A NDIR Mid-Infrared Methane Sensor with a Compact Pentahedron Gas-Cell
Abstract
:1. Introduction
2. Pentahedron Gas-Cell Structure
2.1. Paraboloid Concentrator
2.2. Pentahedron Structure
3. NDIR Sensor Configuration
3.1. Sensor Architecture
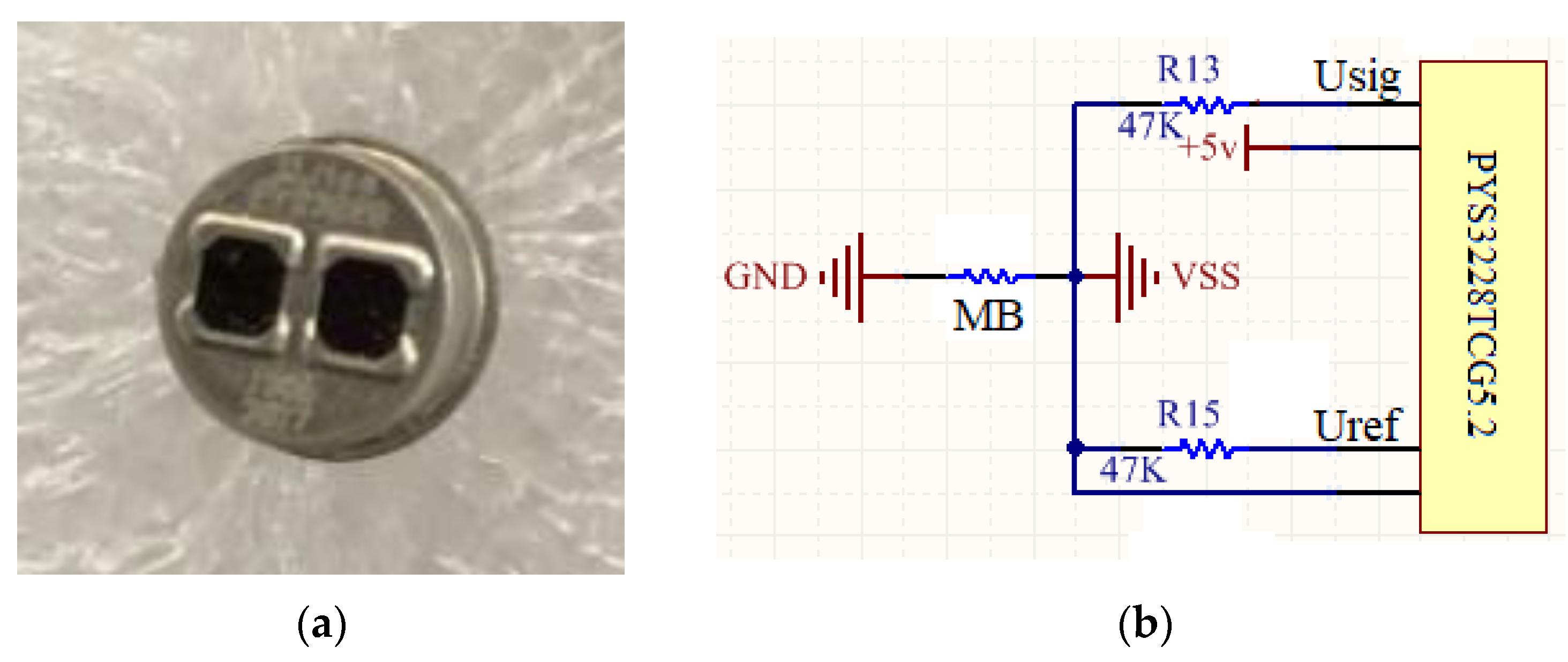
3.2. Hardware Design
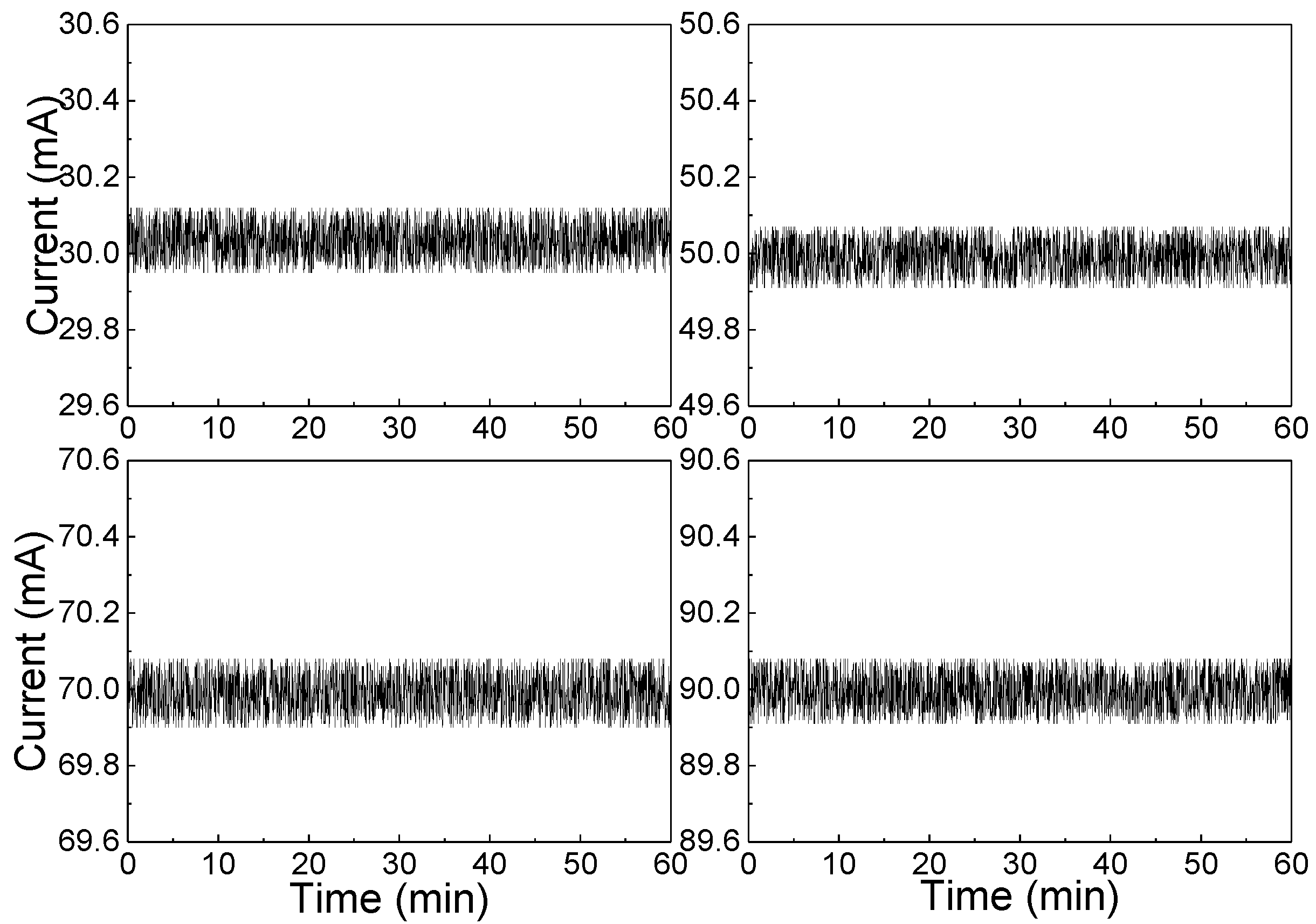
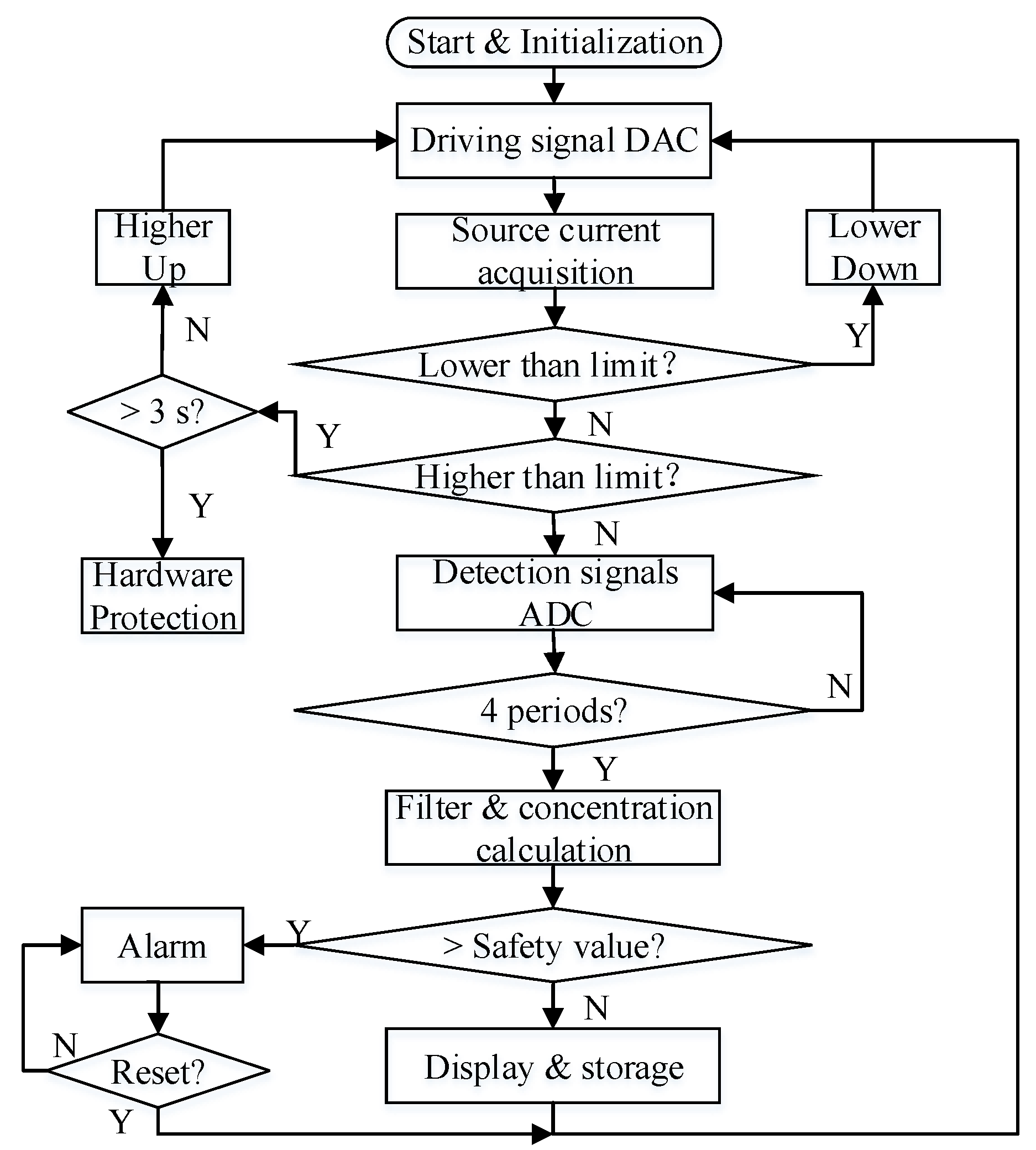
3.3. Signal Acquisition and Processing
4. Experimental Tests and Results
4.1. Expeirmental Setup
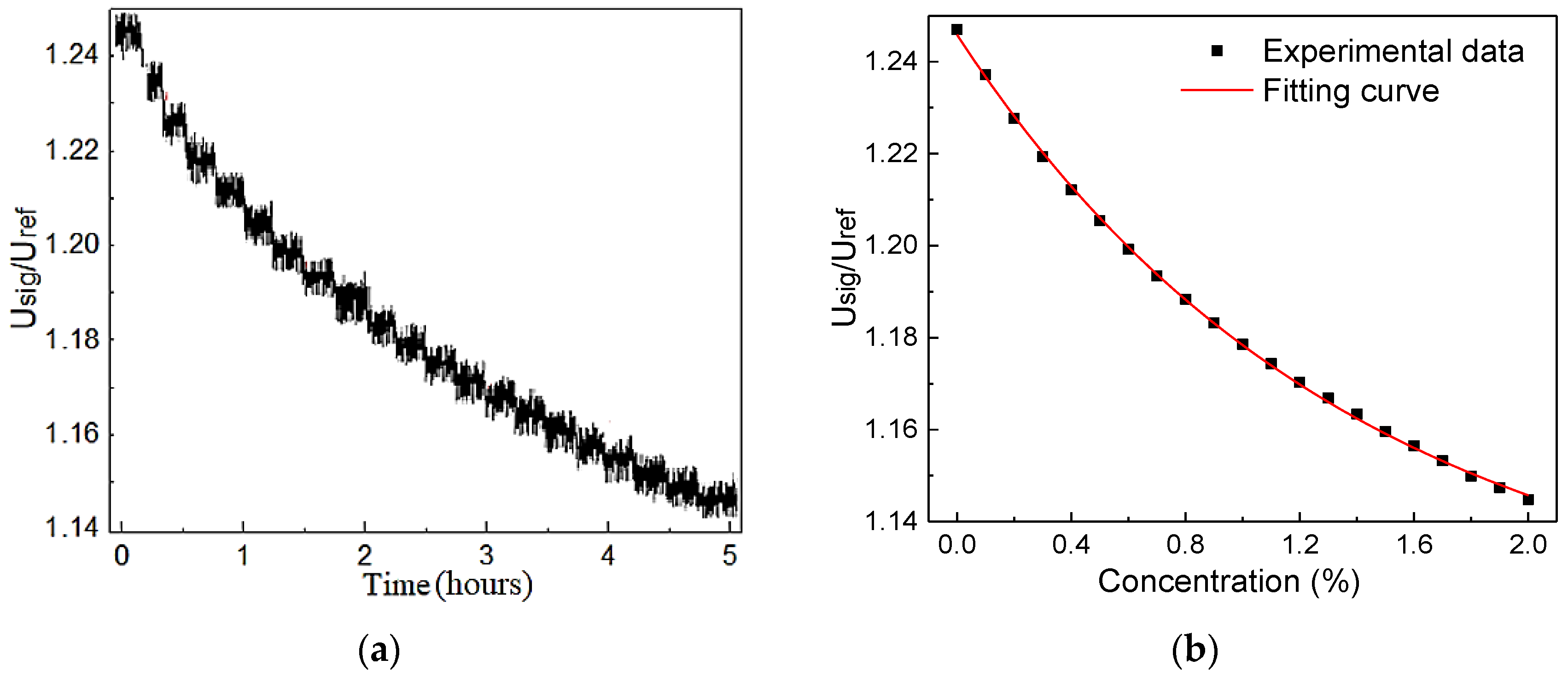
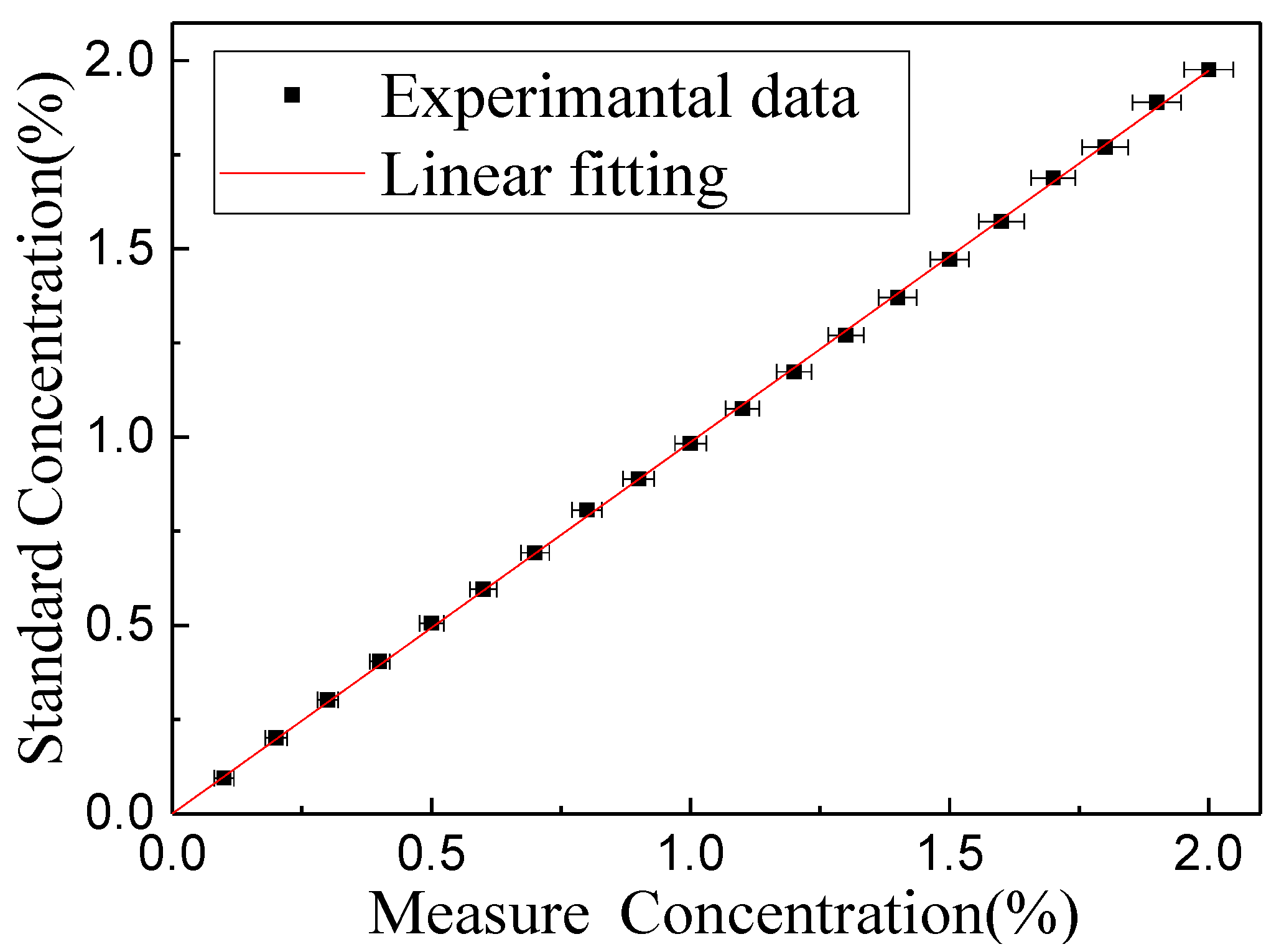
4.2. Sensor Evaluation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ye, W.; Zhou, B.; Tu, Z.; Xiao, X.; Yan, J.; Wu, T.; Wu, F.; Zheng, C.; Tittel, K.F. Leakage source location based on Gaussian plume diffusion model using a near-infrared sensor. Infrared Phys. Technol. 2020, 109, 103411. [Google Scholar] [CrossRef]
- Brandt, A.R.; Heath, G.A.; Cooley, D. Methane leaks from natural gas systems follow extreme distributions. Environ. Sci. Technol. 2016, 50, 12512–12520. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, I.; Stieger, J.; Buchmann, N.; Eugster, W. Spatial variability of methane: Attributing atmospheric concentrations to emissions. Environ. Pollut. 2014, 190, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, Y.; Lin, H.; Kan, R.; Patimisco, P.; Sampaolo, A.; Giglio, M.; Zhu, W.; Yu, J.; Tittle, K.F. Sub-ppb-level CH4 detection by exploiting a low-noise differential photoacoustic resonator with a room-temperature interband cascade laser. Opt. Express 2020, 28, 19446. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, P.; Noori, A.; Parry, J.S.; Davis, J.; Lucieer, A.; Macka, M. Continuous and real-time indoor and outdoor methane sensing with portable optical sensor using rapidly pulsed IR LEDs. Talanta 2020, 218, 121144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ye, W.; Sanchez, P.N.; Li, C.; Dong, L.; Wang, Y.; Griffin, J.R.; Tittel, K.F. Development and field deployment of a mid-infrared methane sensor without pressure control using interband cascade laser absorption spectroscopy. Sens. Actuators B Chem. 2017, 244, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Zheng, C.; Sanchez, P.N.; Girija, V.A.; He, Q.; Zheng, H.; Griffin, J.R.; Tittel, K.F. Thermal effects of an ICL-based mid-infrared CH4 sensor within a wide atmospheric temperature range. Infrared Phys. Technol. 2018, 89, 299–303. [Google Scholar] [CrossRef]
- Avetisov, V.; Bjoroey, O.; Wang, J.; Geiser, P.; Paulsen, K. Hydrogen Sensor Based on Tunable Diode Laser Absorption Spectroscopy. Sensors 2019, 19, 5313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, T.; Wu, H.; Dong, L.; Tittel, K.F. Acoustic Detection Module Design of a Quartz-Enhanced Photoacoustic Sensor. Sensors 2019, 19, 1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasz, S.; Piotr, A. A High Sensitivity Preamplifier for Quartz Tuning Forks in QEPAS (Quartz Enhanced PhotoAcoustic Spectroscopy) Applications. Sensors 2017, 17, 2528. [Google Scholar]
- Christensen, J.B.; Hogstedt, L.; Friis, S.M.M.; Lai, J.; Chou, M.; Balslev, H.D.; Petersen, J.C.; Lassen, M. Intrinsic Spectral Resolution Limitations of QEPAS Sensors for Fast and Broad Wavelength Tuning. Sensors 2020, 20, 4725. [Google Scholar] [CrossRef] [PubMed]
- Norbert, L.; Uwe, M.; Henrik, Z.; Glitsch, S.; Wiese, M.; Ropcke, J.; van Helden, J. RES-Q-Trace: A Mobile CEAS-Based Demonstrator for Multi-Component Trace Gas Detection in the MIR. Sensors 2018, 18, 2058. [Google Scholar]
- He, Q.; Feng, Q.; Li, J. Long-Term Stable Online Acetylene Detection by a CEAS System with Suppression of Cavity Length Drift. Sensors 2019, 19, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alquaity, A.B.S.; Utsav, K.C.; Popov, A.; Farooq, A. Detection of shock-heated hydrogen peroxide (H2O2) by off-axis cavity-enhanced absorption spectroscopy (OA-CEAS). Appl. Phys. B 2017, 123, 280. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; He, Z.; Li, X.; Xu, J.; Liu, B.; Jiang, Y. Enhanced ammonia response of Ti3C2Tx nanosheets supported by TiO2 nanoparticles at room temperature. Sens. Actuators B Chem. 2019, 298, 126874. [Google Scholar] [CrossRef]
- Chen, T.; Chang, S.; Hung, F.; Chang, S.; Hu, Z.; Chen, K. Simple Fabrication Process for 2D ZnO Nanowalls and Their Potential Application as a Methane Sensor. Sensors 2013, 13, 3941–3950. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; Wang, Y.; Wang, S.; Jiang, Y. Paper-Based Sensors for Gas, Humidity, and Strain Detections: A Review. ACS Appl. Mater. Interfaces 2020, 12, 31037–31053. [Google Scholar] [CrossRef]
- PerkinElmer Optoelectronics. IRL715, Product Data Infrared Sources. pp. 1–8. Available online: http://www.hofoo.com.cn/uploadfiles/irl715.pdf (accessed on 15 December 2000).
- Zhao, Z.; Liu, N.; Zhang, J.; Wang, Z.; Li, X.; Tian, E. Design of Non-Dispersed Infrared (NDIR) Methane Gas Sensor. Spectrosc. Spectr. Anal. 2011, 31, 570–573. [Google Scholar]
- Liu, H.; Shi, Y.; Wang, T. Design of a six-gas NDIR gas sensor using an integrated optical gas chamber. Opt. Express 2020, 28, 11451. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Smith, R.; Ho, W.O.; Saffell, R.J.; Tatam, P.R. Non-dispersive infra-red (NDIR) measurement of carbon dioxide at 4.2 μm in a compact and optically efficient sensor. Sens. Actuators B Chem. 2013, 186, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Vincent, T.A.; Gardner, J.W. A low cost MEMS based NDIR system for the monitoring of carbon dioxide in breath analysis at ppm levels. Sens. Actuators B Chem. 2016, 236, 954–964. [Google Scholar] [CrossRef] [Green Version]
- ISO 6144:2003, Gas Analysis—Preparation of Calibration Gas Mixtures—Static Volumetric Method; ISO: Geneva, Switzerland; Available online: https://www.iso.org/standard/24666.html (accessed on 1 February 2003).
- Mayerhofer, T.G.; Mutschke, H.; Popp, J. Employing Theories Far beyond Their Limits—The Case of the (Boguer-) Beer–Lambert Law. ChemPhysChem 2016, 17, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Allan, D.W. Statistics of Atomic Frequency Standards. Proc. IEEE 1966, 54, 221–230. [Google Scholar] [CrossRef] [Green Version]
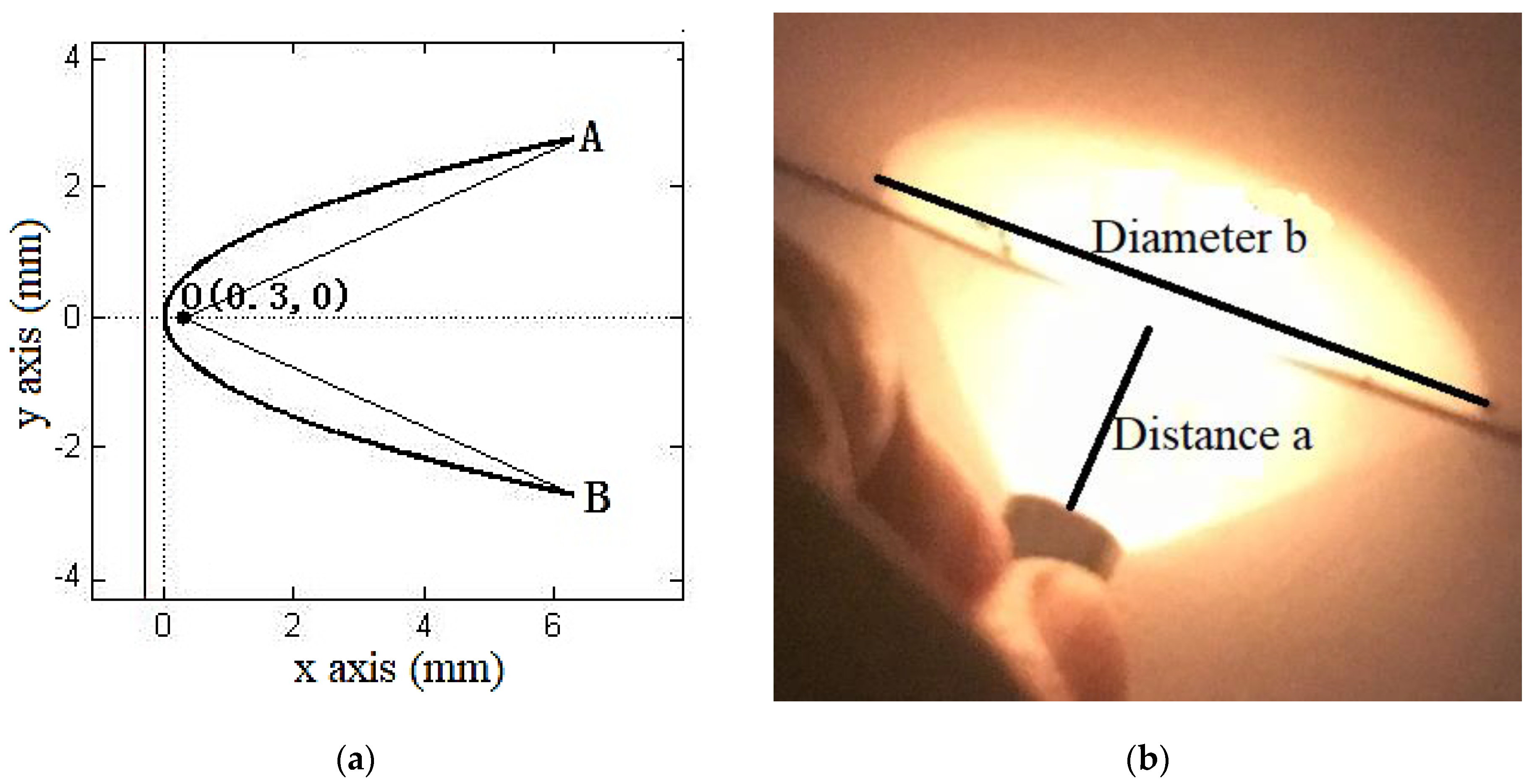

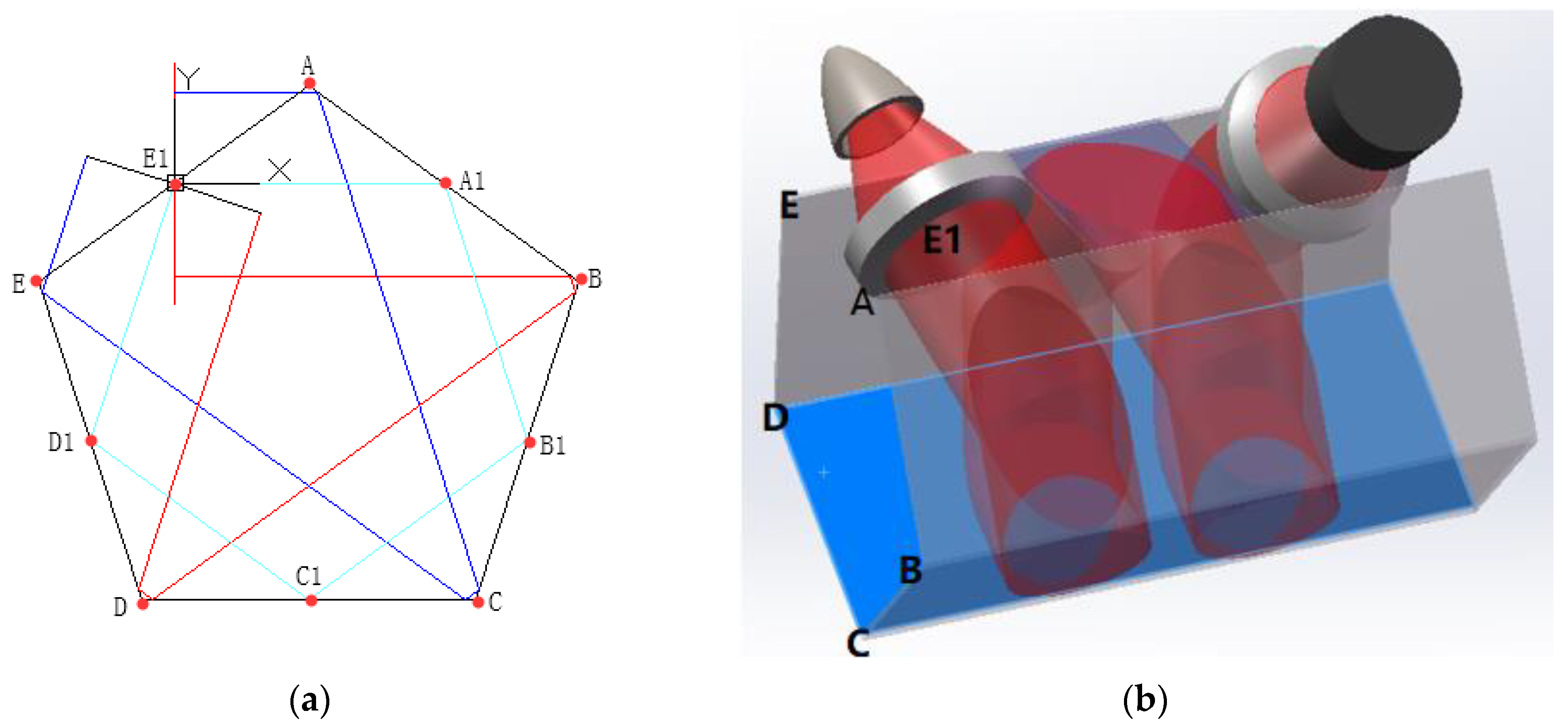
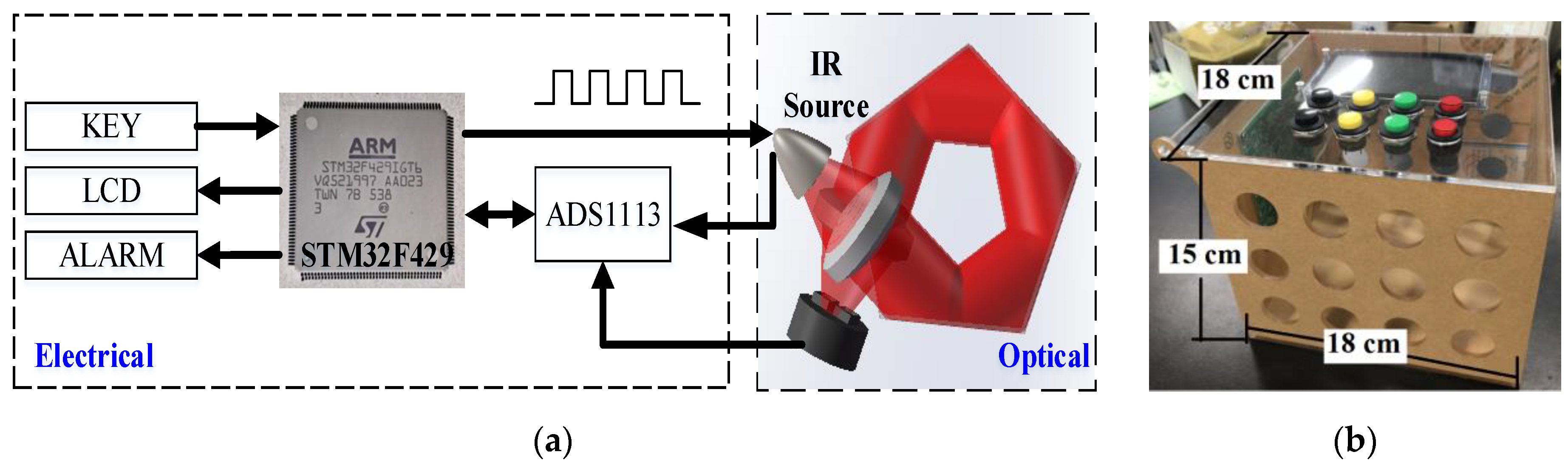


| Distance a (mm) | Diameter b (mm) |
|---|---|
| 5 | 21.5 |
| 10 | 31 |
| 20 | 49.5 |
| 30 | 68.5 |
| 40 | 87.1 |
| 60 | 12.65 |
| C (%) | V (mL) | C (%) | V (mL) | C (%) | V (mL) | C (%) | V (mL) | C (%) | V (mL) |
|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 48.1 | 0.2 | 96.2 | 0.3 | 144.4 | 0.4 | 192.8 | 0.5 | 241.7 |
| 0.6 | 289.7 | 0.7 | 339.7 | 0.8 | 387.1 | 0.9 | 435.9 | 1.0 | 484.8 |
| 1.1 | 533.9 | 1.2 | 583.0 | 1.3 | 632.2 | 1.4 | 681.5 | 1.5 | 731.0 |
| 1.6 | 780.5 | 1.7 | 830.1 | 1.8 | 880.0 | 1.9 | 929.7 | 2.0 | 979.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, W.; Tu, Z.; Xiao, X.; Simeone, A.; Yan, J.; Wu, T.; Wu, F.; Zheng, C.; Tittel, F.K. A NDIR Mid-Infrared Methane Sensor with a Compact Pentahedron Gas-Cell. Sensors 2020, 20, 5461. https://doi.org/10.3390/s20195461
Ye W, Tu Z, Xiao X, Simeone A, Yan J, Wu T, Wu F, Zheng C, Tittel FK. A NDIR Mid-Infrared Methane Sensor with a Compact Pentahedron Gas-Cell. Sensors. 2020; 20(19):5461. https://doi.org/10.3390/s20195461
Chicago/Turabian StyleYe, Weilin, Zihan Tu, Xupeng Xiao, Alessandro Simeone, Jingwen Yan, Tao Wu, Fupei Wu, Chuantao Zheng, and Frank K. Tittel. 2020. "A NDIR Mid-Infrared Methane Sensor with a Compact Pentahedron Gas-Cell" Sensors 20, no. 19: 5461. https://doi.org/10.3390/s20195461







