Stannate Thin Films. Solid State Chem. 1997, 128, 305–309. [Google Scholar] [CrossRef]
- Zheng, Y.; Takei, H.; Kawazoe, H. Electrical Conductivity in Transparent ZnGa2O4: Reduction and Surface-Layer Structure Transformation. J. Am. Ceram. Soc. 1998, 81(1), 180–186. [Google Scholar]
- Satyanarayana, L. Liquid-petroleum-gas sensor based on a spinel semiconductor, ZnGa2O4. Sensors and Actuators B 1998, 46, 1–7. [Google Scholar] [CrossRef]
- Gnanasekar, K. I. Electrical and sensor properties of FeNbO4: a new sensor material. Sensors and Actuators B 1999, 55, 170–174. [Google Scholar] [CrossRef]
- Yang, XJ.; Chen, NS.; Shen, SF.; Liu, ES; Huang, JL. Preparation, characterization and gas- sensitive properties of nano-crystalline Cr2O3-Fe2O3 mixed oxides; Science in China (Series Bchemistry) Vol.41 No.4 (1998); pp. 442–448.
- Chang, F. Y. Manganese-activated luminescence in ZnGa2O4. J. Appl. Phys 1996, 79(9), 7191–7197. [Google Scholar]
- Gopal Reddy, C. V. Semiconducting gas sensor for chlorine based on inverse spinel nickel ferrite. Sensors and Actuators B 1999, 55, 90–95. [Google Scholar] [CrossRef]
- Nakatani, Y.; Sakai, M.; Matsuoka, M. Enhancement of gas sensitivity by controlling microstructure of alpha-Fe2O3 ceramics. Jpn. J. Appl. Phys. Part 1 1983, 22, 912. [Google Scholar] [CrossRef]
- Davis, S. R.; Chadwick, A. V; Wright, J. D. The effects of crystallite growth and dopant migration on the carbon monoxide sensing characteristics of nanocrystalline tin oxide based sensor materials. J. Mater. Chem. 1998, 8, 2065–2072. [Google Scholar] [CrossRef]
- Lantto, V.; Kohl, D.; Demarne, V.; Sanjines, R. Sberveglieri, G., Ed.; Gas Sensors, Kluwer, Dordrecht; 1992; pp. 117–167.
- Williams, D. E. Moseley, P. T., Tofield, B.C., Eds.; Solid State Gas Sensors, Adam Hilger, Bristol; 1987; p. 71.
- Davis, S.; Chadwick, A.; Wright, J. A. Combined EXAFS and Diffraction Study of Pure and Doped Nanocrystalline Tin Oxide. J. Phys. Chem. 1997, 101, 9901. [Google Scholar] [CrossRef]
- Gopel, W.; Schierbaum, K. SnO2 sensor: Current status and future prospects. Sens. Actuators B 1995, 26–27, 1. [Google Scholar] [CrossRef]
- Kohl, D. Surface Processes in the Detection of Reducing Gases with SnO2-based Devices. Sens. Actuators B 1989, 18, 71. [Google Scholar] [CrossRef]
- Lantto, V.; Romppainen, P.; Leppavouri, S. A Study of the Temperature-dependence of the Barrier Energy in Porous tin Dioxide. Sens. Actuators B 1988, 14, 149. [Google Scholar] [CrossRef]
- Galdikas, A.; Mironas, A.; Ssetkus, A. Copper-doping level effect on sensitivity and selectivity of tin oxide thin-film gas sensor. Sens. Actuators B 1995, 26–27, 29. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jiao, Z.; Ye, G.; Chen, F.; Li, M.; Liu, J. The Preparation of ZnGa2O4 Nano Crystals by Spray Coprecipitation and Its Gas Sensitive Characteristics. Sensors 2002, 2, 71-78. https://doi.org/10.3390/s20300071
Jiao Z, Ye G, Chen F, Li M, Liu J. The Preparation of ZnGa2O4 Nano Crystals by Spray Coprecipitation and Its Gas Sensitive Characteristics. Sensors. 2002; 2(3):71-78. https://doi.org/10.3390/s20300071
Chicago/Turabian StyleJiao, Zheng, Gang Ye, Feng Chen, Mingqiang Li, and Jinhuai Liu. 2002. "The Preparation of ZnGa2O4 Nano Crystals by Spray Coprecipitation and Its Gas Sensitive Characteristics" Sensors 2, no. 3: 71-78. https://doi.org/10.3390/s20300071



