Preparation and Application of Metal Nanoparticals Elaborated Fiber Sensors
Abstract
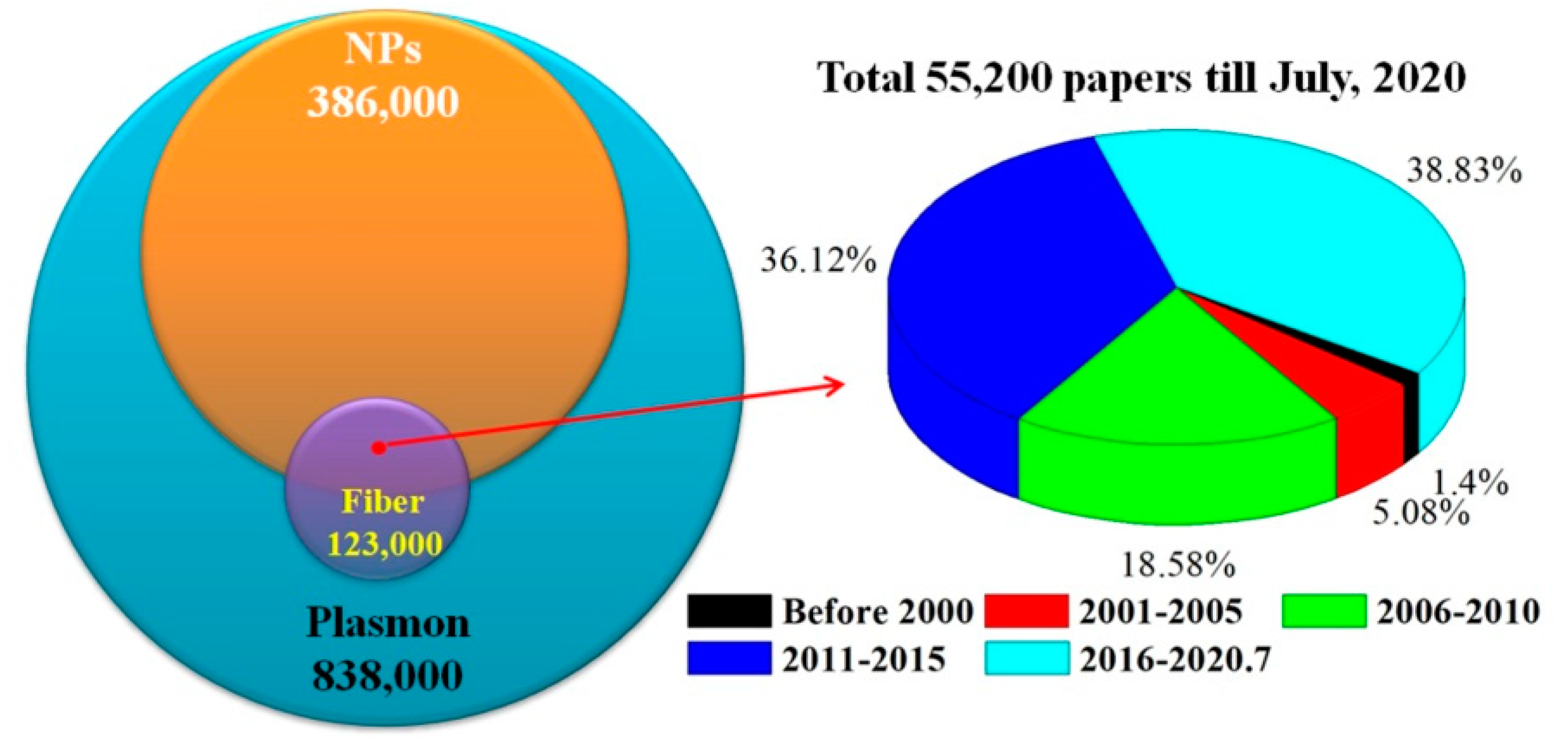
1. Introduction
2. Common Optical Fiber Structure and Probe Fabrication Method
3. Fiber-LSPR Refractive Index Sensors
4. Fiber-LSPR Chemical Sensors
4.1. Chemical Molecules
4.2. Heavy Metal Ions
4.3. Gas
5. Optical Fiber LSPR Biosensors
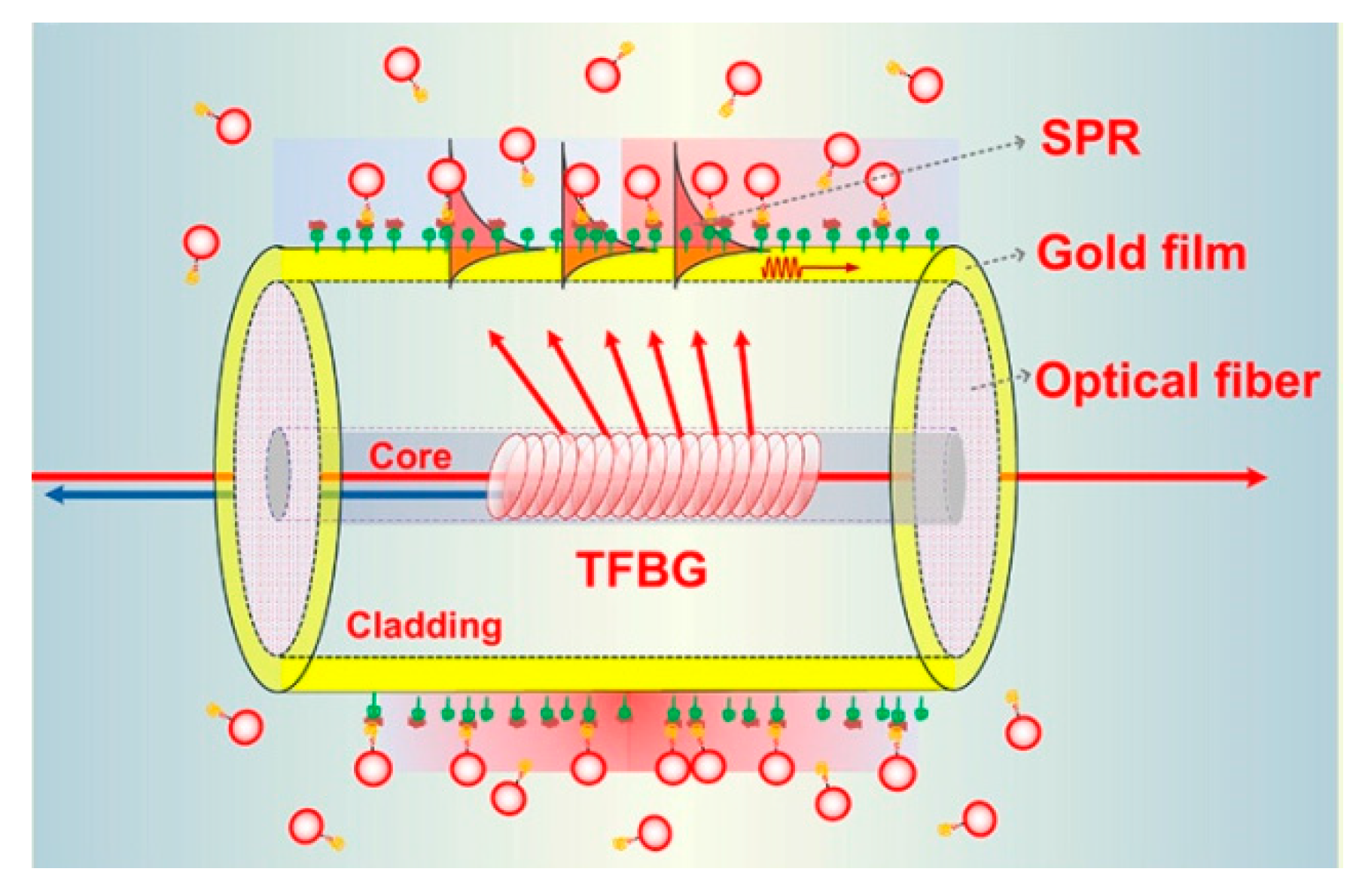
5.1. Typical Fiber Structures and Properties
5.2. Metal NPs Role and Optimization
6. Discussion and Perspective
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Homola, J.; Koudela, I.; Yee, S.S. Surface plasmon resonance sensors based on diffraction gratings and prism couplers: Sensitivity comparison. Sens. Actuators B-Chem. 1999, 54, 16–24. [Google Scholar] [CrossRef]
- Barnes, W.L.; Dereux, A.; Ebbesen, T.W. Surface plasmon subwavelength optics. Nature 2003, 424, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Ma, Y.F.; Sung, M.J.; Huang, C.P. Approach the angular sensitivity limit in surface plasmon resonance sensors with low index prism and large resonant angle. Opt. Eng. 2010, 49, 054403. [Google Scholar] [CrossRef]
- Wang, G.Q.; Wang, C.N.; Yang, R.; Liu, W.L.; Sun, S.Q. A Sensitive and Stable Surface Plasmon Resonance Sensor Based on Monolayer Protected Silver Film. Sensors 2017, 17, 2777. [Google Scholar] [CrossRef]
- Morsin, M.; Salleh, M.M.; Umar, A.A.; Sandan, M.Z. Gold Nanoplates for a localized surface plasmon resonance-based boric acid sensor. Sensors 2017, 17, 947. [Google Scholar] [CrossRef]
- Kazuma, E.; Tatsuma, T. Localized surface plasmon resonance sensors based on wavelength-tunable spectral dips. Nanoscale 2014, 6, 2397–2405. [Google Scholar] [CrossRef]
- Shalabney, A.; Abdulhalim, I. Prism dispersion effects in near-guided-wave surface plasmon resonance sensors. Ann. Phys. 2012, 524, 680–686. [Google Scholar] [CrossRef]
- Kang, Y.Q.; Gao, P.; Liu, H.M.; Zhang, J. Large tunable lateral shift from guided wave surface plasmon resonance. Plasmonics 2019, 14, 1289–1293. [Google Scholar] [CrossRef]
- Alharbi, R.; Irannejad, M.; Yavuz, M. A short review on the role of the metal-graphene hybrid nanostructure in promoting the localized surface plasmon resonance sensor performance. Sensors 2019, 19, 862. [Google Scholar] [CrossRef]
- Jeong, H.H.; Erdene, N.; Park, J.H.; Jeong, D.H.; Lee, S.K. Analysis of fiber-optic localized surface plasmon resonance sensor by controlling formation of gold nanoparticles and its bio-application. J. Nanosci. Nanotechnol. 2012, 12, 7815–7821. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Dona, A.; Dacarro, G.; Pallavicini, P.; Pesavento, M.; Zeni, L. Localized surface plasmon resonance with five-branched gold nanostars in a plastic optical fiber for bio-chemical sensor implementation. Sensors 2013, 13, 14676–14686. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Ji, M.G.; Kim, T.R.; Shim, C.H.; Lee, S.; Woo, D.; Choi, Y.W. Localized surface plasmon resonance in two-dimensional silver nanodot array fabricated using nanoporous alumina mask for chemical sensor platform. Opt. Eng. 2016, 55, 087107. [Google Scholar] [CrossRef]
- Park, J.H.; Byun, J.Y.; Shim, W.B.; Kim, S.U.; Kim, M.G. High-sensitivity detection of ATP using a localized surface plasmon resonance (LSPR) sensor and split aptamers. Biosens. Bioelectron. 2015, 73, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y. Localized surface plasmon resonance sensor based at metallic sphere dimer particle. J. Nanosci. Nanotechnol. 2017, 17, 1443–1446. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lin, K.C.; Tsai, W.H. Side-polished optical fiber sensor coated with a metal/oxide surface-plasmon-resonance sensing film. Optoelectron. Adv. Mat. 2015, 9, 903–906. [Google Scholar]
- He, Y.J. High-performance localized surface plasmon resonance fiber sensor based on nano-metal-gear array. Sens. Actuators B-Chem. 2014, 193, 778–787. [Google Scholar] [CrossRef]
- Yuan, Y.Q.; Hu, D.; Hua, L.; Li, M. Theoretical investigations for surface plasmon resonance based optical fiber tip sensor. Sens. Actuators B-Chem. 2013, 188, 757–760. [Google Scholar] [CrossRef]
- Ortega-Mendoza, J.G.; Padilla-Vivanco, A.; Toxqui-Quitl, C.; Zaca-Moran, P.; Villegas-Hernandez, D.; Chavez, F. Optical fiber sensor based on localized surface plasmon resonance using silver nanoparticles photodeposited on the optical fiber end. Sensors 2014, 14, 18701–18710. [Google Scholar] [CrossRef]
- Moayyed, H.; Leite, I.T.; Coelho, L.; Santos, J.L.; Viegas, D. Theoretical study of phase-interrogated surface plasmon resonance based on optical fiber sensors with metallic and oxide layers. Plasmonics 2015, 10, 979–987. [Google Scholar] [CrossRef]
- Uh, M.; Kim, J.S.; Park, J.H.; Jeong, D.H.; Lee, H.Y.; Lee, S.M.; Lee, S.K. Fabrication of localized surface plasmon resonance sensor based on optical fiber and micro fluidic channel. J. Nanosci. Nanotechnol. 2017, 17, 1083–1091. [Google Scholar] [CrossRef]
- Chang, C.Y.; Lin, H.T.; Lai, M.S.; Shieh, T.Y.; Peng, C.C.; Shih, M.H.; Tung, Y.C. Flexible localized surface plasmon resonance sensor with metal-insulator-metal nanodisks on PDMS substrate. Sci. Rep. 2018, 8, 11812. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.X.; Zheng, J.; Liang, Q.Y.; Deng, S.J.; Deng, H.C.; Liu, H.Q.; Yuan, L.B. The influence of structural parameters on the surface plasmon resonance sensor based on a side-polished macrobending plastic optical fiber. IEEE Sens. J. 2020, 20, 4245–4250. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mohr, G.J. On the application of different bimetallic alloy nanoparticle combinations in fiber optic surface plasmon resonance salinity sensor and its performance optimization against thermal effects. J. Nanosci. Nanotechnol. 2010, 10, 3145–3154. [Google Scholar] [CrossRef]
- Tu, M.H.; Sun, T.; Grattan, K.T.V. Optimization of gold-nanoparticle-based optical fibre surface plasmon resonance (SPR)-based sensors. Sens. Actuators B-Chem. 2012, 164, 43–53. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Mukherji, S. Gold nanoparticle coated U-bend fibre optic probe for localized surface plasmon resonance based detection of explosive vapours. Sens. Actuators B-Chem. 2014, 192, 804–811. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, G.C.; Sharma, G.; Singh, V. Study of surface plasmon resonance sensors based on silver-gold nanostructure alloy film coated tapered optical fibers. Appl. Phys. A 2018, 124, 695. [Google Scholar] [CrossRef]
- Liu, H.; Chen, C.; Zhang, Y.Z.; Bai, B.B.; Tang, S.F. A high-sensitivity methane sensor with localized surface plasmon resonance behavior in an improved hexagonal gold nanoring array. Sensors 2019, 19, 4803. [Google Scholar] [CrossRef]
- Muri, H.I.; Bano, A.; Hjelme, D.R. A single-point, multiparameter, fiber optic sensor based on a combination of interferometry and LSPR. J. Lightwave Technol. 2018, 36, 1159–1167. [Google Scholar] [CrossRef]
- Liu, Y.; Guang, J.Y.; Liu, C.; Bi, S.; Liu, Q.; Li, P.; Zhang, N.; Chen, S.M.; Yuan, H.Z.; Zhou, D.P.; et al. Simple and low-cost plasmonic fiber-optic probe as SERS and biosensing platform. Adv. Opt. Mater. 2019, 7, 1900337. [Google Scholar] [CrossRef]
- Pisco, M.; Galeotti, F.; Quero, G.; Grisci, G.; Micco, A.; Mercaldo, L.V.; Veneri, P.D.; Cutolo, A.; Cusano, A. Nanosphere lithography for optical fiber tip nanoprobes. Light-Sci. Appl. 2017, 6, e16229. [Google Scholar] [CrossRef]
- Polley, N.; Basak, S.; Hass, R.; Pacholski, C. Fiber optic plasmonic sensors: Providing sensitive biosensor platforms with minimal lab equipment. Biosens. Bioelectron. 2019, 132, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Muri, H.I.; Hjelme, D.R. LSPR coupling and distribution of interparticle distances between Nanoparticles in hydrogel on optical fiber end face. Sensors 2017, 17, 2723. [Google Scholar] [CrossRef] [PubMed]
- Rivero, P.J.; Goicoechea, J.; Arregui, F.J. Layer-by-layer nano-assembly: A powerful tool for optical fiber sensing applications. Sensors 2019, 19, 683. [Google Scholar] [CrossRef]
- Liu, T.; Wang, W.Q.; Liu, F.; Wang, S.Y. Photochemical deposition fabricated highly sensitive localized surface plasmon resonance based optical fiber sensor. Opt. Commun. 2018, 427, 301–305. [Google Scholar] [CrossRef]
- Spasopoulos, D.; Kaziannis, S.; Karantzalis, A.E.; Lidorikis, E.; Ikiades, A.; Kosmidis, C. Tailored aggregate-free Au nanoparticle decorations with sharp plasmonic peaks on a U-type optical fiber sensor by nanosecond laser irradiation. Plasmonics 2017, 12, 535–543. [Google Scholar] [CrossRef]
- Qayyum, H.; Ali, R.; Rehman, Z.U.; Ullah, S.; Shafique, B.; Dogar, A.H.; Shah, A.; Qayyum, A. Synthesis of silver and gold nanoparticles by pulsed laser ablation for nanoparticle enhanced laser-induced breakdown spectroscopy. J. Laser Appl. 2019, 31, 022014. [Google Scholar] [CrossRef]
- Lin, S.K.; Cheng, W.T. Fabrication and characterization of colloidal silver nanoparticle via photochemical synthesis. Mater. Lett. 2020, 261, 127077. [Google Scholar] [CrossRef]
- Menazea, A.A.; Ahmed, M.K. Wound healing activity of chitosan/polyvinyl alcohol embedded by gold nanoparticles prepared by nanosecond laser ablation. J. Mol. Struct. 2020, 1217, 128401. [Google Scholar] [CrossRef]
- Ran, Y.; Strobbia, P.; Cupil-Garcia, V.; Vo-Dinh, T. Fiber-optrode SERS probes using plasmonic silver-coated gold nanostars. Sens. Actuators B-Chem. 2019, 287, 95–101. [Google Scholar] [CrossRef]
- Danny, C.G.; Subrahmanyam, A.; Sai, V.V.R. Development of plasmonic U-bent plastic optical fiber probes for surface enhanced Raman scattering based biosensing. J. Raman Spectrosc. 2018, 49, 1607–1616. [Google Scholar] [CrossRef]
- Doherty, B.; Csaki, A.; Thiele, M.; Zeisberger, M.; Schwuchow, A.; Kobelke, J.; Fritzsche, W.; Schmidt, M.A. Nanoparticle functionalised small-core suspended-core fibre-a novel platform for efficient sensing. Biomed. Opt. Express 2017, 8, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.J.J.; Ho, H.P. Recent advances in plasmonic photonic crystal fibers: Design, fabrication and applications. Adv. Opt. Photonics 2017, 9, 257–314. [Google Scholar] [CrossRef]
- Gao, S.S.; Shang, S.B.; Liu, X.Y.; Li, Z.; Sheng, Y.Q.; Zhang, C.; Yang, C.; Qiu, H.W.; Huo, Y.Y.; Jiang, S.Z. An optical fiber SERS sensor based on GO/AgNPs/rGO sandwich structure hybrid films. RSC Adv. 2016, 6, 81750–81756. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.J.; Wang, X.Z.; Yang, Y.; Hu, Z.; Liu, L.; Huang, Y.D. Highly conductive PVA/Ag coating by aqueous in situ, reduction and its stretchable structure for strain sensor. ACS Appl. Mater. Interfaces 2020, 12, 1427–1435. [Google Scholar] [CrossRef]
- Jiang, S.Z.; Li, Z.; Zhang, C.; Gao, S.S.; Li, Z.; Qiu, H.W.; Li, C.H.; Yang, C.; Liu, M.; Liu, Y.J. A novel U-bent plastic optical fibre local surface plasmon resonance sensor based on a graphene and silver nanoparticle hybrid structure. J. Phys. D Appl. Phys. 2017, 50, 165105. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Jiang, S.Z.; Li, C.H.; Xu, S.C.; Yu, J.; Li, Z.; Wang, M.H.; Liu, A.H.; Man, B.Y. U-bent fiber optic SPR sensor based on graphene/AgNPs. Sens. Actuators B-Chem. 2017, 251, 127–133. [Google Scholar] [CrossRef]
- Minz, R.A.; Pal, S.S.; Sinha, R.K.; Mondal, S.K. Plasmonic coating on chemically treated optical fiber probe in the presence of evanescent wave: A novel approach for designing sensitive plasmonic sensor. Plasmonics 2016, 11, 653–658. [Google Scholar] [CrossRef]
- Tang, Y.W.; Yuan, H.; Chen, J.P.; Xing, Q.G.; Su, R.X.; Qi, W.; He, Z.M. Polydopamine-assisted fabrication of stable silver nanoparticles on optical fiber for enhanced plasmonic sensing. Photonic Sens. 2020, 10, 97–104. [Google Scholar] [CrossRef]
- Urrutia, A.; Goicoechea, J.; Rivero, P.J.; Pildain, A.; Arregui, F.J. Optical fiber sensors based on gold nanorods embedded in polymeric thin films. Sens. Actuators B-Chem. 2018, 255, 2105–2112. [Google Scholar] [CrossRef]
- Wieduwilt, T.; Zeisberger, M.; Thiele, M.; Doherty, B.; Chemnitz, M.; Csaki, A.; Fritzsche, W.; Schmidt, M.A. Gold-reinforced silver nanoprisms on optical fiber tapers-A new base for high precision sensing. APL Photonics 2016, 1, 066102. [Google Scholar] [CrossRef]
- Mos, J.E.; Korec, J.; Stasiewicz, K.A.; Jankiewicz, B.; Bartosewicz, B.; Jaroszewicz, L.R. Research on optical properties of tapered optical fibers with liquid crystal cladding doped with gold nanoparticles. Crystals 2019, 9, 306. [Google Scholar] [CrossRef]
- Guo, Y.; Song, B.B.; Huang, W.; Chen, S.Y. LSPR sensor employing side-polished suspend-core microstructured optical fiber with a silver nanorod. IEEE Sens. J. 2019, 19, 956–961. [Google Scholar] [CrossRef]
- Garcia, J.A.; Monzon-Hernandez, D.; Manriquez, J.; Bustos, E. One step method to attach gold nanoparticles onto the surface of an optical fiber used for refractive index sensing. Opt. Mater. 2016, 51, 208–212. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Basumallick, N.; Bysakh, S.; Dey, T.K.; Biswas, P.; Bandyopadhyay, S. Design of turn around point long period fiber grating sensor with Au-nanoparticle self monolayer. Opt. Laser Technol. 2018, 102, 254–261. [Google Scholar] [CrossRef]
- Liu, C.L.; Gao, Y.; Gao, Y.C.; Wei, Y.; Wu, P.; Su, Y.D. Enhanced sensitivity of fiber SPR sensor by metal nanoparticle. Sens. Rev. 2020, 40, 355–361. [Google Scholar] [CrossRef]
- Song, H.; Zhang, H.X.; Sun, Z.; Ren, Z.Y.; Yang, X.Y.; Wang, Q. Triangular silver nanoparticle U-bent fiber sensor based on localized surface plasmon resonance. AIP Adv. 2019, 9, 085307. [Google Scholar] [CrossRef]
- Miliutina, E.; Kalachyova, Y.; Postnikov, P.; Svorc?k, V.; Lyutakov, O. Enhancement of surface plasmon fiber sensor sensitivity through the grafting of gold nanoparticles. Photonic Sens. 2020, 10, 105–112. [Google Scholar] [CrossRef]
- Spasopoulos, D.; Kaziannis, S.; Danakas, S.; Ikiades, A.; Kosmidis, C. LSPR based optical fiber sensors treated with nanosecond laser irradiation for refractive index sensing. Sens. Actuators B-Chem. 2018, 256, 359–366. [Google Scholar] [CrossRef]
- Dash, S.P.; Patnaik, S.K.; Tripathy, S.K. Investigation of a low cost tapered plastic fiber optic biosensor based on manipulation of colloidal gold nanoparticles. Opt. Commun. 2019, 437, 388–391. [Google Scholar] [CrossRef]
- Wu, W.T.; Chen, C.H.; Chiang, C.Y.; Chau, L.K. Effect of Surface Coverage of Gold Nanoparticles on the Refractive Index Sensitivity in Fiber-Optic Nanoplasmonic Sensing. Sensors 2018, 18, 1759. [Google Scholar] [CrossRef]
- Niu, L.Y.; Wang, Q.; Jing, J.Y.; Zhao, W.M. Sensitivity enhanced D-type large-core fiber SPR sensor based on Gold nanoparticle/Au film co-modification. Opt. Commun. 2019, 450, 287–295. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Y.; Zhu, W.J.; Lai, T.T.; Peng, J.K.; Lyu, D.J.; Guo, D.L.; Yuan, Y.Q.; Lewis, E.; Yang, M.H. Graphene-gold-Au@Ag NPs-PDMS films coated fiber optic for refractive index and temperature sensing. IEEE Photonic Technol. Lett. 2019, 31, 1205–1208. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Q. Refractive index sensitivity enhancement of optical fiber SPR sensor utilizing layer of MWCNT/PtNPs composite. Opt. Fiber Technol. 2019, 51, 118–124. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.M.; Gong, T.C.; Zhang, X.L.; Zhu, Y. Tapered fiber probe modified by Ag nanoparticles for SERS detection. Plasmonics 2016, 11, 743–751. [Google Scholar] [CrossRef]
- Xu, W.J.; Chen, Z.Y.; Chen, N.; Zhang, H.; Liu, S.P.; Hu, X.M.; Wen, J.X.; Wang, T.Y. SERS taper-fiber nanoprobe modified by gold nanoparticles wrapped with ultrathin alumina film by atomic layer deposition. Sensors 2017, 17, 467. [Google Scholar] [CrossRef]
- Paul, D.; Dutta, S.; Biswas, R. LSPR enhanced gasoline sensing with a U-bent optical fiber. J. Phys. D Appl. Phys. 2016, 49, 305104. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Zhao, Y.F.; Zhang, C.; Jiang, S.Z.; Yang, C.; Xiu, X.W.; Li, C.H.; Li, Z.; Zhao, X.F.; Man, B.Y. In-situ growth of AuNPs on WS2@U-bent optical fiber for evanescent wave absorption sensor. Appl. Surf. Sci. 2018, 441, 1072–1078. [Google Scholar] [CrossRef]
- Wang, J.Q.; Geng, Y.J.; Shen, Y.T.; Shi, W.; Xu, W.Q.; Xu, S.P. SERS-active fiber tip for intracellular and extracellular pH sensing in living single cells. Sens. Actuators B-Chem. 2019, 290, 527–534. [Google Scholar] [CrossRef]
- Islam, S.; Bakhtiara, H.; Aziz, M.; Riaz, S.; Abd Aziz, M.S.; Naseem, S.; Elshikeri, N. Optically active phenolphthalein encapsulated gold nanodendrites for fiber optic pH sensing. Appl. Surf. Sci. 2019, 485, 323–331. [Google Scholar] [CrossRef]
- Yin, Z.; Geng, Y.F.; Li, X.J.; Tan, X.L.; Hong, X.M. Sensitivity-enhanced U-shaped fiber SERS probe with photoreduced silver nanoparticles. IEEE Photonics J. 2016, 8, 6803607. [Google Scholar] [CrossRef]
- Zhou, H.W.; Liu, J.S.; Liu, H.T.; Zheng, Z. Compact dual-fiber surface-enhanced Raman scattering sensor with monolayer gold nanoparticles self-assembled on optical fiber. Appl. Opt. 2018, 57, 7931–7937. [Google Scholar] [CrossRef] [PubMed]
- Turasan, H.; Cakmak, M.; Kokini, J. Fabrication of zein-based electrospun nanofiber decorated with gold nanoparticles as a SERS platform. J. Mater. Sci. 2019, 54, 8872–8891. [Google Scholar] [CrossRef]
- Sanchez-Solis, A.; Karim, F.; Alam, M.S.; Zhan, Q.W.; Lopez-Luke, T.; Zhao, C.L. Print metallic nanoparticles on a fiber probe for 1064-nm surface-enhanced Raman scattering. Opt. Lett. 2019, 44, 4997–5000. [Google Scholar] [CrossRef]
- Parveen, S.; Pathak, A.; Gupta, B.D. Fiber optic SPR nanosensor based on synergistic effects of CNT/Cu-nanoparticles composite for ultratrace sensing of nitrate. Sens. Actuators B-Chem. 2017, 246, 910–919. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Lai, Y.M.; Wu, C.; Sun, S.Q.; He, Y.H.; Ma, H. Ultrasensitive and selective detection of copper (II) and mercury (II) ions by dye-coded silver nanoparticle-based SERS probes. Biosens. Bioelectron. 2013, 39, 82–87. [Google Scholar] [CrossRef]
- Su, D.Y.; Yang, X.; Xia, Q.D.; Zhang, Q.; Chai, F.; Wang, C.G.; Qu, F.Y. Folic acid functionalized silver nanoparticles with sensitivity and selectivity colorimetric and fluorescent detection for Hg2+ and efficient catalysis. Nanotechnology 2014, 25, 355702. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.M.; Punjabi, N.; Kundu, T.; Mukherji, S. Optimization of plasmonic U-shaped optical fiber sensor for mercury ions detection using glucose capped silver nanoparticles. IEEE Sens. J. 2019, 19, 3224–3231. [Google Scholar] [CrossRef]
- Martinez-Hernandez, M.E.; Goicoechea, J.; Arregui, F.J. Hg2+ optical fiber sensor based on LSPR generated by gold nanoparticles embedded in LBL nano-assembled coatings. Sensors 2019, 19, 4906. [Google Scholar] [CrossRef]
- Raj, D.R.; Prasanth, S.; Vineeshkumar, T.V.; Sudarsanakumar, C. Surface plasmon resonance based fiber optic sensor for mercury detection using gold nanoparticles PVA hybrid. Opt. Commun. 2016, 367, 102–107. [Google Scholar]
- Tan, S.Y.; Lee, S.C.; Okazaki, T.; Kuramitz, H.; Abd-Rahman, F. Detection of mercury (II) ions in water by polyelectrolyte-gold nanoparticles coated long period fiber grating sensor. Opt. Commun. 2018, 419, 18–24. [Google Scholar] [CrossRef]
- Prakashan, V.P.; George, G.; Sanu, M.S.; Sajna, M.S.; Saritha, A.C.; Sudarsanakumar, C.; Biju, P.R.; Joseph, C.; Unnikrishnan, N.V. Investigations on SPR induced Cu@Ag core shell doped SiO2-TiO2-ZrO2 fiber optic sensor for mercury detection. Appl. Surf. Sci. 2020, 507, 144957. [Google Scholar] [CrossRef]
- Boruah, B.S.; Biswas, R. Localized surface plasmon resonance based U-shaped optical fiber probe for the detection of Pb2+ in aqueous medium. Sens. Actuators B-Chem. 2018, 276, 89–94. [Google Scholar] [CrossRef]
- Jia, S.; Bian, C.; Sun, J.Z.; Tong, J.H.; Xia, S.H. A wavelength-modulated localized surface plasmon resonance (LSPR) optical fiber sensor for sensitive detection of mercury(II) ion by gold nanoparticles-DNA conjugates. Biosens. Bioelectron. 2018, 114, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sadani, K.; Nag, P.; Mukherji, S. LSPR based optical fiber sensor with chitosan capped gold nanoparticles on BSA for trace detection of Hg (II) in water, soil and food samples. Biosens. Bioelectron. 2019, 134, 90–96. [Google Scholar] [CrossRef]
- Halkare, P.; Punjabi, N.; Wangchuk, J.; Nair, A.; Kondabagil, K.; Mukherji, S. Bacteria functionalized gold nanoparticle matrix based fiber-optic sensor for monitoring heavy metal pollution in water. Sens. Actuators B-Chem. 2019, 281, 643–651. [Google Scholar] [CrossRef]
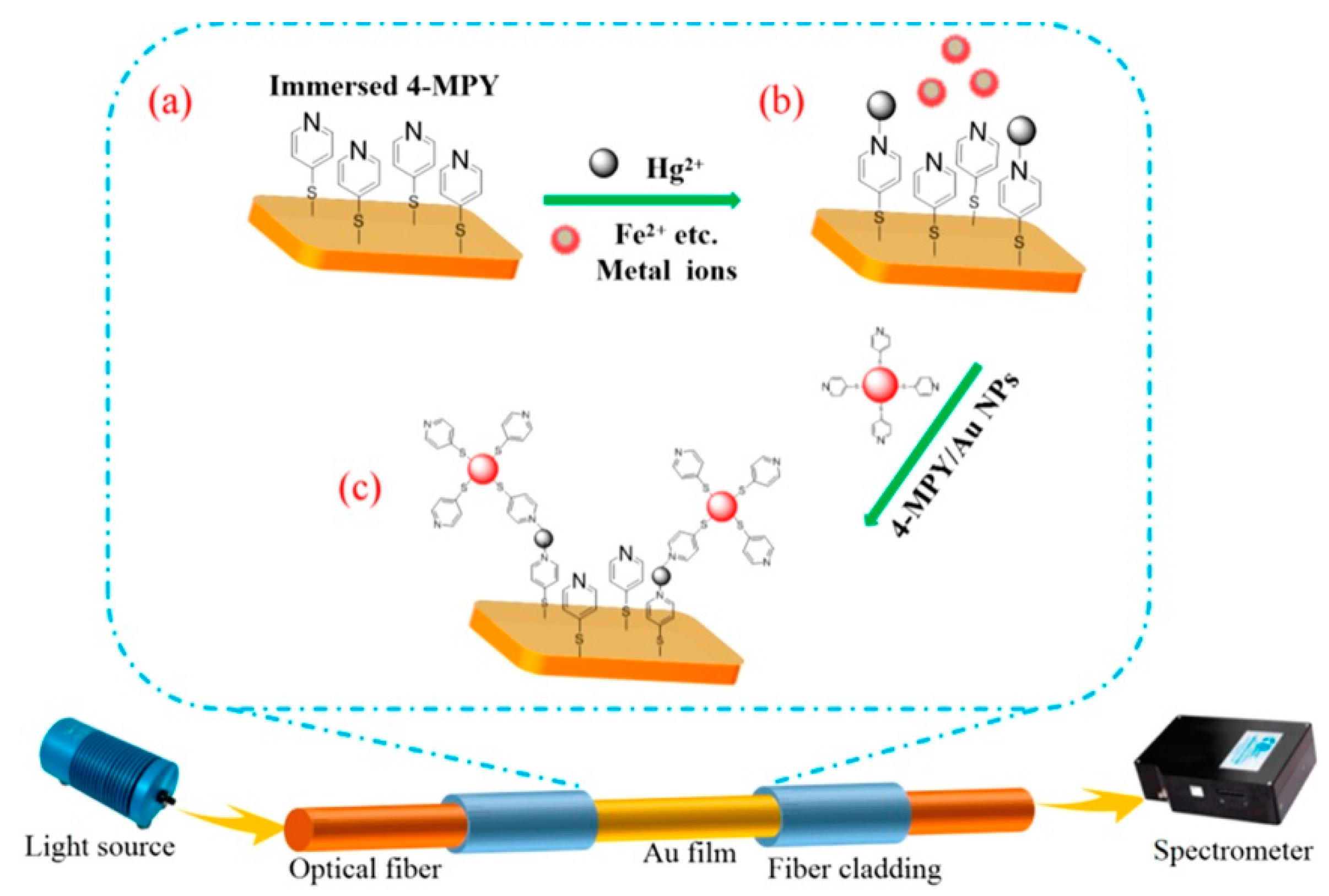
- Yuan, H.Z.; Ji, W.; Chu, S.W.; Liu, Q.; Qian, S.Y.; Guang, J.Y.; Wang, J.B.; Han, X.Y.; Masson, J.F.; Peng, W. Mercaptopyridine-functionalized gold nanoparticles for fiber-optic surface plasmon resonance Hg2+ sensing. ACS Sens. 2019, 4, 704–710. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Gupta, B.D. Ion-imprinted nanoparticles for the concurrent estimation of Pb(II) and Cu(II) ions over a two channel surface plasmon resonance-based fiber optic platform. J. Biomed. Opt. 2018, 23, 017001. [Google Scholar]
- Dhara, P.; Kumar, R.; Binetti, L.; Nguyen, H.T.; Alwis, L.S.; Sun, T.; Grattan, K.T.V. Optical fiber-based heavy metal detection using the localized surface plasmon resonance Technique. IEEE Sens. J. 2019, 19, 8720–8726. [Google Scholar] [CrossRef]
- Yap, S.H.K.; Chien, Y.H.; Tan, R.; Alauddin, A.R.B.; Ji, W.B.; Tjin, S.C.; Yong, K.T. An advanced hand-held microfiber-based sensor for ultrasensitive lead ion detection. ACS Sens. 2018, 3, 2506–2512. [Google Scholar] [CrossRef]
- Mironenko, A.Y.; Sergeev, A.A.; Nazirov, A.E.; Modin, E.B.; Voznesenskiy, S.S.; Bratskaya, S.Y. H2S optical waveguide gas sensors based on chitosan/Au and chitosan/Ag nanocomposites. Sens. Actuators B-Chem. 2016, 225, 348–353. [Google Scholar] [CrossRef]
- Paul, D.; Dutta, S.; Saha, D.; Biswas, R. LSPR based Ultra-sensitive low cost U-bent optical fiber for volatile liquid sensing. Sens. Actuators B-Chem. 2017, 250, 198–207. [Google Scholar] [CrossRef]
- Yu, C.B.; Wu, Y.; Liu, X.L.; Fu, F.; Gong, Y.; Rao, Y.J.; Chen, Y.F. Miniature fiber-optic NH3 gas sensor based on Pt nanoparticle-incorporated graphene oxide. Sens. Actuators B-Chem. 2017, 244, 107–113. [Google Scholar] [CrossRef]
- Gao, N.; Mu, Z.Z.; Li, J. Palladium nanoparticles doped polymer microfiber functioned as a hydrogen probe. Int. J. Hydrog. Energy 2019, 44, 14085–14091. [Google Scholar] [CrossRef]
- Chao, J.F.; Chen, Y.H.; Xing, S.M.; Zhang, D.L.; Shen, W.L. Facile fabrication of ZnO/C nanoporous fibers and ZnO hollow spheres for high performance gas sensor. Sens. Actuators B-Chem. 2019, 298, 126927. [Google Scholar] [CrossRef]
- Zhong, X.X.; Yang, M.H.; Huang, C.J.; Wang, G.P.; Dai, J.X.; Bai, W. Water photolysis effect on the long-term stability of a fiber optic hydrogen sensor with Pt/WO3. Sci. Rep. 2016, 6, 39160. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Sun, C.Y.; Yu, S.L.; Pu, Z.H.; Zhang, P.H.; Xu, K.X.; Song, Z.Q.; Li, D.C. Flattened fiber-optic ATR sensor enhanced by silver nanoparticles for glucose measurement. Biomed. Microdevices 2018, 20, 104. [Google Scholar] [CrossRef]
- Arcas, A.D.; Dutra, F.D.; Allil, R.C.S.B.; Werneck, M.M. Surface plasmon resonance and bending loss-based U-shaped plastic optical giber biosensors. Sensors 2018, 18, 648. [Google Scholar] [CrossRef]
- Chen, K.C.; Li, Y.L.; Wu, C.W.; Chiang, C.C. Glucose sensor using U-Shaped optical fiber probe with gold nanoparticles and glucose oxidase. Sensors 2018, 18, 1217. [Google Scholar] [CrossRef]
- Raj, D.R.; Prasanth, S.; Sudarsanakumar, C. Development of LSPR-based optical fiber dopamine sensor using l-tyrosine-capped silver nanoparticles and its nonlinear optical properties. Plasmonics 2017, 12, 1227–1234. [Google Scholar]
- Urrutia, A.; Bojan, K.; Marques, L.; Mullaney, K.; Goicoechea, J.; James, S.; Clark, M.; Tatam, R.; Korposh, S. Novel highly sensitive protein sensors based on tapered optical fibres modified with Au-based nanocoatings. J. Sens. 2016, 2016, 8129387. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, B.K.; Singh, R.; Chen, N.K.; Yang, Q.S.; Zhang, X.; Wang, W.J.; Zhang, B.Y. LSPR-based cholesterol biosensor using a tapered optical fiber structure. Biomed. Opt. Express 2019, 10, 2150–2160. [Google Scholar] [CrossRef]
- Singh, L.; Zhu, G.; Singh, R.; Zhang, B.Y.; Wang, W.J.; Kaushik, B.K.; Kumar, S. Gold nanoparticles and uricase functionalized tapered fiber sensor for uric acid detection. IEEE Sens. J. 2020, 20, 219–226. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, D.; Mao, Q.H. A highly reproducible and sensitive fiber SERS probe fabricated by direct synthesis of closely packed AgNPs on the silanized fiber taper. Analyst 2017, 142, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Deng, S.X.; Wang, H.; Zhang, R.H.; Zhu, K.; Lu, Y.; Wang, Z.L.; Zong, S.F.; Wang, Z.Y.; Cui, Y.P. A SERS fiber probe fabricated by layer-by-layer assembly of silver sphere nanoparticles and nanorods with a greatly enhanced sensitivity for remote sensing. Nanotechnology 2019, 30, 255503. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, B.R.; Chiamenti, I.; Oliveira, M.M.; Muller, M.; Fabris, J.L. Functionalized long period grating-plasmonic fiber sensor applied to the detection of glyphosate in water. J. Lightwave Technol. 2018, 36, 863–870. [Google Scholar] [CrossRef]
- Lao, J.J.; Han, L.Z.; Wu, Z.; Zhnag, X.J.; Huang, Y.Y.; Tang, Y.; Guo, T. Gold nanoparticle functionalized surface plasmon resonance optical fiber biosensor: In situ detection of thrombin with 1 nM detection Limit. J. Lightwave Technol. 2019, 37, 2748–2755. [Google Scholar] [CrossRef]
- Chen, H.M.; Zhao, L.; Chen, D.Q.; Hu, W.H. Stabilization of gold nanoparticles on glass surface with polydopamine thin film for reliable LSPR sensing. J. Colloid Interface Sci. 2015, 460, 258–263. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Wang, B.T.; Wang, Q. Sensitivity-enhanced optical fiber biosensor based on coupling effect between SPR and LSPR. IEEE Sens. J. 2018, 18, 8303–8310. [Google Scholar] [CrossRef]
- Li, K.W.; Zhou, W.C.; Zeng, S.W. Optical micro/nanofiber-based localized surface plasmon resonance biosensors: Fiber diameter dependence. Sensors 2018, 18, 3295. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Kaushik, B.K.; Chen, N.K.; Yang, Q.S.; Zhang, X. LSPR-based cholesterol biosensor using hollow core fiber structure. IEEE Sens. J. 2019, 19, 7399–7406. [Google Scholar] [CrossRef]
- Singh, L.; Singh, R.; Zhang, B.Y.; Kaushik, B.K.; Kumar, S. Localized surface plasmon resonance based hetero-core optical fiber sensor structure for the detection of l-cysteine. IEEE Trans. Nanotechnol. 2020, 19, 201–208. [Google Scholar]
- Zhu, G.; Agrawal, N.; Singh, R.; Kumar, S.; Zhang, B.Y.; Saha, C.; Kumar, C. A novel periodically tapered structure-based gold nanoparticles and graphene oxide-Immobilized optical fiber sensor to detect ascorbic acid. Opt. Laser Technol. 2020, 127, 106156. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, J.H.; Jeong, D.H.; Lee, H.Y.; Lee, S.K. Real-time detection of prostate-specific antigens using a highly reliable fiber-optic localized surface plasmon resonance sensor combined with micro fluidic channel. Sens. Actuators B-Chem. 2018, 273, 891–898. [Google Scholar] [CrossRef]
- Kim, H.M.; Uh, M.; Jeong, D.H.; Lee, H.Y.; Park, J.H.; Lee, S.K. Localized surface plasmon resonance biosensor using nanopatterned gold particles on the surface of an optical fiber. Sens. Actuators B-Chem. 2019, 280, 183–191. [Google Scholar] [CrossRef]
- Liu, L.L.; Marques, L.; Correia, R.; Morgan, S.P.; Lee, S.W.; Tighe, P.; Fairclough, L.; Korposh, S. Highly sensitive label-free antibody detection using a long period fibre grating sensor. Sens. Actuators B-Chem. 2018, 271, 24–32. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Mukherji, S.; Mukherji, S. Probing the localized surface plasmon field of a gold nanoparticle-based fibre optic biosensor. Plasmonics 2016, 11, 753–761. [Google Scholar] [CrossRef]
- Baliyan, A.; Usha, S.P.; Gupta, B.D.; Gupta, R.; Sharma, E.K. Localized surface plasmon resonance-based fiber-optic sensor for the detection of triacylglycerides using silver nanoparticles. J. Biomed. Opt. 2017, 22, 107001. [Google Scholar] [CrossRef]
- Raj, D.R.; Sudarsanakumar, C. Surface plasmon resonance based fiber optic sensor for the detection of cysteine using diosmin capped silver nanoparticles. Sens. Actuators A-Phys. 2017, 253, 41–48. [Google Scholar]
- Lu, M.D.; Zhu, H.; Bazuin, C.G.; Peng, W.; Masson, J.F. Polymer-templated gold nanoparticles on optical fibers for enhanced-sensitivity localized surface plasmon resonance biosensors. ACS Sens. 2019, 4, 613–622. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.H.; Byun, J.Y.; Kim, J.H.; Kim, M.G. An optical fiber-based LSPR aptasensor for simple and rapid in-situ detection of ochratoxin A. Biosens. Bioelectron. 2018, 102, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.R.; Prasanth, S.; Vineeshkumar, T.V.; Sudarsanakumar, C. Surface plasmon resonance based fiber optic dopamine sensor using green synthesized silver nanoparticles. Sens. Actuators B-Chem. 2016, 224, 600–606. [Google Scholar]
- Yuan, H.Z.; Ji, W.; Chu, S.W.; Liu, Q.; Guang, J.Y.; Sun, G.Y.; Zhang, Y.; Han, X.Y.; Masson, J.F.; Peng, W. Au nanoparticles as label-free competitive reporters for sensitivity enhanced fiber-optic SPR heparin sensor. Biosens. Bioelectron. 2020, 154, 112039. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.Z.; Song, H.; Zhao, W.M.; Jing, J.Y. A dual channel self-compensation optical fiber biosensor based on coupling of surface plasmon polariton. Opt. Laser Technol. 2020, 124, 106002. [Google Scholar] [CrossRef]
- Kim, H.M.; Jeong, D.H.; Lee, H.Y.; Park, J.H.; Lee, S.K. Improved stability of gold nanoparticles on the optical fiber and their application to refractive index sensor based on localized surface plasmon resonance. Opt. Laser Technol. 2019, 114, 171–178. [Google Scholar] [CrossRef]
- Loyez, M.; Ribaut, C.; Caucheteur, C.; Wattiez, R. Functionalized gold electroless-plated optical fiber gratings for reliable surface biosensing. Sens. Actuators B-Chem. 2019, 280, 54–61. [Google Scholar] [CrossRef]
- Singh, L.; Singh, R.; Zhang, B.Y.; Cheng, S.; Kaushik, B.K.; Kumar, S. LSPR based uric acid sensor using graphene oxide and gold nanoparticles functionalized tapered fiber. Opt. Fiber Technol. 2019, 53, 102043. [Google Scholar] [CrossRef]
- Nayak, J.K.; Parhi, P.; Jha, R. Experimental and theoretical studies on localized surface plasmon resonance based fiber optic sensor using graphene oxide coated silver nanoparticles. J. Phys. D Appl. Phys. 2016, 49, 285101. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Li, S.L.; Zhang, Y.N.; Sun, B.P.; Yu, Y.H.; Ren, H.Y.; Jiang, S.Z.; Yue, W.W. LSPR optical fiber biosensor based on a 3D composite structure of gold nanoparticles and multilayer graphene films. Opt. Express 2020, 28, 6071–6083. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, J.H.; Lee, S.K. Fiber optic sensor based on ZnO nanowires decorated by Au nanoparticles for improved plasmonic biosensor. Sci. Rep. 2019, 9, 15605. [Google Scholar] [CrossRef]
- Yuan, H.Z.; Ji, W.; Chu, S.W.; Qian, S.Y.; Wang, F.; Masson, J.F.; Han, X.Y.; Peng, W. Fiber-optic surface plasmon resonance glucose sensor enhanced with phenylboronic acid modified Au nanoparticles. Biosens. Bioelectron. 2018, 117, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.M.; Usha, S.P.; Gupta, B.D. Highly sensitive and selective erythromycin nanosensor employing fiber optic SPR/ERY imprinted nanostructure: Application in milk and honey. Biosens. Bioelectron. 2017, 90, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Semwal, V.; Shrivastav, A.M.; Gupta, B.D. Surface plasmon resonance based fiber optic trichloroacetic acid sensor utilizing layer of silver nanoparticles and chitosan doped hydrogel. Nanotechnology 2017, 28, 065503. [Google Scholar] [CrossRef] [PubMed]
- Cepeda-Perez, E.; Moreno-Hernandez, C.; Luke, T.L.; Monzon-Hernandez, D.; Plascencia-Villa, G.; de la Rosa, E. Reusable fiber taper sensor based on the metastability of gold nanoparticles. Mater. Res. Express 2019, 6, 026207. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, B.D. Surface plasmon resonance based highly selective fiber optic dopamine sensor fabricated using molecular imprinted GNP/SnO2 nanocomposite. J. Lightwave Technol. 2018, 36, 5956–5962. [Google Scholar] [CrossRef]
- Gupta, B.D.; Shrivastav, A.M.; Usha, S.P. Surface plasmon resonance-based fiber optic sensors utilizing molecular imprinting. Sensors 2016, 16, 1381. [Google Scholar] [CrossRef]
- Semwal, V.; Gupta, B.D. LSPR- and SPR-based fiber-optic cholesterol sensor using immobilization of cholesterol oxidase over silver nanoparticles coated graphene oxide nanosheets. IEEE Sens. J. 2018, 18, 1039–1046. [Google Scholar] [CrossRef]
- Qian, S.Y.; Lin, M.; Ji, W.; Yuan, H.Z.; Zhang, Y.; Jing, Z.G.; Zhao, J.Z.; Masson, J.F.; Peng, W. Boronic acid functionalized Au nanoparticles for selective microRNA signal amplification in fiber-optic surface plasmon resonance sensing system. ACS Sens. 2018, 3, 929–935. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Chen, P.W.; Liang, H.; Xiao, A.X.; Zeng, S.K.; Guan, B.O. Nucleic acid hybridization on a plasmonic nanointerface of optical microfiber enables ultrahigh-sensitive detection and potential photothermal therapy. Biosens. Bioelectron. 2020, 156, 112147. [Google Scholar] [CrossRef]
- Sharma, P.; Semwal, V.; Gupta, B.D. A highly selective LSPR biosensor for the detection of taurine realized on optical fiber substrate and gold nanoparticles. Opt. Fiber Technol. 2019, 52, 101962. [Google Scholar] [CrossRef]
- Fang, Y.H.; Wen, K.H.; Li, Z.F.; Wu, B.Y.; Chen, L.; Zhou, J.Y.; Zhou, D.Y. Multiple Fano Resonances Based on End-Coupled Semi-Ring Rectangular Resonator. IEEE Photonics J. 2019, 11, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, J.; Zhang, Z.; Wu, H.; Yao, N.; Cai, D.W.; Xu, Y.X.; Zhang, J.; Sun, G.F.; Wang, L.Q.; et al. Ultrasensitive skin-like wearable optical sensors based on glass micro/nanofibers. Opto-Electron. Adv. 2020, 3, 190022. [Google Scholar] [CrossRef]
- Li, Z.F.; Wen, K.H.; Fang, Y.H.; Guo, Z.C. Refractive Index Sensing Research on Multi-Fano-Based Plasmonic MDM Resonant System With Water-Based Dielectric. IEEE J. Quantum Electron. 2020, 56, 1–7. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, L.; Zheng, Q.L.; Zhang, Y.X.; Wen, L. On-chip readout plasmonic mid-IR gas sensor. Opto-Electron. Adv. 2020, 3, 190040. [Google Scholar] [CrossRef]




| Fiber Structures and Elaborate Materials | Sensitivity | Working Range | Reference |
|---|---|---|---|
| U-fiber/PVA/G/AgNPs@Ag film | 700.3 nm/RIU | 1.330–1.3657 | [45] |
| U-fiber/Ag NPs-Graphene | 1198 nm/RIU | 1.3657–1.3557 | [46] |
| MMF/Au NPs | ~987.85 nm/RIU | 1.328–1.377 | [47] |
| MMF/PDA-Ag NPs-PDA | 961 nm/RIU | 1.33–1.40 | [48] |
| MMF/Au NRs | 75.69 dB/RIU | 1.33–1.408 | [49] |
| Microfiber taper/Au-Ag nanoprisms | 900 nm/RIU | 1.333–1.383 | [50] |
| Side-polished PCF/Ag NR | 8600 nm/RIU | 1.33–1.40 | [51] |
| MMF-SMF-MMF/Au NPs | 765 nm/RIU | 1.333–1.365 | [52] |
| LPFG/Au NPs | ~3928 nm/RIU | 1.3333–1.3428 | [53] |
| Core angle SMF-MMF/Au NPs-Au film | 5140nm/RIU | 1.32–1.37 | [54] |
| U-fiber/Spherical Ag NPs | 342.7 nm/RIU | 1.3317–1.3640 | [55] |
| U-fiber/Triangular Ag NPs | 1116.8 nm/RIU | ||
| MMF/Au Multibranched NPs | 3164 nm/RIU | 1.333 to 1.393 | [56] |
| MMF/Au NPs | 349.1 nm/RIU | 1.333–1.403 | [57] |
| D fiber/Au NPs-Au film | 3074 nm/RIU | 1.3332–1.3710 | [58] |
| MMF-SMF-MMF/Graphene-Au-Au@Ag NPs-PDMS | 1591 nm/RIU | 1.3330–1.4005 | [59] |
| MMF end/MWCNT-Pt NPs | 5923 nm/RIU | 1.3385–1.3585 | [60] |
| Fiber Structures and Elaborate Materials | Measurement Target | Sensitivity/Resolution | Working Range | Reference |
|---|---|---|---|---|
| Fiber nanotaper/Au NPs | R6G | 0.1 μM | 0.1–100 μM | [64] |
| R6G | 1 μM/L | 0.1–100 μM | [65] | |
| U-fiber/Au NPs | Gasoline | 0.1198 mV/ppm | 0–153.38 ppm | [66] |
| 0.08244 mV/ppm | 0–144.09 ppm | |||
| U-fiber/Au NPs-WS2 film | Ethanol | 0.65/% | 10–80% | [67] |
| NaCl | 1.5/% | 5–25% | ||
| Fiber tip/Ag NPs-4 MPY | PH | 0–1 cm−1/PHU | 5.01–9.10 | [68] |
| NCF/Au NPs-PHPH | PH | ~25 counts/PHU | 1–12 | [69] |
| Dual fiber tips/Au NPs | Rh B | 10 ppm (LOD) | -- | [71] |
| Zein nanofiber/Au NPs | Rh 6G | 100 μM | 100 μM–1 mM | [72] |
| MMF tip/Au NPs | Rh 6G | -- | 50 μM–1 mM | [73] |
| NCF/CNT-Cu NPs | Nitrate | 80.62 × 106 nm/M | 10−6 M–5 × 10−3 M | [74] |
| U-fiber/Glucose-Ag NPs | Hg2+ | 2 ppb | 0–200 ppb | [77] |
| MMF/PAA-capped AuNPs | Hg2+ | 0.7 ppb | 1–20 ppb | [78] |
| NCF/PVA-Au NPs | Hg2+ | 1 μM | 0–25 μM | [79] |
| LPG/PE-Au NPs | Hg2+ | 0.5 ppm | 0.5–10 ppm | [80] |
| NCF/ternary system-Cu@Ag NPs | Hg2+ | 0.01 μM | 0.01–1000 μM | [81] |
| U-fiber/Oxalic acid-Au NPs | Pb2+ | 1.75 ppb | 1–20 ppb | [82] |
| Fiber end/DNA-Au NPs | Hg2+ | 0.7 nM | 1–50 nM | [83] |
| U fiber/BSA-Chitosan-Au NPs | Hg2+ | 0.1 ppb | 0.1–540 ppb | [84] |
| U-fiber/Au NPs-PSS-PAH-E.coli | Hg2+/Cd2+ | 0.5 ppb | 2–2000 ppb | [85] |
| NCF/Au NPs-4 MPY | Hg2+ | 8 nM | 8–100 nM | [86] |
| NCF/Au NPs-MUA | Pb2+ | 800 μM (65 ppm) | 10–100mM | [88] |
| Planar waveguide/ chitosan-Au/Ag NPs | H2S | 5 ppm | 5–300 ppm | [90] |
| 0.1 ppm | 0.1–100 ppm | |||
| U-fiber/Au(Ag) NPs | methanol | ~26.46 (2.12) ppm | 0–228.65 ppm | [91] |
| acetone | ~1.00 (0.43) ppm | 0–202.72 ppm | ||
| ethanol | ~3.53 (3.79) ppm | 0–304.26 ppm | ||
| propanol | ~3.81 (11.09) ppm | 0–148.89 ppm | ||
| Microfiber taper/Pt NPs-GO | NH3 | 10.2 pm/ppm | 0–120 ppm | [92] |
| Microfiber/Pd NPs | H2 | 52 pm/ppm | 0.2–1 × 104 ppm | [93] |
| Ag-Pt NPs-ZnO | Ethanol/acetone | 0.5 ppm | 0–100 ppm | [94] |
| U-fiber/Pt-WO3 nanosheets | H2 | 43.5 ppm | 0–1.6 × 104 ppm | [95] |
| Fiber Structure/Materials | Target | Limit of Detection | Sensitivity | Working Range | Reference |
|---|---|---|---|---|---|
| Flattened fiber/Ag NPs | Glucose | 4.42 mg/dL | -- | 0–500 mg/dL | [96] |
| U-fiber/Au NPs-GOD | Glucose | 0.16 mg/dL | 2.899 nm/% | 0.1–0.5% | [98] |
| PMMA U-fiber/Tyr-AgNPs | Dopamine | 0.16 μM | 0–50 μM | [99] | |
| Microfiber Taper/SiO2-Au NPs | SV | 271 pM | -- | 2.5 nM–1.33 μM | [100] |
| Microfiber taper/Au NPs | Cholesterol | 53.1 nM | 0.125%/mM | 10 nM–1 μM | [101] |
| Microfiber taper/Au NPs | UA | 175.89 μM | 0.0131 nm/μM | 10–800 μM | [102] |
| BSA | 0.3263 gm/dL | ~25 μA/mM | 0.05–0.2 mM | [59] | |
| HCF Tip/Ag NSs-NRs | MB | 10 fM | -- | -- | [104] |
| Melamine | 100 nM | ||||
| LPG/Au NPs-Cys | Glyphosate | 0.02 μM | 3.5 nm/μM | 0.5–100 μM | [105] |
| TFBG/Au NPs-film | Thrombin | 1 nM | 3.21 × 107 dB/M | 1–33.75 nM | [106] |
| PCF/Au NPs-film | Human IgG | 37 ng/mL | ~0.54 pm/(ng/mL) | 1–30 μg/mL | [109] |
| Microfiber taper/Au NPs | SV | 1 pg/mL | 0 pg–1 ng/mL | [110] | |
| HCF/Ag NPs | Cholesterol | 25.5 nM | 16.149 nm/μM | 50 nM–1 μM | [111] |
| MMF/Au-Ag NPs-GO | L-Cysteine | 126.6 μM | 0.0012 nm/μM | 50 μM–1 mM | [112] |
| Microfiber taper/Au NPs | AA | 51.94 μM | 8.3 nm/mM | 10 μM–1 mM | [113] |
| MMF End/Au NPs | PSA | 124 fg/mL | -- | 1 pg–10μg/mL | [114] |
| MMF/SMF End/Au NPs | 0.1 pg/mL | -- | 0.1–100 pg/mL | [115] | |
| LPG/SiO2-Au NPs | IgM | 15 pg/mm2 | 11 nm/(ng/mm2) | 15 μg–1mg/mL | [116] |
| LPG/SiO2-Au NPs | SV | 0.86 pg/mm2 | 3.88/(ng/mm2) | 1.25 nM–2.7 μM | [117] |
| NCF/Ag NPs | TG | 0.016 mM | 28.5 nm/mM | 0–7 nM | [118] |
| NCF/Dios-Ag NPs | Cysteine | 0.0077 μM | 0–100 μM | [119] | |
| MMF/PS-b-P4VP-Au NPs | Human IgG | 0.3nM | 38 pm/(ng/cm2) | 6.7–66.7 nM | [120] |
| NCF/Au NRs | OTA | 12.0 pM | -- | 10 pM–100 nM, | [121] |
| MMF/PVA-Ag NPs-GO | Dopamine | 0.2 μM | -- | 0.2–80 μM | [122] |
| NCF/PDDA-Au NPs | Heparin | 0.0257ng/mL | 1 nm/(ng/mL) | 0.1ng-1μg/mL | [123] |
| MMF-PCF/Ag-Au film-NPs-GO | Human IgG | 15 ng/mL | ~13.6 μm/RIU | 1–40 μg/mL | [124] |
| MMF end/Au NPs | Tg | 0.19 pg/mL | -- | 1 pg–10 ng/mL | [125] |
| Microfiber Taper/GO-Au NPs | UA | 259 μM | 8.9 pm/μM | 10–800 μM | [127] |
| U-MMF/Graphene-Au NPs | DNA | 0.1 nM | 1.25 μm/RIU | 0.1–100nM | [129] |
| Flattened fiber/Au NPs or ZnO NWs-Au NPs | PSA | 2.06 pg/mL | 35 V/RIU | 0.01 pg–1 ng/mL | [130] |
| PSA | 0.51 pg/mL | 60 V/RIU | |||
| NCF/Au NPs | Glucose | 80 nM | ~1.44 nm/nM | 0.01–30 mM | [131] |
| NCF/Ag NPs | ERY | 1.62 nM | 205 nm/μM | 0–100 μM | [132] |
| MMF/Ag NPs | TCA | 10 μM | 0.587 nm/μM | 40–100 μM | [133] |
| NCF/Au NPs-SnO2 | Dopamine | 0.031 μM, | -- | 0–100 μM | [135] |
| NCF/Ag NPs-GO | Cholesterol | 1.131 mM | 5.068 nm/mM | 0–10 mM | [137] |
| NCF/PBA-Au NPs | MicroRNA | 0.27 pM | -- | 10 pM–10 μM | [138] |
| U-Microfiber/GO-CuS NPs | MicroRNA | 0.0156 aM | 0.62 nm/lgM | 0.1 aM–10 pM | [139] |
| MMF/Au NPs | Taurine | 53 μM | 0.0190 AU/mM | 0–1 mM | [140] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, H.; Li, Z.; Su, Z.; Zhu, Y. Preparation and Application of Metal Nanoparticals Elaborated Fiber Sensors. Sensors 2020, 20, 5155. https://doi.org/10.3390/s20185155
Li J, Wang H, Li Z, Su Z, Zhu Y. Preparation and Application of Metal Nanoparticals Elaborated Fiber Sensors. Sensors. 2020; 20(18):5155. https://doi.org/10.3390/s20185155
Chicago/Turabian StyleLi, Jin, Haoru Wang, Zhi Li, Zhengcheng Su, and Yue Zhu. 2020. "Preparation and Application of Metal Nanoparticals Elaborated Fiber Sensors" Sensors 20, no. 18: 5155. https://doi.org/10.3390/s20185155
APA StyleLi, J., Wang, H., Li, Z., Su, Z., & Zhu, Y. (2020). Preparation and Application of Metal Nanoparticals Elaborated Fiber Sensors. Sensors, 20(18), 5155. https://doi.org/10.3390/s20185155






