Microfluidic Production of Autofluorescent BSA Hydrogel Microspheres and Their Sequential Trapping for Fluorescence-Based On-Chip Permanganate Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microfluidic Device Fabrication
2.3. Synthesis of Autofluorescent BSA Hydrogel Microspheres
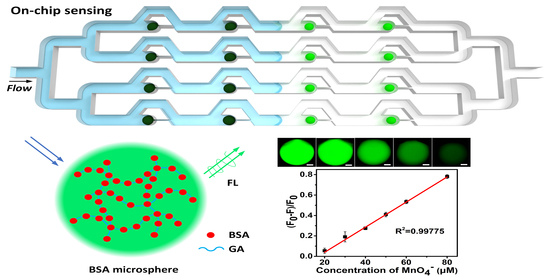
2.4. Immobilization of BSA Hydrogel Microspheres into Microchannels
2.5. Fluorescence-Based Anion Sensing
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Heiland, J.J.; Geissler, D.; Piendl, S.K.; Warias, R.; Belder, D. Supercritical-Fluid Chromatography On-Chip with Two-Photon-Excited-Fluorescence Detection for High-Speed Chiral Separations. Anal. Chem. 2019, 91, 6134–6140. [Google Scholar] [CrossRef] [PubMed]
- Tjong, V.; Yu, H.; Hucknall, A.; Rangarajan, S.; Chilkoti, A. Amplified On-Chip Fluorescence Detection of DNA Hybridization by Surface-Initiated Enzymatic Polymerization. Anal. Chem. 2011, 83, 5153–5159. [Google Scholar] [CrossRef]
- Arpali, S.A.; Arpali, C.; Coskun, A.F.; Chiang, H.H.; Ozcan, A. High-throughput screening of large volumes of whole blood using structured illumination and fluorescent on-chip imaging. Lab Chip 2012, 12, 4968–4971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janasek, D.; Franzke, J.; Manz, A. Scaling and the design of miniaturized chemical-analysis systems. Nature 2006, 442, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Hitzbleck, M.; Delamarche, E. Reagents in microfluidics: An ’in’ and ’out’ challenge. Chem. Soc. Rev. 2013, 42, 8494–8516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.L.; Hong, Z.Y.; Tang, S.Y.; Li, W.H.; Inglis, D.W.; Hosokawa, Y.; Yalikun, Y.; Li, M. Focusing of sub-micrometer particles in microfluidic devices. Lab Chip 2020, 20, 35–53. [Google Scholar] [CrossRef]
- Armbrecht, L.; Muller, R.; Nikoloff, J.; Dittrich, P. Single-cell protein profiling in microchambers with barcoded beads. Microsyst. Nanoeng. 2019, 5, 55. [Google Scholar] [CrossRef]
- Xuan, X.C. Recent Advances in Continuous-Flow Particle Manipulations Using Magnetic Fluids. Micromachines 2019, 10, 744. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yang, C.; Li, C.M. On-demand microfluidic droplet trapping and fusion for on-chip static droplet assays. Lab Chip 2009, 9, 1504–1506. [Google Scholar] [CrossRef]
- Xuan, X.C. Recent advances in direct current electrokinetic manipulation of particles for microfluidic applications. Electrophoresis 2019, 40, 2484–2513. [Google Scholar] [CrossRef]
- Tan, W.H.; Takeuchi, S. A trap-and-release integrated microfluidic system for dynamic microarray applications. Proc. Natl. Acad. Sci. USA 2007, 104, 1146–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wlodkowic, D.; Faley, S.; Zagnoni, M.; Wikswo, J.P.; Cooper, J.M. Microfluidic Single-Cell Array Cytometry for the Analysis of Tumor Apoptosis. Anal. Chem. 2009, 81, 5517–5523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, L.; Seshia, A.; Lando, D.; Laue, E.; Palayret, M.; Lee, S.F.; Klenerman, D. A microfluidic device for the hydrodynamic immobilisation of living fission yeast cells for super-resolution imaging. Sens. Actuators B 2014, 192, 36–41. [Google Scholar] [CrossRef] [Green Version]
- DiCarlo, D.; Wu, L.Y.; Lee, L.P. Dynamic single cell culture array. Lab Chip 2006, 6, 1445–1449. [Google Scholar]
- Kumano, I.; Hosoda, K.; Suzuki, H.; Hirata, K.; Yomo, T. Hydrodynamic trapping of Tetrahymena thermophila for the long-term monitoring of cell behaviors. Lab Chip 2012, 12, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.D.; Luo, K.; Chang, W.H.; Lee, G.B. A microfluidic chip capable of generating and trapping emulsion droplets for digital loop-mediated isothermal amplification analysis. Lab Chip 2018, 18, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.A.; Srijanto, B.; Collier, C.P.; Retterer, S.T.; Sarles, S.A. Hydrodynamic trapping for rapid assembly and in situ electrical characterization of droplet interface bilayer arrays. Lab Chip 2016, 16, 3576–3588. [Google Scholar] [CrossRef]
- Courtney, M.; Chen, X.; Chan, S.; Mohamed, T.; Rao, P.P.; Ren, C.L. Droplet Microfluidic System with On-Demand Trapping and Releasing of Droplet for Drug Screening Applications. Anal. Chem. 2017, 89, 910–915. [Google Scholar] [CrossRef]
- Kim, H.; Choi, I.H.; Lee, S.; Won, D.J.; Oh, Y.S.; Kwon, D.; Sung, H.J.; Jeon, S.; Kim, J. Deterministic bead-in-droplet ejection utilizing an integrated plug-in bead dispenser for single bead-based applications. Sci. Rep. 2017, 7, 46260. [Google Scholar] [CrossRef]
- Yu, L.F.; Chen, M.C.W.; Cheung, K.C. Droplet-based microfluidic system for multicellular tumor spheroid formation and anticancer drug testing. Lab Chip 2010, 10, 2424–2432. [Google Scholar] [CrossRef]
- Amselem, G.; Guermonprez, C.; Drogue, B.; Michelin, S.; Baroud, C.N. Universal microfluidic platform for bioassays in anchored droplets. Lab Chip 2016, 16, 4200–4211. [Google Scholar] [CrossRef] [PubMed]
- Sabhachandani, P.; Motwani, V.; Cohen, N.; Sarkar, S.; Torchilinab, V.; Konry, T. Generation and functional assessment of 3D multicellular spheroids in droplet based microfluidics platform. Lab Chip 2016, 16, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Kuster, S.K.; Pabst, M.; Jefimovs, K.; Zenobi, R.; Dittrich, P.S. High-Resolution Droplet-Based Fractionation of Nano-LC Separations onto Microarrays for MALDI-MS Analysis. Anal. Chem. 2014, 86, 4848–4855. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.H.; Xu, J.; Zhang, A.; Lee, H.; et al. Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 2018, 47, 5646–5683. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Wang, K.; Fan, K.; Feng, Z.L.; Zhang, Y.X.; Zhao, Q.B.; Yun, G.L.; Yuan, D.; Jiang, L.M.; Li, M.; et al. High-Throughput, Off-Chip Microdroplet Generator Enabled by a Spinning Conical Frustum. Anal. Chem. 2019, 91, 3725–3732. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Min, N.G.; Ku, M.; Yang, J.; Kim, S.H. Microfluidic Production of Uniform Microcarriers with Multicompartments through Phase Separation in Emulsion Drops. Chem. Mater. 2016, 28, 1430–1438. [Google Scholar] [CrossRef]
- Abalde-Cela, S.; Taladriz-Blanco, P.; de Oliveira, M.G.; Abell, C. Droplet microfluidics for the highly controlled synthesis of branched gold nanoparticles. Sci. Rep. 2018, 8, 2440. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Wang, H.; Sun, L.; Liu, Y.; Zhao, Y. Quantum-dot-encapsulated core–shell barcode particles from droplet microfluidics. J. Mater. Chem. B 2018, 6, 7257–7262. [Google Scholar] [CrossRef]
- Cai, Q.W.; Ju, X.J.; Zhang, S.Y.; Chen, Z.H.; Hu, J.Q.; Zhang, L.P.; Xie, R.; Wang, W.; Liu, Z.; Chu, L.Y. Controllable Fabrication of Functional Microhelices with Droplet Microfluidics. ACS Appl. Mater. Interfaces 2019, 11, 46241–46250. [Google Scholar] [CrossRef]
- Campbell, Z.S.; Parker, M.; Bennett, J.A.; Yusuf, S.; Al-Rashdi, A.K.; Lustik, J.; Li, F.; Abolhasani, M. Continuous Synthesis of Monodisperse Yolk–Shell Titania Microspheres. Chem. Mater. 2018, 30, 8948–8958. [Google Scholar] [CrossRef]
- Kanai, T.; Nakai, H.; Yamada, A.; Fukuyama, M.; Weitz, D.A. Preparation of monodisperse hybrid gel particles with various morphologies via flow rate and temperature control. Soft Matter 2019, 15, 6934–6937. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Mansson, L.K.; Holm, S.H.; Ghosh, S.; Carlstrom, G.; Crassous, J.J.; Schurtenberger, P.; Tegenfeldt, J.O. A Droplet-Based Microfluidics Route to Temperature-Responsive Colloidal Molecules. J. Phys. Chem. B 2019, 123, 9260–9271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Liu, J.D.; Liu, C.; Wu, X.; Li, Q.; Chen, S.; Zhao, X.; Weitz, D.A. Microfluidics-Assisted Assembly of Injectable Photonic Hydrogels toward Reflective Cooling. Small 2019, 16, e1903939. [Google Scholar] [CrossRef]
- Sabhachandania, P.; Sarkara, S.; Mckenneya, S.; Ravib, D.; Evensb, A.M.; Konry, T. Microfluidic assembly of hydrogel-based immunogenic tumor spheroids for evaluation of anticancer therapies and biomarker release. J. Control. Release 2019, 295, 21–30. [Google Scholar] [CrossRef]
- Mugherli, L.; Lety-Stefanska, A.; Landreau, N.; Tomasib, R.F.-X.; Baroud, C.N. Quantifying the sol–gel process and detecting toxic gas in an array of anchored microfluidic droplets. Lab Chip 2020, 20, 236–243. [Google Scholar] [CrossRef]
- Park, H.I.; Park, S.Y. Smart Fluorescent Hydrogel Glucose Biosensing Microdroplets with Dual-Mode Fluorescence Quenching and Size Reduction. ACS Appl. Mater. Interfaces 2018, 10, 30172–30179. [Google Scholar] [CrossRef]
- Ji, J.J.; Lu, W.B.; Zhu, Y.; Jin, H.; Yao, Y.Y.; Zhang, H.D.; Zhao, Y.J. Porous Hydrogel-Encapsulated Photonic Barcodes for Multiplex Detection of Cardiovascular Biomarkers. ACS Sens. 2019, 4, 1384–1390. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, D.J.; Lee, S.; Kim, D.H.; Park, S.G.; Kim, S.H. Microfluidic Designing Microgels Containing Highly Concentrated Gold Nanoparticles for SERS Analysis of Complex Fluids. Small 2019, 15, 1905076. [Google Scholar] [CrossRef]
- Deshapriya, I.K.; Stromer, B.S.; Pattammattel, A.; Kim, C.S.; Iglesias-Bartolome, R.; Gonzalez-Fajardo, L.; Patel, V.; Gutkind, J.S.; Lu, X.; Kumar, C.V. Fluorescent, bioactive protein nanoparticles (prodots) for rapid, improved cellular uptake. Bioconjug. Chem. 2015, 26, 396–404. [Google Scholar] [CrossRef]
- He, H.; Yang, C.; Wang, F.; Wei, Z.; Shen, J.; Chen, D.; Fan, C.; Zhang, H.; Liu, K. Mechanically Strong Globular-Protein-Based Fibers Obtained Using a Microfluidic Spinning Technique. Angew. Chem. Int. Ed. 2020, 59, 1–6. [Google Scholar]
- Qin, W.; Ding, D.; Liu, J.; Yuan, W.Z.; Hu, Y.; Liu, B.; Tang, B.Z. Biocompatible Nanoparticles with Aggregation-Induced Emission Characteristics as Far-Red/Near-Infrared Fluorescent Bioprobes for In Vitro and In Vivo Imaging Applications. Adv. Funct. Mater. 2012, 22, 771–779. [Google Scholar] [CrossRef]
- Ma, X.; Wang, T.; Song, D.; Hargrove, D.; Dong, Q.; Luo, Z.; Chen, J.; Lu, X.; Luo, Y.; Fan, T.-H.; et al. Protein Microspheres with Unique Green and Red Fluorescence for Noninvasively Tracking and Modeling Their in Vivo Biodegradation. ACS Biomater. Sci. Eng. 2016, 2, 954–962. [Google Scholar] [CrossRef]
- Ma, X.; Li, J.Q.; O’Connell, C.; Fan, T.H.; Lei, Y. Integrated Experimental and Modeling Study of Enzymatic Degradation Using Novel Fluorescent BSA Microspheres. Langmuir 2018, 34, 191–197. [Google Scholar] [CrossRef]
- Ye, Z.J.; Weng, R.; Ma, Y.H.; Wang, F.Y.; Liu, H.; Wei, L.; Xiao, L.H. Label-Free, Single-Particle, Colorimetric Detection of Permanganate by GNPs@Ag Core–Shell Nanoparticles with Dark-Field Optical Microscopy. Anal. Chem. 2018, 90, 13044–13050. [Google Scholar] [CrossRef]
- Zhu, K.; Fan, R.; Zheng, X.; Wang, P.; Chen, W.; Sun, T.; Gai, S.; Zhou, X.; Yang, Y. Dual-emitting dye-CDs@MOFs for selective and sensitive identification of antibiotics and MnO4− in water. J. Mater. Chem. C 2019, 7, 15057–15065. [Google Scholar] [CrossRef]
- Shi, G.; Shahid, M.A.; Yousuf, M.; Mahmood, F.; Rasheed, L.; Bielawski, C.W.; Kim, K.S. A “turn-on” fluorescent probe for the detection of permanganate in aqueous media. Chem. Commun. 2019, 55, 1470–1473. [Google Scholar] [CrossRef]
- Liu, H.; Rong, J.; Shen, G.; Song, Y.; Gu, W.; Liu, X. A fluorescent probe for sequential sensing of MnO4− and Cr2O72− ions in aqueous medium based on a UCNS/TMB nanosystem. Dalton Trans. 2019, 48, 4168–4175. [Google Scholar] [CrossRef]
- Zhu, Z.; Xue, J.; Wen, B.; Ji, W.; Du, B.; Nie, J. Ultrasensitive and selective detection of MnO4− in aqueous solution with fluorescent microgels. Sens. Actuators B 2019, 291, 441–450. [Google Scholar] [CrossRef]
- Cha, C.E.Y.; Oh, J.; Kim, K.; Qiu, Y.L.; Joh, M.; Shin, S.R.; Wang, X.; Camci-Unal, G.; Wan, K.T.; Liao, R.L.; et al. Microfluidics-Assisted Fabrication of Gelatin-Silica Core-Shell Microgels for Injectable Tissue Constructs. Biomacromolecules 2014, 15, 283–290. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Li, G.; Xiang, N.; Huang, X.; Shiba, K. Microfluidic Production of Autofluorescent BSA Hydrogel Microspheres and Their Sequential Trapping for Fluorescence-Based On-Chip Permanganate Sensing. Sensors 2020, 20, 5886. https://doi.org/10.3390/s20205886
Liu L, Li G, Xiang N, Huang X, Shiba K. Microfluidic Production of Autofluorescent BSA Hydrogel Microspheres and Their Sequential Trapping for Fluorescence-Based On-Chip Permanganate Sensing. Sensors. 2020; 20(20):5886. https://doi.org/10.3390/s20205886
Chicago/Turabian StyleLiu, Linbo, Guangming Li, Nan Xiang, Xing Huang, and Kota Shiba. 2020. "Microfluidic Production of Autofluorescent BSA Hydrogel Microspheres and Their Sequential Trapping for Fluorescence-Based On-Chip Permanganate Sensing" Sensors 20, no. 20: 5886. https://doi.org/10.3390/s20205886