Gyrocardiography: A Review of the Definition, History, Waveform Description, and Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
3. Results
3.1. History
3.1.1. Before 2015
3.1.2. 2015–2016
3.1.3. 2017
3.1.4. 2018
3.1.5. 2019
3.1.6. 2020
3.2. Number of Works on Gyrocardiography
3.3. The Definition and Signal Characteristics
3.3.1. Signal Registration
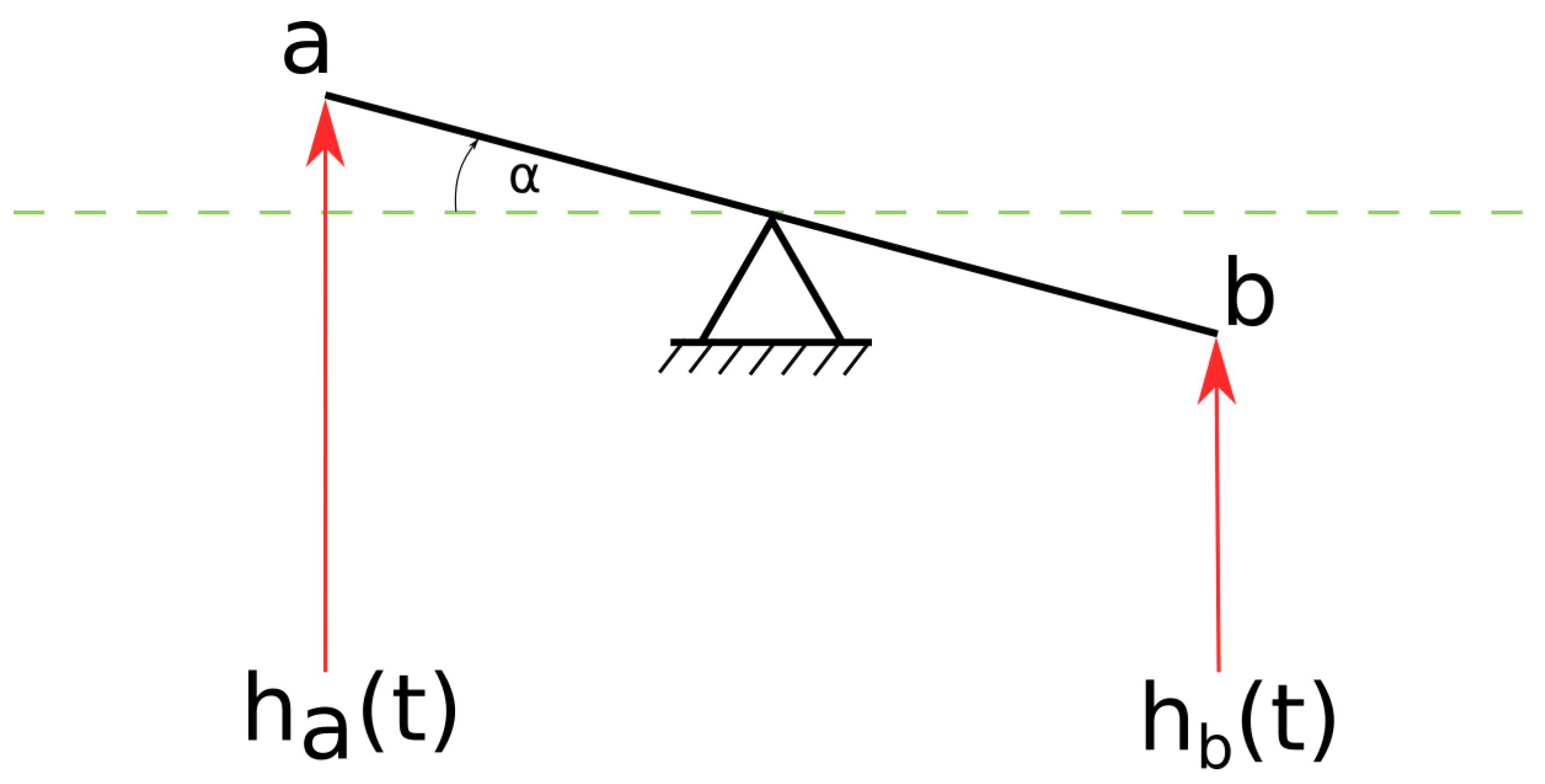
3.3.2. The physics of Gyrocardiography
3.3.3. Physiological Sources of Gyrocardiography
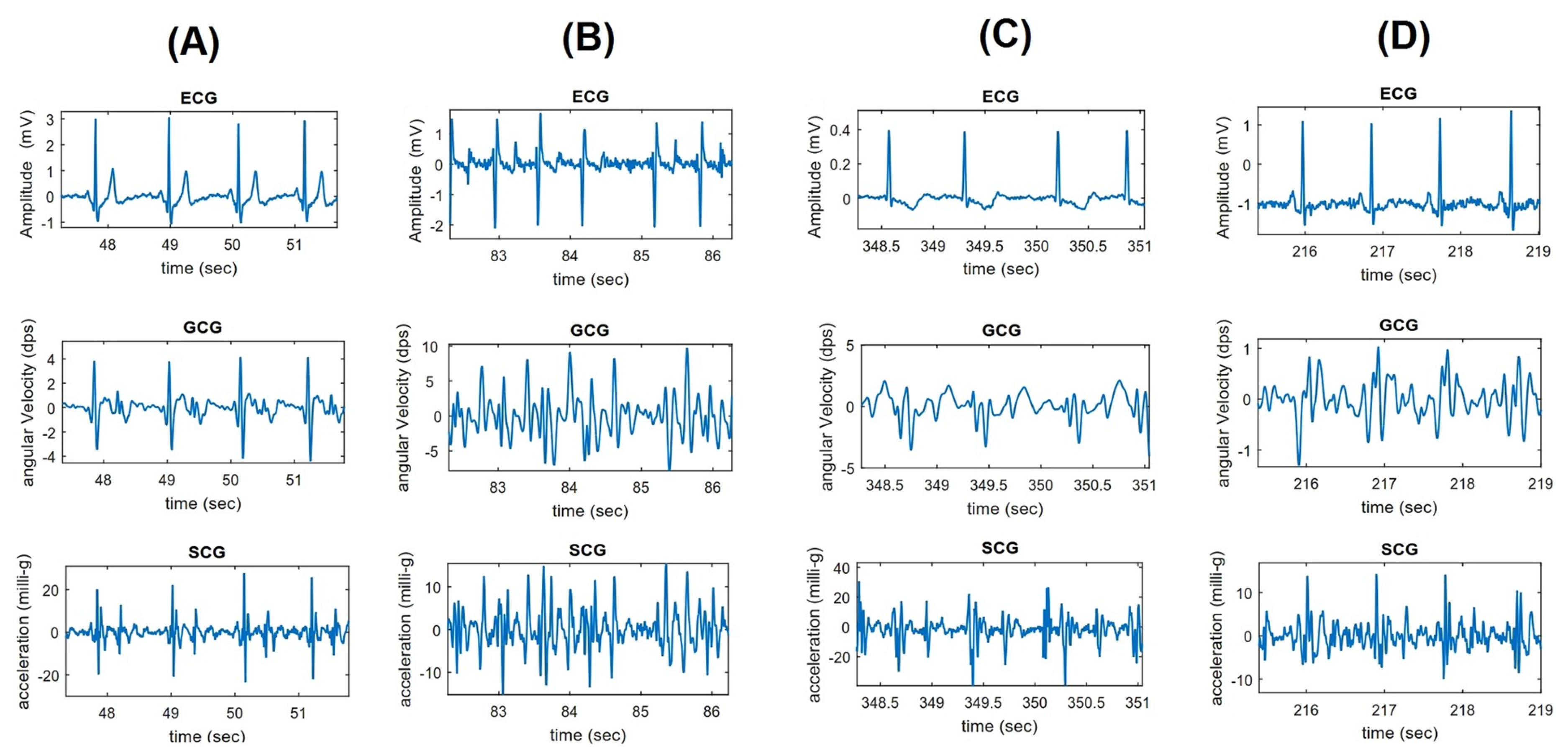
3.4. Waveform Description
3.4.1. The Periods in Gyrocardiography
3.4.2. Signal Morphology in Cardiac Diseases
3.5. Applications
- fetal heart rate extraction [72],
- the analysis of hemodynamics [66], including:
- annotation of seismocardiograms [39],
- annotation of heart sounds [80],
- diagnosing of various cardiovascular diseases, including:
- sleep monitoring [114],
- identification of heart sounds [80],
- estimation of lung volume [73],
- cardiac monitoring of dogs [47],
- cardiac monitoring in workplace [74].
3.5.1. Heart Beat Detection
3.5.2. HRV Analysis
4. Discussion
- Small in size, accurate and readily available sensors [32],
- Only one sensor is required to perform the registration [32],
- The signal is not affected by gravity [32],
- Signal registration is insensitive to the location of sensor relative to the heart [32],
- The possibility of:
- Better performance in PEP estimation than in SCG [112].
- Lack of widely accepted standard of waveform description,
- Lower temporal accuracy of GCG peaks than in SCG [39].
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| °/s | degrees per second |
| 1D | one-dimensional |
| 3D | three-dimensional |
| AC | Aortic valve closure |
| ADA | Autocorrelated Differential Algorithm |
| AFib | Atrial fibrillation |
| ANN | Artificial Neural Network |
| AO | Aortic valve opening |
| AVNN | mean inter-beat interval |
| BCG | ballistocardiography |
| BMI | Body Mass Index |
| BPM | Beats per minute |
| CAD | Coronary artery disease |
| CNN | Convolutional neural network |
| CWT | Continuous Wavelet Transform |
| CT | Computed Tomography |
| dps | degrees per second |
| DPV | Diastolic peak velocity |
| Ea | Early diastolic velocity |
| ECG | Electrocardiography |
| EMD | Empirical Mode Decomposition |
| FHR | Fetal Heart Rate |
| GCG | Gyrocardiography |
| HABIT | Hilbert adaptive beat identification technique |
| HF | the power of the HRV spectrum in the high frequency range |
| HR | Heart rate |
| HRV | Heart rate variability |
| ICA | Independent Component Analysis |
| IMU | Inertial Measurement Unit |
| IVCT | Isovolumetric contraction time |
| IVRT | Isovolumetric relaxation time |
| KSVM | Kernel Support Vector Machine |
| LBP | Local Binary Patterns |
| LOOCV | Leave-one-out Cross-Validation |
| LF | the power of the HRV spectrum in the low frequency range |
| LF/HF | LF/HF power ratio |
| LR | Logistic regression |
| LV | Left ventricular |
| LVET | Left Ventricular Ejection Time |
| MC | Mitral valve closures |
| MCG | Mechanocardiography |
| MEMS | Microelectromechanical systems |
| MO | Mitral valve opening |
| MRI | Magnetic Resonance Imaging |
| NN50 | number of successive RR intervals differing more than 50 ms |
| PCG | Phonocardiogram |
| PEP | Pre-ejection Period |
| PET | Positron Emission Tomography |
| pNN50 | probability of NN50 against total number of inter-beat intervals |
| PWD | Pulse Wave Doppler |
| QS2 | Total electromechanical systole |
| RF | Random Forest |
| RKE | Rotational Kinetic Energy |
| RMSSD | Root mean square of successive differences between inter-beat intervals |
| Sa | Systolic myocardial velocity |
| SCG | Seismocardiography |
| SD | Standard deviation |
| SDNN | Standard deviation of the inter-beat interval |
| SFFT | Sparse Fast Fourier Transform |
| SMQT | successive mean quantization transform |
| SPV | Systolic peak velocity |
| STI | Systolic time interval |
| SVM | Support Vector Machine |
| TAVR | Transcatheter aortic valve replacement |
| TDI | Tissue Doppler Imaging |
| TIMM | Baseline width of the RR interval histogram |
| VLF | the power of the HRV spectrum in the very low frequency range |
| XGB | Extreme Gradient Boosting |
References
- Moore, K.; Dalley, A.; Agur, A. Clinically Oriented Anatomy; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
- Venes, D. Taber’s Cyclopedic Medical Dictionary; F.A. Davis Company: Philadelphia, PA, USA, 2009. [Google Scholar]
- Waldman, L.K.; Fung, Y.C.; Covell, J.W. Transmural myocardial deformation in the canine left ventricle. Normal in vivo three-dimensional finite strains. Circ. Res. 1985, 57, 152–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rademakers, F.E.; Buchalter, M.B.; Rogers, W.J.; Zerhouni, E.A.; Weisfeldt, M.L.; Weiss, J.L.; Shapiro, E.P. Dissociation between left ventricular untwisting and filling. Accentuation by catecholamines. Circulation 1992, 85, 1572–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taber, L.A.; Yang, M.; Podszus, W.W. Mechanics of ventricular torsion. J. Biomech. 1996, 29, 745–752. [Google Scholar] [CrossRef]
- Marcelli, E.; Plicchi, G.; Cercenelli, L.; Bortolami, F. First Experimental Evaluation of Cardiac Apex Rotation with an Epicardial Coriolis Force Sensor. ASAIO J. 2005, 51. [Google Scholar] [CrossRef]
- Arts, T.; Meerbaum, S.; Reneman, R.S.; Corday, E. Torsion of the left ventricle during the ejection phase in the intact dog. Cardiovasc. Res. 1984, 18, 183–193. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Pankäälä, M.; Saraste, A.; Vasankari, T.; Teräs, M.; Koivisto, T. Gyrocardiography: A new non-invasive approach in the study of mechanical motions of the heart. Concept, method and initial observations. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2034–2037. [Google Scholar] [CrossRef]
- D’Mello, Y.; Skoric, J.; Xu, S.; Roche, P.J.R.; Lortie, M.; Gagnon, S.; Plant, D.V. Real-Time Cardiac Beat Detection and Heart Rate Monitoring from Combined Seismocardiography and Gyrocardiography. Sensors 2019, 19, 3472. [Google Scholar] [CrossRef] [Green Version]
- Salerno, D.; Zanetti, J. Seismocardiography: A new technique for recording cardiac vibrations. Concept, method, and initial observations. J. Cardiovasc. Technol. 1990, 9, 111–118. [Google Scholar]
- Tadi, M.J.; Lehtonen, E.; Saraste, A.; Tuominen, J.; Koskinen, J.; Teräs, M.; Airaksinen, J.; Pänkäälä, M.; Koivisto, T. Gyrocardiography: A New Non-invasive Monitoring Method for the Assessment of Cardiac Mechanics and the Estimation of Hemodynamic Variables. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Ingels, N.B.; Daughters, G.T.; Stinson, E.B.; Alderman, E.L. Measurement of midwall myocardial dynamics in intact man by radiography of surgically implanted markers. Circulation 1975, 52, 859–867. [Google Scholar] [CrossRef] [Green Version]
- Beyar, R.; Kroeker, C.A.G.; ter Keurs, H.E.D.J.; Knudtson, M.L.; Tyberg, J.V. An optical device to measure the dynamics of apex rotation of the left ventricle. In Proceedings of the 1992 14th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Paris, France, 29 October–1 November 1992; Volume 2, pp. 436–438. [Google Scholar] [CrossRef] [Green Version]
- Gibbons Kroeker, C.A.; Ter Keurs, H.E.; Knudtson, M.L.; Tyberg, J.V.; Beyar, R. An optical device to measure the dynamics of apex rotation of the left ventricle. Am. J. Physiol. Heart Circ. Physiol. 1993, 265, H1444–H1449. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.C.; Lugo-Olivieri, C.H.; McVeigh, E.R.; Zerhouni, E.A. Three-dimensional Systolic Strain Patterns in the Normal Human Left Ventricle: Characterization with Tagged MR Imaging. Radiology 2000, 214, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Notomi, Y.; Setser, R.M.; Shiota, T.; Martin-Miklovic, M.G.; Weaver, J.A.; Popović, Z.B.; Yamada, H.; Greenberg, N.L.; White, R.D.; Thomas, J.D. Assessment of Left Ventricular Torsional Deformation by Doppler Tissue Imaging. Circulation 2005, 111, 1141–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amundsen, B.H.; Helle-Valle, T.; Edvardsen, T.; Torp, H.; Crosby, J.; Lyseggen, E.; Støylen, A.; Ihlen, H.; Lima, J.A.; Smiseth, O.A.; et al. Noninvasive Myocardial Strain Measurement by Speckle Tracking Echocardiography: Validation Against Sonomicrometry and Tagged Magnetic Resonance Imaging. J. Am. Coll. Cardiol. 2006, 47, 789–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, J.; McDuff, D.J.; Picard, R.W. BioInsights: Extracting personal data from “Still” wearable motion sensors. In Proceedings of the 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Cambridge, MA, USA, 9–12 June 2015. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, J.; McDuff, D.J.; Picard, R.W. Biophone: Physiology monitoring from peripheral smartphone motions. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Li, Y.; Bai, Y.; Mao, Z.H.; Sun, M.; Zhao, Q. Estimation of heart rate from a chest-worn inertial measurement unit. In Proceedings of the 2015 International Symposium on Bioelectronics and Bioinformatics (ISBB), Beijing, China, 14–17 October 2015. [Google Scholar] [CrossRef]
- Migeotte, P.F.; Mucci, V.; Delière, Q.; Lejeune, L.; van de Borne, P. Multi-dimensional Kineticardiography a New Approach for Wearable Cardiac Monitoring Through Body Acceleration Recordings. In Proceedings of the XIV Mediterranean Conference on Medical and Biological Engineering and Computing 2016, Paphos, Cyprus, 31 March–2 April 2016; Kyriacou, E., Christofides, S., Pattichis, C.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1125–1130. [Google Scholar]
- Casanella, R.; Khosrow-khavar, F.; Schmidt, S.; Zanetti, J.; Tavakolian, K. Cardiac Mechanical Signals. In Series in BioEngineering; Springer Singapore: Singapore, 2019; pp. 63–79. [Google Scholar]
- Gordon, J. Certain Molar Movements of the Human Body produced by the Circulation of the Blood. J. Anat. Physiol. 1877, 11, 533–536. [Google Scholar]
- Starr, I.; Rawson, A.J.; Schroeder, H.A.; Joseph, N.R. Studies on The Estimation of Cardiac Ouptut in Man, And of Abnormalities In Cardiac Function, from The Heart’s Recoil and The Blood’s Impacts: The Ballistocardiogram. Am. J. Physiol. Leg. Content 1939, 127, 1–28. [Google Scholar] [CrossRef]
- Bozhenko, B. Seismocardiography—A new method in the study of the functional condition of the heart. Ter. Arkhiv 1961, 33, 55–64. [Google Scholar]
- Zanetti, J.M.; Salerno, D.M. Seismocardiography: A technique for recording precordial acceleration. Computer-Based Medical Systems. In Proceedings of the Fourth Annual IEEE Symposium, Baltimore, MD, USA, 12–14 May 1991; pp. 4–9. [Google Scholar] [CrossRef]
- Inan, O.T.; Migeotte, P.F.; Park, K.S.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.; Tank, J.; Funtova, I.; Prisk, G.K.; et al. Ballistocardiography and Seismocardiography: A Review of Recent Advances. IEEE J. Biomed. Health Inform. 2015, 19, 1414–1427. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, J.M.; Tavakolian, K. Seismocardiography: Past, present and future. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 7004–7007. [Google Scholar] [CrossRef]
- Kim, C.S.; Ober, S.L.; McMurtry, M.S.; Finegan, B.A.; Inan, O.T.; Mukkamala, R.; Hahn, J.O. Ballistocardiogram: Mechanism and Potential for Unobtrusive Cardiovascular Health Monitoring. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Mounsey, P. Praecordial ballistocardiography. Br. Heart J. 1957, 19, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Baevskii, R.M.; Egorov, A.D.; Kazarian, L.A. The Method of Seismocardiography. Kardiologiia 1964, 18, 87–89. [Google Scholar]
- Meriheinä, U.; Juppo, M.; Koivisto, T.; Mikko, P.; Sairanen, K.; Grönholm, M. Heart Monitoring System. WIPO Patent WO 2015/036925 A1, 19 March 2015. [Google Scholar]
- Tadi, M.J.; Lehtonen, E.; Teuho, J.; Saraste, A.; Pänkäälä, M.; Teräs, M.; Koivisto, T. A Miniaturized MEMS Motion Processing System for Nuclear Medicine Imaging Applications. In Proceedings of the 2016 Computing in Cardiology Conference (CinC), Computing in Cardiology, Vancouver, BC, USA, 11–14 September 2016. [Google Scholar] [CrossRef]
- Maxim Integrated. Ultra-Accurate, Low Power, 3-Axis Digital Output Gyroscope, MAX21000 Datasheet, 1st ed.; Maxim Integrated: San Jose, CA, USA, February 2013. [Google Scholar]
- Yang, C.; Tavassolian, N. Combined Seismo- and Gyro-Cardiography: A More Comprehensive Evaluation of Heart-Induced Chest Vibrations. IEEE J. Biomed. Health Inform. 2018, 22, 1466–1475. [Google Scholar] [CrossRef]
- Yang, C.; Tavassolian, N. An Independent Component Analysis Approach to Motion Noise Cancelation of Cardio-Mechanical Signals. IEEE Trans. Biomed. Eng. 2019, 66, 784–793. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.; Whang, M. An Enhanced Method to Estimate Heart Rate from Seismocardiography via Ensemble Averaging of Body Movements at Six Degrees of Freedom. Sensors 2018, 18, 238. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Whang, M. Heart Rate Estimated from Body Movements at Six Degrees of Freedom by Convolutional Neural Networks. Sensors 2018, 18, 1392. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Tang, S.; Tavassolian, N. Utilizing Gyroscopes Towards the Automatic Annotation of Seismocardiograms. IEEE Sens. J. 2017, 17, 2129–2136. [Google Scholar] [CrossRef]
- Crow, R.S.; Hannan, P.; Jacobs, D.; Hedquist, L.; Salerno, D.M. Relationship between Seismocardiogram and Echocardiogram for Events in the Cardiac Cycle. Am. J. Noninvasive Cardiol. 1994, 8, 39–46. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Smedsrud, M.K.; Steen, T.; Kongsgaard, E.; Loennechen, J.P.; Skjaerpe, T.; Voigt, J.U.; Willems, R.; Smith, G.; Smiseth, O.A.; et al. Mechanical Dispersion Assessed by Myocardial Strain in Patients After Myocardial Infarction for Risk Prediction of Ventricular Arrhythmia. JACC Cardiovasc. Imaging 2010, 3, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Dehkordi, P.; Khosrow-Khavar, F.; Rienzo, M.D.; Inan, O.T.; Schmidt, S.E.; Blaber, A.P.; Sørensen, K.; Struijk, J.J.; Zakeri, V.; Lombardi, P.; et al. Comparison of Different Methods for Estimating Cardiac Timings: A Comprehensive Multimodal Echocardiography Investigation. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Marcus, F.I.; Sorrell, V.; Zanetti, J.; Bosnos, M.; Baweja, G.; Perlick, D.; Ott, P.; Indik, J.; He, D.S.; Gear, K. Accelerometer-derived time intervals during various pacing modes in patients with biventricular pacemakers: Comparison with normals. Pacing Clin. Electrophysiol. PACE 2007, 30, 1476–1481. [Google Scholar] [CrossRef]
- Hyler, S.; Espinoza, A.; Skulstad, H.; Fosse, E.; Halvorsen, P.S. Left ventricular function can be continuously monitored with an epicardially attached accelerometer sensor. Eur. J. Cardio Thorac. Surg. 2014, 46, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Grymyr, O.J.H.; Remme, E.W.; Espinoza, A.; Skulstad, H.; Elle, O.J.; Fosse, E.; Halvorsen, P.S. Assessment of 3D motion increases the applicability of accelerometers for monitoring left ventricular function. Interact. Cardio Vascular Thorac. Surg. 2014, 20, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Tadi, M.J.; Teuho, J.; Lehtonen, E.; Saraste, A.; Koivisto, T.; Pänkäälä, M.; Teräs, M. MEMS gating: A new dual gating technique for eliminating motion-related inaccuracies in PET imaging. In Proceedings of the 2016 IEEE Nuclear Science Symposium, Medical Imaging Conference and Room-Temperature Semiconductor Detector Workshop (NSS/MIC/RTSD), Strasbourg, France, 29 October–6 November 2016; pp. 1–5. [Google Scholar] [CrossRef]
- Lahdenoja, O.; Hurnanen, T.; Tadi, M.J.; Pänkäälä, M.; Koivisto, T. Heart Rate Variability Estimation with Joint Accelerometer and Gyroscope Sensing. Comput. Cardiol. 2016, 43, 717–720. [Google Scholar]
- Yang, C.; Tavassolian, N. A feasibility study on a low-cost, smartphone-based solution of pulse transit time measurement using cardio-mechanical signals. In Proceedings of the 2017 IEEE Healthcare Innovations and Point of Care Technologies (HI-POCT), Bethesda, MD, USA, 6–8 November 2017; pp. 93–96. [Google Scholar] [CrossRef]
- Hurnanen, T.; Kaisti, M.; Tadi, M.J.; Vähä-Heikkilä, M.; Nieminen, S.; Iftikhar, Z.; Paukkunen, M.; Pänkäälä, M.; Koivisto, T. Heartbeat Detection Using Multidimensional Cardiac Motion Signals and Dynamic Balancing. In EMBEC & NBC 2017; Springer: Singapore, 2017; pp. 896–899. [Google Scholar]
- Migeotte, P.F.; Delière, Q. Improvements in or Relating to Heart Monitoring. WIPO Patent WO 2,017,036,877 A1, 9 March 2017. [Google Scholar]
- Tuominen, J.; Lehtonen, E.; Tadi, M.J.; Koskinen, J.; Pänkäälä, M.; Koivisto, T. A miniaturized low power biomedical sensor node for clinical research and long term monitoring of cardiovascular signals. In Proceedings of the 2017 IEEE International Symposium on Circuits and Systems (ISCAS), Baltimore, MD, USA, 28–31 May 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Lahdenoja, O.; Tadi, M.J.; Kaisti, M.; Knuutila, T.; Pänkäälä, M.; Koivisto, T. Biomedical Signal Quality Assessment via Learning to Rank with an Application to Mechanical Heart Signals. In Proceedings of the 2017 Computing in Cardiology Conference (CinC), Computing in Cardiology, Rennes, France, 24–27 September 2017. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lahdenoja, O.; Humanen, T.; Koskinen, J.; Pänkaälä, M.; Koivisto, T. Automatic identification of signal quality for heart beat detection in cardiac MEMS signals. In Proceedings of the 2017 IEEE EMBS International Conference on Biomedical and Health Informatics (BHI), Orland, FL, USA, 16–19 February 2017; pp. 137–140. [Google Scholar] [CrossRef]
- Lahdenoja, O.; Koivisto, T.; Tadi, M.J.; Iftikhar, Z.; Hurnanen, T.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; Pänkäälä, M. A smartphone-only solution for detecting indications of acute myocardial infarction. In Proceedings of the 2017 IEEE EMBS International Conference on Biomedical Health Informatics (BHI), Orland, FL, USA, 16–19 February 2017; pp. 197–200. [Google Scholar] [CrossRef]
- Iftikhar, Z.; Lahdenoja, O.; Tadi, M.J.; Hurnanen, T.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; Koivisto, T.; Pänkäälä, M. Multiclass Classifier based Cardiovascular Condition Detection Using Smartphone Mechanocardiography. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Yang, C.; Aranoff, N.D.; Green, P.; Tavassolian, N. A Binary Classification of Cardiovascular Abnormality Using Time-Frequency Features of Cardio-mechanical Signals. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 5438–5441. [Google Scholar] [CrossRef]
- Hernandez, J.E.; Cretu, E. Simple Heart Rate Monitoring System with a MEMS Gyroscope for Sleep Studies. In Proceedings of the 2018 IEEE 9th Annual Information Technology, Electronics and Mobile Communication Conference (IEMCON), Vancouver, BC, Canada, 1–3 November 2018; pp. 61–67. [Google Scholar] [CrossRef]
- Kaisti, M.; Tadi, M.J.; Lahdenoja, O.; Hurnanen, T.; Saraste, A.; Pankaala, M.; Koivisto, T. Stand-Alone Heartbeat Detection in Multidimensional Mechanocardiograms. IEEE Sens. J. 2019, 19, 234–242. [Google Scholar] [CrossRef]
- Yang, C.; Dong, Y.; Chen, Y.; Tavassolian, N. A Low-cost, Smartphone-only Pulse Transit Time Measurement System Using Cardio-mechanical Signals and Optical Sensors*. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Vancouver, BC, Canada, 1–3 November 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Mehrang, S.; Jafari Tadi, M.; Kaisti, M.; Lahdenoja, O.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; Koivisto, T.; Pänkäälä, M. Machine Learning Based Classification of Myocardial Infarction Conditions Using Smartphone-Derived Seismo- and Gyrocardiography. In Proceedings of the 2018 Computing in Cardiology Conference (CinC), Maastricht, The Netherlands, 23–26 September 2018; Volume 45, pp. 1–4. [Google Scholar] [CrossRef]
- Lahdenoja, O.; Hurnanen, T.; Iftikhar, Z.; Nieminen, S.; Knuutila, T.; Saraste, A.; Kiviniemi, T.; Vasankari, T.; Airaksinen, J.; Pänkäälä, M.; et al. Atrial Fibrillation Detection via Accelerometer and Gyroscope of a Smartphone. IEEE J. Biomed. Health Inform. 2018, 22, 108–118. [Google Scholar] [CrossRef]
- Vega-Martinez, G.; Ramos-Becerril, F.J.; Mirabent-Amor, D.; Franco-Sánchez, J.G.; Vera-Hernández, A.; Alvarado-Serrano, C.; Leija-Salas, L. Analysis of heart rate variability and its application in sports medicine: A review. In Proceedings of the 2018 Global Medical Engineering Physics Exchanges/Pan American Health Care Exchanges (GMEPE/PAHCE), Porto, Portuga, 19–24 March 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Kaisti, M.; Tadi, M.J.; Lahdenoja, O.; Hurnanen, T.; Pänkäälä, M.; Koivisto, T. Mechanocardiograms with ECG Reference; IEEE DataPort: New Tork, NY, USA, 2018. [Google Scholar] [CrossRef]
- Jafari Tadi, M.; Mehrang, S.; Kaisti, M.; Lahdenoja, O.; Hurnanen, T.; Jaakkola, J.; Jaakkola, S.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; et al. Comprehensive Analysis of Cardiogenic Vibrations for Automated Detection of Atrial Fibrillation Using Smartphone Mechanocardiograms. IEEE Sens. J. 2019, 19, 2230–2242. [Google Scholar] [CrossRef]
- Mehrang, S.; Tadi, M.J.; Hurnanen, T.; Knuutila, T.; Lahdenoja, O.; Jaakkola, J.; Jaakkola, S.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; et al. Reliability of Self-Applied Smartphone Mechanocardiography for Atrial Fibrillation Detection. IEEE Access 2019, 7, 146801–146812. [Google Scholar] [CrossRef]
- Dehkordi, P.; Tavakolian, K.; Zhao, T.; Zakeri, V.; Khosrow-khavar, F. Estimation of Cardiac Time Intervals from the Mechanical Activity of the Heart Using Machine Learning. In Proceedings of the 2019 Computing in Cardiology Conference (CinC), Singapore, 8–11 September 2019. [Google Scholar] [CrossRef]
- Zia, J.; Kimball, J.; Shandhi, M.H.; Inan, O.T. Automated Identification of Persistent Time-Domain Features in Seismocardiogram Signals. In Proceedings of the 2019 IEEE EMBS International Conference on Biomedical Health Informatics (BHI), Chicago, IL, USA, 19–22 May 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Yang, C.; Dong, Y.; Chen, Y.; Tavassolian, N. A Smartphone-Only Pulse Transit Time Monitor Based on Cardio-Mechanical and Photoplethysmography Modalities. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1462–1470. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Teuho, J.; Koskinen, J.; Schultz, J.; Siekkinen, R.; Koivisto, T.; Pänkäälä, M.; Teräs, M.; Klén, R. A Computational Framework for Data Fusion in MEMS-Based Cardiac and Respiratory Gating. Sensors 2019, 19, 4137. [Google Scholar] [CrossRef] [Green Version]
- Taebi, A.; Solar, B.E.; Bomar, A.J.; Sandler, R.H.; Mansy, H.A. Recent Advances in Seismocardiography. Vibration 2019, 2, 64–86. [Google Scholar] [CrossRef] [Green Version]
- Lahdenoja, O.; Hurnanen, T.; Kaisti, M.; Koskinen, J.; Tuominen, J.; Vähä-Heikkilä, M.; Parikka, L.; Wiberg, M.; Koivisto, T.; Pänkäälä, M. Cardiac monitoring of dogs via smartphone mechanocardiography: A feasibility study. BioMed. Eng. OnLine 2019, 18, 47. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Antoine, C.; Young, B.K.; Tavassolian, N. A Pilot Study on Fetal Heart Rate Extraction from Wearable Abdominal Inertial Sensors. IEEE Sens. J. 2019, 19, 10773–10781. [Google Scholar] [CrossRef]
- Skoric, J.; D’Mello, Y.; Lortie, M.; Gagnon, S.; Plant, D.V. Effect of Static Respiratory Volume on the Waveform of Cardiac-induced Sternal Vibrations. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 3–27 July 2019. [Google Scholar] [CrossRef]
- Hernandez, J.; McDuff, D.; Quigley, K.; Maes, P.; Picard, R.W. Wearable Motion-Based Heart Rate at Rest: A Workplace Evaluation. IEEE J. Biomed. Health Inform. 2019, 23, 1920–1927. [Google Scholar] [CrossRef]
- McHugh, L.C.; Snyder, K.; Yager, T.D. The effect of uncertainty in patient classification on diagnostic performance estimations. PLoS ONE 2019, 14, e0217146. [Google Scholar] [CrossRef]
- Achi’ldiev, V.; Bedro, N.; Uspenckiy, V.; Gruzevich, Y.; Komarova, M.; Rulev, M.; Evseeva, Y. Gyrocardiography Unit for Non-Invasive Human Diseases Diagnosis. In Proceedings of the 2020 27th Saint Petersburg International Conference on Integrated Navigation Systems (ICINS), Saint Petersburg, Russia, 25–27 May 2020; pp. 1–7. [Google Scholar] [CrossRef]
- Mehrang, S.; Lahdenoja, O.; Kaisti, M.; Tadi, M.J.; Hurnanen, T.; Airola, A.; Knuutila, T.; Jaakkola, J.; Jaakkola, S.; Vasankari, T.; et al. Classification of Atrial Fibrillation and Acute Decompensated Heart Failure Using Smartphone Mechanocardiography: A Multilabel Learning Approach. IEEE Sens. J. 2020, 20, 7957–7968. [Google Scholar] [CrossRef]
- Yang, C.; Aranoff, N.D.; Green, P.; Tavassolian, N. Classification of Aortic Stenosis Using Time–Frequency Features From Chest Cardio-Mechanical Signals. IEEE Trans. Biomed. Eng. 2020, 67, 1672–1683. [Google Scholar] [CrossRef]
- Clairmonte, N.; Skoric, J.; D’Mello, Y.; Hakim, S.; Aboulezz, E.; Lortie, M.; Plant, D. Neural Network-based Classification of Static Lung Volume States using Vibrational Cardiography*. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Montréal, QC, Canada, 20–24 July 2020; pp. 221–224. [Google Scholar] [CrossRef]
- D’Mello, Y.; Skoric, J.; Hakim, S.; Aboulezz, E.; Clairmonte, N.; Lortie, M.; Plant, D.V. Identification of the Vibrations Corresponding with Heart Sounds using Vibrational Cardiography*. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Montréal, QC, Canada, 20–24 July 2020; pp. 17–20. [Google Scholar] [CrossRef]
- Aboulezz, E.; Skoric, J.; D’Mello, Y.; Hakim, S.; Clairmonte, N.; Lortie, M.; Plant, D.V. Analyzing Heart Rate Estimation from Vibrational Cardiography with Different Orientations*. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Montréal, QC, Canada, 20–24 July 2020; pp. 2638–2641. [Google Scholar] [CrossRef]
- D’Mello, Y.; Skoric, J.; Xu, S.; Akhras, M.; Roche, P.J.R.; Lortie, M.A.; Gagnon, S.; Plant, D.V. Autocorrelated Differential Algorithm for Real-Time Seismocardiography Analysis. IEEE Sens. J. 2019, 19, 5127–5140. [Google Scholar] [CrossRef]
- Sieciński, S.; Kostka, P.S.; Tkacz, E.J. Heart Rate Variability Analysis on Electrocardiograms, Seismocardiograms and Gyrocardiograms on Healthy Volunteers. Sensors 2020, 20, 4522. [Google Scholar] [CrossRef]
- Siecinski, S.; Kostka, P.S.; Tkacz, E.J. Time Domain And Frequency Domain Heart Rate Variability Analysis on Gyrocardiograms. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Montréal, QC, Canada, 20–24 July 2020; pp. 2630–2633. [Google Scholar] [CrossRef]
- Ramos-Castro, J.; Moreno, J.; Miranda-Vidal, H.; García-González, M.A.; Fernández-Chimeno, M.; Rodas, G.; Capdevila, L. Heart rate variability analysis using a seismocardiogram signal. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 5642–5645. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Koivisto, T.; Pänkäälä, M.; Paasio, A.; Teräs, M. Seismocardiography: Toward heart rate variability (HRV) estimation. In Proceedings of the 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA) Proceedings, Gatineau, QC, Canada, 4–5 May 2015; pp. 261–266. [Google Scholar] [CrossRef]
- Siecinski, S.; Kostka, P.S.; Tkacz, E.J. Heart Rate Variability Analysis on CEBS Database Signals. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Honolulu, HI, USA, 18–21 July 2018; pp. 5697–5700. [Google Scholar] [CrossRef]
- Sarlabous, L.; Torres, A.; Fiz, J.A.; Jané, R. Evidence towards Improved Estimation of Respiratory Muscle Effort from Diaphragm Mechanomyographic Signals with Cardiac Vibration Interference Using Sample Entropy with Fixed Tolerance Values. PLoS ONE 2014, 9, e88902. [Google Scholar] [CrossRef]
- Ruohio, J. Ring Gyroscope Structure and Gyroscope. US Patent No. 9,612,118 B2, 4 April 2017. [Google Scholar]
- Holbrow, C. Modern Introductory Physics; Springer: New York, NY, USA, 2010. [Google Scholar]
- Maquet, P.; Nayler, M.; Ziggelaar, A.; Croone, W. William Croone: On the Reason of the Movement of the Muscles. Trans. Am. Philos. Soc. 2000, 90, 1–130. [Google Scholar] [CrossRef]
- Sørensen, K.; Schmidt, S.E.; Jensen, A.S.; Søgaard, P.; Struijk, J.J. Definition of Fiducial Points in the Normal Seismocardiogram. Sci. Rep. 2018, 8, 15455. [Google Scholar] [CrossRef] [Green Version]
- Taebi, A.; Mansy, H.A. Effect of Noise on Time-frequency Analysis of Vibrocardiographic Signals. J. Bioeng. Biomed. Sci. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Tadi, M.J.; Koivisto, T.; Pänkäälä, M.; Paasio, A.; Knuutila, T.; Teräs, M.; Hänninen, P. A new algorithm for segmentation of cardiac quiescent phases and cardiac time intervals using seismocardiography. In Proceedings of the Sixth International Conference on Graphic and Image Processing (ICGIP 2014), Beijing, China, 24–26 October 2014; Wang, Y., Jiang, X., Zhang, D., Eds.; SPIE: Bellingham, WA, USA, 2015. [Google Scholar] [CrossRef]
- Gurev, V.; Tavakolian, K.; Constantino, J.C.; Kaminska, B.; Blaber, A.P.; Trayanova, N. Mechanisms underlying isovolumic contraction and ejection peaks in seismocardiogram morphology. J. Med. Biol. Eng. 2012, 32, 103. [Google Scholar] [CrossRef]
- Tavakolian, K.; Khosrow-Khavar, F.; Kajbafzadeh, B.; Marzencki, M.; Rohani, S.; Kaminska, B.; Menon, C. Seismocardiographic adjustment of diastolic timed vibrations. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 3797–3800. [Google Scholar] [CrossRef]
- di Rienzo, M.; Vaini, E.; Bruno, B.; Castiglioni, P.; Lombardi, P.; Parati, G.; Lombardi, C.; Meriggi, P.; Rizzo, F. Wearable Seismocardiography: Towards the beat-to-beat assessment of cardiac mechanics during sleep in microgravity. In Proceedings of the 2014 8th Conference of the European Study Group on Cardiovascular Oscillations (ESGCO), Trento, Italy, 25–28 May 2014; pp. 239–240. [Google Scholar] [CrossRef]
- Garrard, C.L.; Weissler, A.M.; Dodge, H.T. The Relationship of Alterations in Systolic Time Intervals to Ejection Fraction in Patients with Cardiac Disease. Circulation 1970, 42, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Weissler, A.M.; Harris, W.S.; Schoenfeld, C.D. Systolic Time Intervals in Heart Failure in Man. Circulation 1968, 37, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [Green Version]
- Fuster, V.; Rydén, L.E.; Cannom, D.S.; Crijns, H.J.; Curtis, A.B.; Ellenbogen, K.A.; Halperin, J.L.; Heuzey, J.Y.L.; Kay, G.N.; Lowe, J.E.; et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation. Circulation 2006, 114. [Google Scholar] [CrossRef] [Green Version]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. Circulation 2014, 130. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Mendis, S.; Puska, P.; Norrving, B. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization in Collaboration with the World Heart Federation and the World Stroke Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Yang, C.; Tang, S.; Tavassolian, N. Annotation of seismocardiogram using gyroscopic recordings. In Proceedings of the 2016 IEEE Biomedical Circuits and Systems Conference (BioCAS), Shanghai, China, 17–19 October 2016; pp. 204–207. [Google Scholar] [CrossRef]
- Taebi, A.; Mansy, H. Noise cancellation from vibrocardiographic signals based on the ensemble empirical mode decomposition. J. Appl. Biotechnol. Bioeng. 2017, 2, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Dash, S.; Chon, K.H.; Lu, S.; Raeder, E.A. Automatic Real Time Detection of Atrial Fibrillation. Ann. Biomed. Eng. 2009, 37, 1701–1709. [Google Scholar] [CrossRef]
- Hurnanen, T.; Lehtonen, E.; Tadi, M.J.; Kuusela, T.; Kiviniemi, T.; Saraste, A.; Vasankari, T.; Airaksinen, J.; Koivisto, T.; Pänkäälä, M. Automated Detection of Atrial Fibrillation Based on Time–Frequency Analysis of Seismocardiograms. IEEE J. Biomed. Health Inform. 2017, 21, 1233–1241. [Google Scholar] [CrossRef]
- Nehmeh, S.A.; Erdi, Y.E.; Ling, C.C.; Rosenzweig, K.E.; Squire, O.D.; Braban, L.E.; Ford, E.; Sidhu, K.; Mageras, G.S.; Larson, S.M.; et al. Effect of respiratory gating on reducing lung motion artifacts in PET imaging of lung cancer. Med. Phys. 2002, 29, 366–371. [Google Scholar] [CrossRef]
- McQuaid, S.J.; Hutton, B.F. Sources of attenuation-correction artefacts in cardiac PET/CT and SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1117–1123. [Google Scholar] [CrossRef]
- Migeotte, P.F.; Lejeune, L.; Deliere, Q.; Caiani, E.; Casellato, C.; Tank, J.; Funtova, I.; Baevsky, R.; Prisk, G.K.; van de Borne, P. Three dimensional Ballistocardiogram and Seismocardiogram: What do they have in common? In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014. [Google Scholar] [CrossRef]
- Shandhi, M.M.H.; Semiz, B.; Hersek, S.; Goller, N.; Ayazi, F.; Inan, O.T. Performance Analysis of Gyroscope and Accelerometer Sensors for Seismocardiography-Based Wearable Pre-Ejection Period Estimation. IEEE J. Biomed. Health Inform. 2019, 23, 2365–2374. [Google Scholar] [CrossRef]
- Tadi, M.J.; Teuho, J.; Lehtonen, E.; Saraste, A.; Pänkäälä, M.; Koivisto, T.; Teräs, M. A novel dual gating approach using joint inertial sensors: Implications for cardiac PET imaging. Phys. Med. Biol. 2017, 62, 8080–8101. [Google Scholar] [CrossRef] [Green Version]
- Gurel, N.Z.; Jeong, H.K.; Kloefkorn, H.; Hochman, S.; Inan, O.T. Unobtrusive Heartbeat Detection from Mice Using Sensors Embedded in the Nest. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 1604–1607. [Google Scholar] [CrossRef]
- Skoric, J.; D’Mello, Y.; Aboulezz, E.; Hakim, S.; Clairmonte, N.; Lortie, M.; Plant, D.V. Relationship of the Respiration Waveform to a Chest Worn Inertial Sensor. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Montréal, QC, Canada, 20–24 July 2020; pp. 2732–2735. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Hurnanen, T.; Koskinen, J.; Eriksson, J.; Pänkäälä, M.; Teräs, M.; Koivisto, T. A real-time approach for heart rate monitoring using a Hilbert transform in seismocardiograms. Physiol. Meas. 2016, 37, 1885–1909. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart Rate Variability. Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Sieciński, S.; Kostka, P.; Piaseczna, N.; Wadas, M. Comparison of Indices Derived from Poincaré Maps on Electrocardiograms and Seismocardiograms. In Current Trends in Biomedical Engineering and Bioimages Analysis; Korbicz, J., Maniewski, R., Patan, K., Kowal, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 15–24. [Google Scholar]
- Thakor, N.V.; Webster, J.G. Electrode studies for the long-term ambulatory ECG. Med. Biol. Eng. Comput. 1985, 23, 116–121. [Google Scholar] [CrossRef]
- Reant, P.; Dijos, M.; Donal, E.; Mignot, A.; Ritter, P.; Bordachar, P.; Santos, P.D.; Leclercq, C.; Roudaut, R.; Habib, G.; et al. Systolic time intervals as simple echocardiographic parameters of left ventricular systolic performance: Correlation with ejection fraction and longitudinal two-dimensional strain. Eur. J. Echocardiogr. 2010, 11, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Castiglioni, P.; Faini, A.; Parati, G.; di Rienzo, M. Wearable Seismocardiography. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 23–26 August 2007; pp. 3954–3957. [Google Scholar] [CrossRef]
- Choudhary, T.; Bhuyan, M.; Sharma, L. Orthogonal subspace projection based framework to extract heart cycles from SCG signal. Biomed. Signal Process. Control 2019, 50, 45–51. [Google Scholar] [CrossRef]









| Database/Search Engine | Number of Articles | |||||
|---|---|---|---|---|---|---|
| Number of Articles Per Year | TOTAL | |||||
| 2016 | 2017 | 2018 | 2019 | 2020 | ||
| Google Scholar | 4 | 12 | 26 | 34 | 31 | 107 |
| Web of Science Core Collection | 2 | 5 | 5 | 5 | 3 | 20 |
| Scopus | 2 | 6 | 5 | 7 | 6 | 26 |
| IEEEXplore | 2 | 3 | 5 | 5 | 4 | 19 |
| PubMed | 1 | 2 | 5 | 6 | 3 | 17 |
| Springer Link | 0 | 0 | 1 | 2 | 0 | 3 |
| Authors | Year | Reference | Performance Metrics |
|---|---|---|---|
| Tadi et al. | 2017 | [113] | TPR 1: 99.6%; PPV 2: 99.8% |
| Yang et al. | 2017 | [39] | Accuracy: 96.8% |
| Hurnanen et al. | 2017 | [49] | Average missed peaks: 0.22% |
| False positive peaks: 0.21% | |||
| Mean errors: 0.47% | |||
| Lee et al. | 2018 | [37] | (standing, relaxed) |
| (sitting, relaxed) | |||
| (standing, aroused) | |||
| (sitting, aroused) | |||
| Hernandez and Cretu | 2018 | [57] | Mean absolute error: BPM 3 |
| Standard deviation of the absolute error: ± 2.7167 BPM | |||
| Kaisti et al. | 2019 | [58] | TPR: 99.9% for healthy subjects and 95.9% for heart disease patients |
| PPV: 99.6% for healthy subjects and for 95.3% for heart disease patients | |||
| Tadi et al. | 2019 | [69] | TPR (Mean ± SD 4): 0.94 ± 0.06 |
| PPV (Mean ± SD): 0.93 ± 0.08 | |||
| F1 (Mean ± SD): 0.93 ± 0.06 | |||
| D’Mello et al. | 2019 | [9] | TPR: 0.9657 (96.57%) |
| PPV: 0.9968 (99.68%) | |||
| Aboulezz et al. | 2020 | [81] | when supine |
| when standing | |||
| across the entire data set |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sieciński, S.; Kostka, P.S.; Tkacz, E.J. Gyrocardiography: A Review of the Definition, History, Waveform Description, and Applications. Sensors 2020, 20, 6675. https://doi.org/10.3390/s20226675
Sieciński S, Kostka PS, Tkacz EJ. Gyrocardiography: A Review of the Definition, History, Waveform Description, and Applications. Sensors. 2020; 20(22):6675. https://doi.org/10.3390/s20226675
Chicago/Turabian StyleSieciński, Szymon, Paweł S. Kostka, and Ewaryst J. Tkacz. 2020. "Gyrocardiography: A Review of the Definition, History, Waveform Description, and Applications" Sensors 20, no. 22: 6675. https://doi.org/10.3390/s20226675





