Coupling Square Wave Anodic Stripping Voltammetry with Support Vector Regression to Detect the Concentration of Lead in Soil under the Interference of Copper Accurately
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Preparation of Bi/GCE
2.3. Collection of Pb2+ and Cu2+ SWASV Signals
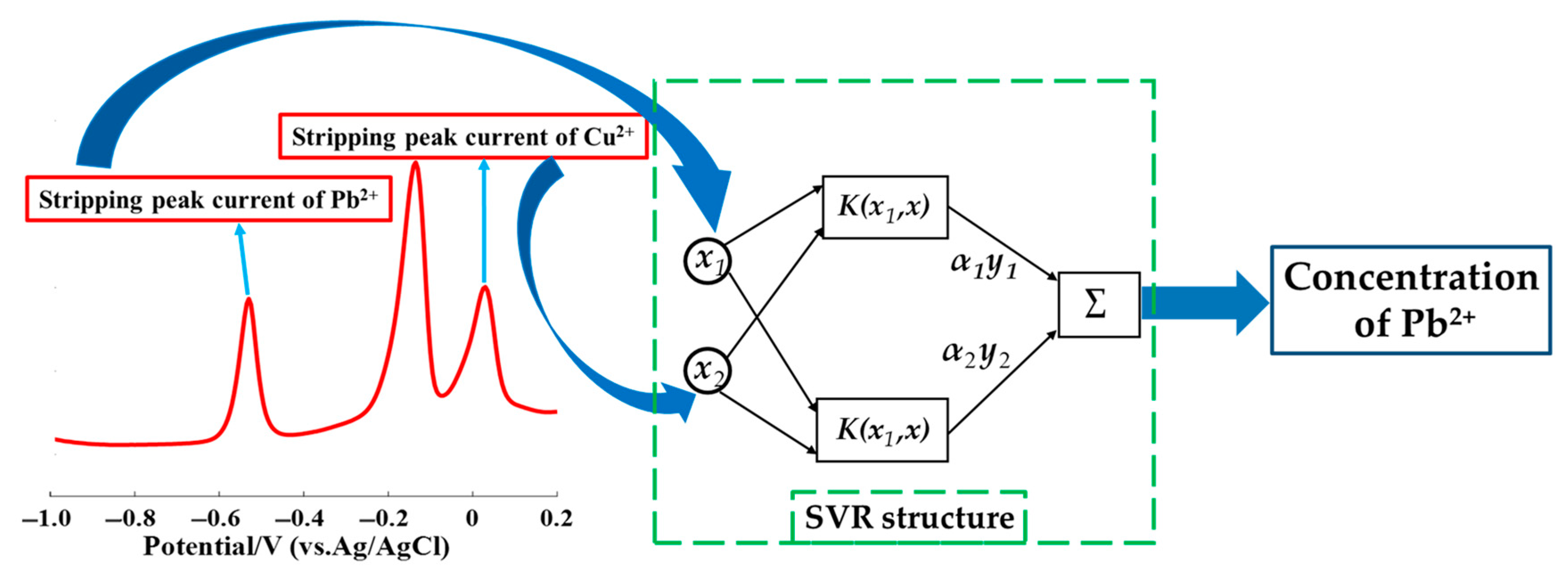
2.4. SVR Modeling
2.5. Preparation of Soil Samples and AAS Measurement
3. Results and Analysis
3.1. Electrochemical Activity of the Bare GCE
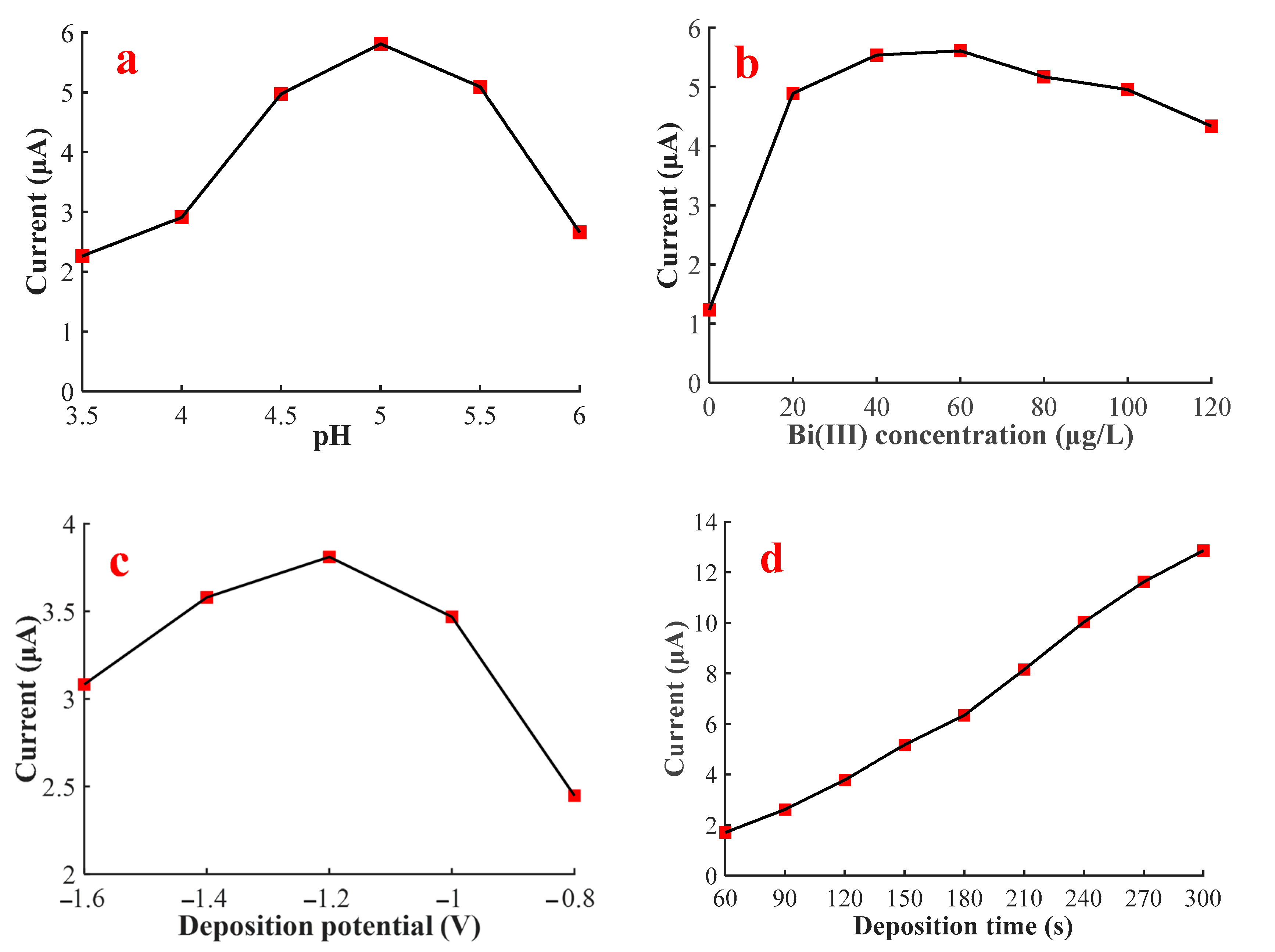
3.2. Optimization of Experimental Conditions
3.3. Electrochemical Responses of Bi/GCE
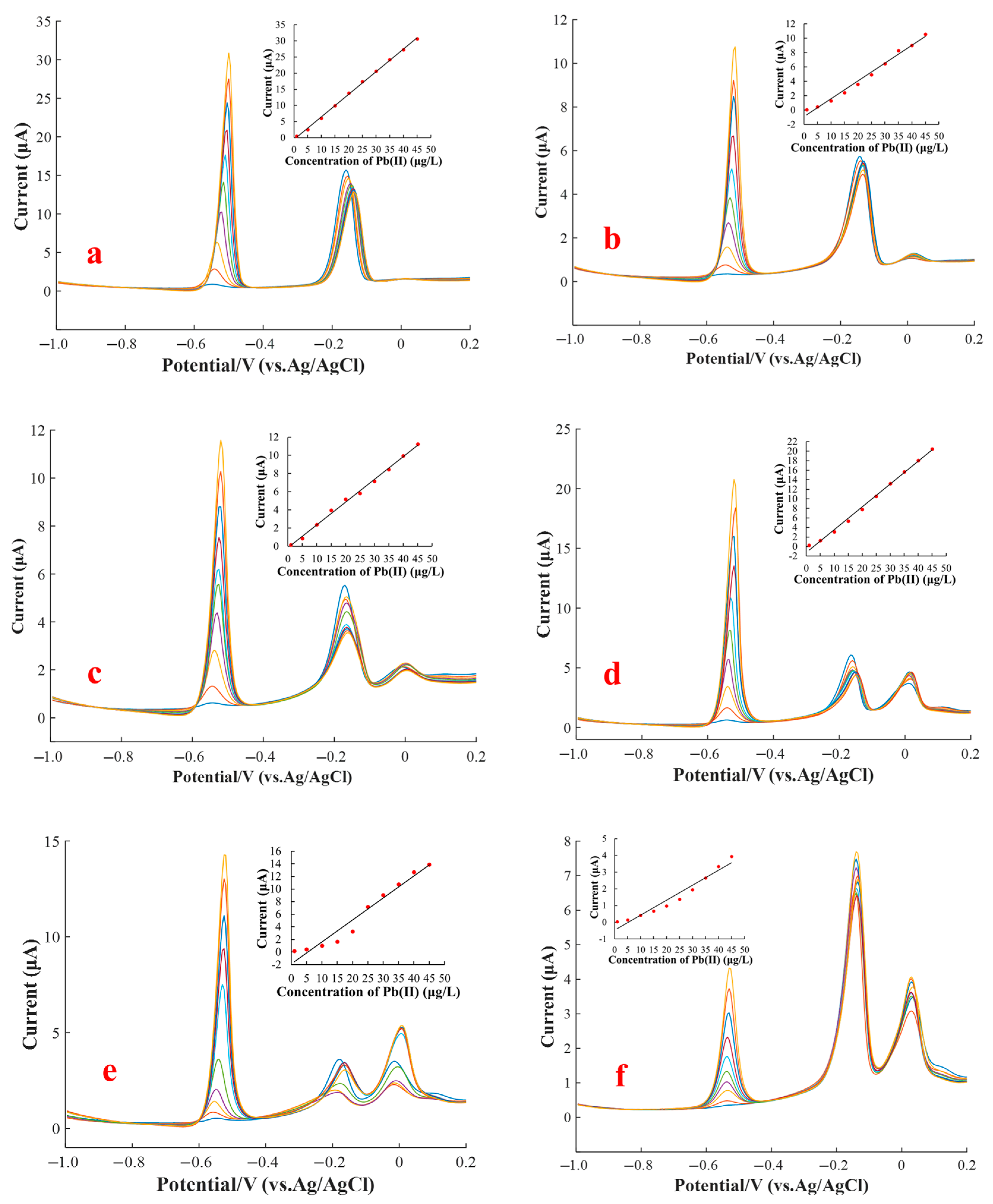
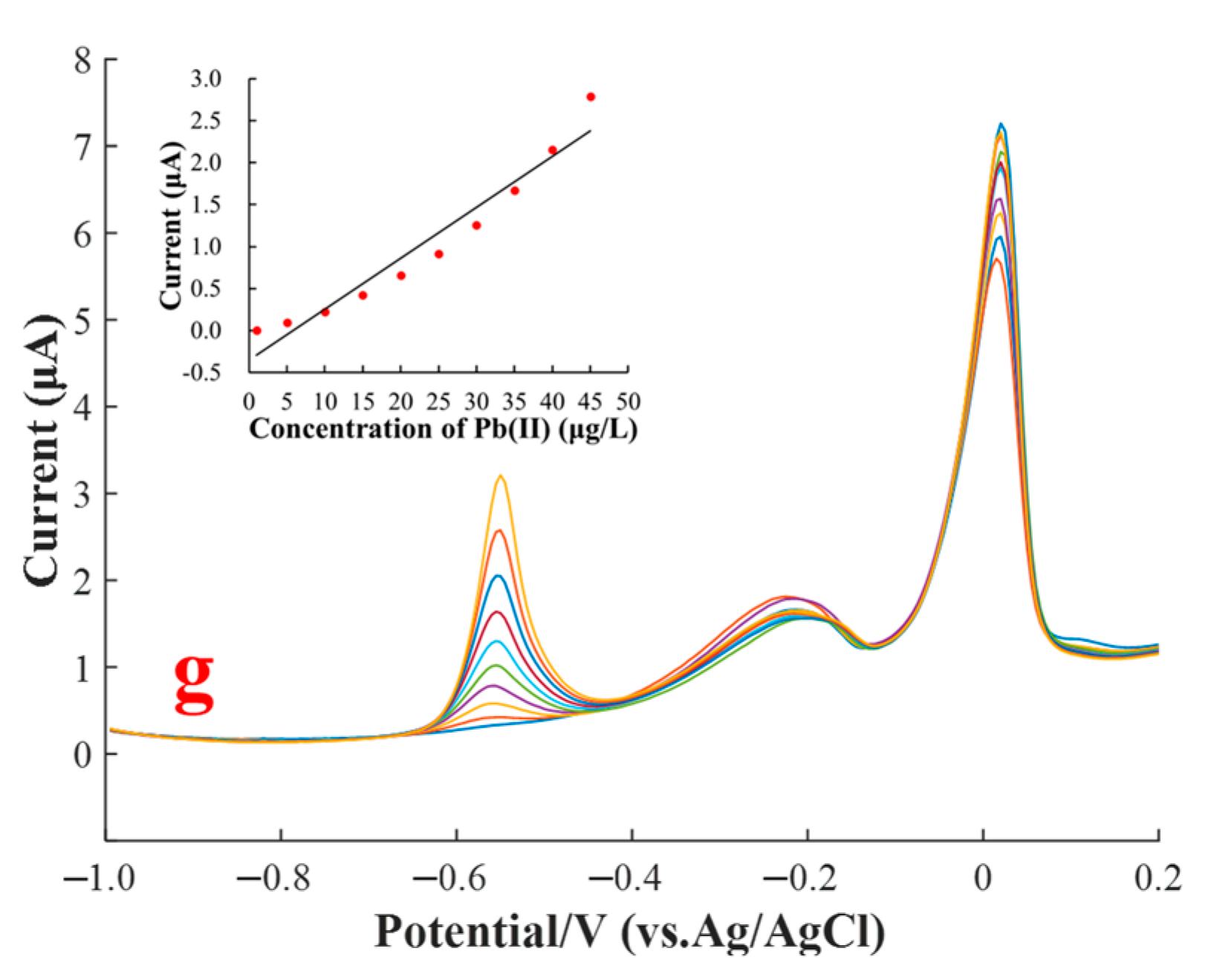
3.4. Interference of Cu(II) on the SWASV Determination of Pb(II)
3.5. Masking Effect of Ferricyanide on the Cu(II) Interference
3.6. Establishment of an SVR Model for the Pb2+ Detection in the Presence of Cu2+
3.6.1. Optimization of the SVR Model Parameters by Using the Grid Search Algorithm
3.6.2. Analysis of the SVR Model Result for Pb(II) Concentration Detection
3.7. Validation of the Proposed SVR Model by Using Real Soil Sample Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gong, H.; Chi, J.; Ding, Z.; Zhang, F.; Huang, J. Removal of lead from two polluted soils by magnetic wheat straw biochars. Ecotoxicol. Environ. Saf. 2020, 205, 111132. [Google Scholar] [CrossRef] [PubMed]
- Conde, P.; Martín Rubí, J.A.; García, R.; Jiménez, B.R. Impacts caused by the addition of wine vinasse on some chemical and mineralogical properties of a Luvisol and a Vertisol in La Mancha (Central Spain). J. Soils Sediments 2009, 9, 121–128. [Google Scholar] [CrossRef]
- Jiménez-Ballesta, R.; Bravo, S.; Amorós, J.A.; Pérez-de los Reyes, C.; Garcia Gimenez, R.; Higueras, P.; García-Navarro, F.J. Mineralogical and Geochemical Nature of Calcareous Vineyard Soils from Alcubillas (La Mancha, Central Spain). Int. J. Environ. Res. Public Health 2020, 17, 6229. [Google Scholar] [CrossRef] [PubMed]
- Gul, I.; Maria, M.; Jean, K.; Arshad, M. Enhanced phytoremediation of lead by soil applied organic and inorganic amendments: Pb phytoavailability, accumulation and metal recovery. Chemosphere 2020, 258, 127405. [Google Scholar] [CrossRef]
- Sharp, R.M.; Brabander, D.J. Lead (Pb) Bioaccessibility and Mobility Assessment of Urban Soils and Composts: Fingerprinting Sources and Refining Risks to Support Urban Agriculture. Geohealth 2017, 1, 333–345. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Lin, C.; Huang, X. Analyzing the anodic stripping square wave voltammetry of heavy metal ions via machine learning: Information beyond a single voltammetric peak. J. Electroanal. Chem. 2020, 872, 113934. [Google Scholar] [CrossRef]
- Zhou, S.; Han, X.; Liu, Y. SWASV performance toward heavy metal ions based on a high-activity and simple magnetic chitosan sensing nanomaterials. J. Alloy. Compd. 2016, 684, 1–7. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, G. Interference Effects of Cu(II) and Pb(II) on the Stripping Voltammetric Detection of Cd(II): Improvement in the Detection Precision and Interference Correction. J. Electrochem. Soc. 2018, 165, H488–H495. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Scarponi, G. Heavy metal distribution in organic and siliceous marine sponge tissues measured by square wave anodic stripping voltammetry. Mar. Pollut. Bull. 2016, 111, 476–482. [Google Scholar] [CrossRef]
- Quiroa, M.C.; Gomez, P.L.; Alvarez, C.R.; Valdez, N.A.; Oropeza, G.M.T. Ordered Mesoporous Carbon Decorated with Magnetite for the Detection of Heavy Metals by Square Wave Anodic Stripping Voltammetry. J. Electrochem. Soc. 2017, 164, B304–B313. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, H.; Liu, G. Direct Quantification of Cd2+ in the Presence of Cu2+ by a Combination of Anodic Stripping Voltammetry Using a Bi-Film-Modified Glassy Carbon Electrode and an Artificial Neural Network. Sensors 2017, 17, 1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaime, P.; Rodrigo, S.; Diego, T.; Freddy, N.; Francesca, F.; María, J.A. Inexpensive and green electrochemical sensor for the determination of Cd(II) and Pb(II) by square wave anodic stripping voltammetry in bivalve mollusks. Food Chem. 2020, 321, 126682. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, G. Synthesis of a three-dimensional (BiO)2CO3@single-walled carbon nanotube nanocomposite and its application for ultrasensitive detection of trace Pb(II) and Cd(II) by incorporating Nafion. Sens. Actuators B Chem. 2019, 288, 71–79. [Google Scholar] [CrossRef]
- María, H.R.; Gismera, M.J.; Procopio, J.R.; Sevilla, M.T. Disposable screen-printed electrode modified with bismuth–PSS composites as high sensitive sensor for cadmium and lead determination. J. Electroanal. Chem. 2016, 767, 114–122. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Raptis, I.; Efstathiou, C.E. Lithographically fabricated disposable bismuth-film electrodes for the trace determination of Pb(II) and Cd(II) by anodic stripping voltammetry. Electrochim. Acta 2008, 53, 5294–5299. [Google Scholar] [CrossRef]
- Matjaž, F.; Laura, K. Copper-bismuth-film in situ electrodes for heavy metal detection. Microchem. J. 2020, 154, 104635. [Google Scholar] [CrossRef]
- Simona, S.; Rita, M.; Abdelhamid, E.; Nicole, J.R. Metal and metal oxide nanoparticles in the voltammetric detection of heavy metals: A review. TrAC Trends Anal. Chem. 2020, 131, 116014. [Google Scholar] [CrossRef]
- Wang, L.; Peng, X.; Fu, H.; Huang, C.; Li, Y.; Liu, Z. Recent advances in the development of electrochemical aptasensors for detection of heavy metals in food. Biosens. Bioelectron. 2020, 147, 111777. [Google Scholar] [CrossRef]
- Cristina, A.; Núria, S.; José, M.D.C.; Miquel, E. Voltammetric determination of metal ions beyond mercury electrodes. A review. Anal. Chim. Acta 2017, 990, 11–53. [Google Scholar] [CrossRef] [Green Version]
- Abdul, W.; Muhammad, M.; Nisar, U. Nanomaterials-based electrochemical detection of heavy metals in water: Current status, challenges and future direction. TrAC Trends Anal. Chem. 2018, 105, 37–51. [Google Scholar] [CrossRef]
- Huma, A.; Aamir, A.A.K.; Muhammad, S.N.; Asim, Y.; Mohd, A.A. Cellulose-hydroxyapatite carbon electrode composite for trace plumbum ions detection in aqueous and palm oil mill effluent: Interference, optimization and validation studies. Environ. Res. 2019, 176, 108563. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, G.; Liu, G. Sensitive Stripping Voltammetric Determination of Pb (II) in Soil Using a Bi/single-walled Carbon Nanotubes-Nafion/ionic Liquid Nanocomposite Modified Screen-Printed Electrode. Int. J. Electrochem. Sci. 2020, 15, 7868–7882. [Google Scholar] [CrossRef]
- Fu, J.H.; Lee, S.L. A multi-class SVM classification system based on learning methods from indistinguishable chinese official documents. Expert Syst. Appl. 2012, 39, 3127–3134. [Google Scholar] [CrossRef]
- Nusrat, P.; Sadaf, Z.; Mohammad, D. Support vector regression model for predicting the sorption capacity of lead (II). Perspect. Sci. 2016, 8, 629–631. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Zhao, R.; Qiao, L. Growth Stages Classification of Potato Crop Based on Analysis of Spectral Response and Variables Optimization. Sensors 2020, 20, 3995. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.; Yan, W.; Zhang, X. Multi-output parameter-insensitive kernel twin SVR model. Neural Netw. 2020, 121, 276–293. [Google Scholar] [CrossRef]
- Wang, J. Stripping analysis at bismuth electrodes: A review. Electroanalysis 2005, 17, 1341–1346. [Google Scholar] [CrossRef]
- Vasko, J.; Samo, B.; Hočevar, B.O. Bismuth electrodes in contemporary electroanalysis. Curr. Opin. Electrochem. 2017, 3, 114–122. [Google Scholar] [CrossRef]
- Yi, W.; Li, Y.; Ran, G.; Luo, H.Q.; Li, N.B. Determination of cadmium(II) by square wave anodic stripping voltammetry using bismuth–antimony film electrode. Sens. Actuators B Chem. 2012, 166–167, 544–548. [Google Scholar] [CrossRef]
- Wang, L.; Xing, P.; Wang, C.; Zhou, X.; Dai, Z.; Bai, L. Maximal Information Coefficient and Support Vector Regression Based Nonlinear Feature Selection and QSAR Modeling on Toxicity of Alcohol Compounds to Tadpoles of Rana temporaria. J. Braz. Chem. Soc. 2019, 30, 279–285. [Google Scholar] [CrossRef]
- Vinicius, G.M.; Thales, K.; Gabriel, Z.E.; Patricia, R.O.; Kathia, M.H. Advances with support vector machines for novel drug discovery. Expert Opin. Drug Discov. 2018, 14, 23–33. [Google Scholar] [CrossRef]
- Kumar, T.M. Nonlinear Support Vector Regression Model Selection Using Particle Swarm Optimization Algorithm. Natl. Acad. Sci. Lett. 2017, 40, 79–85. [Google Scholar] [CrossRef]
- Ying, Y.; Xu, S.; Li, J.; Zhang, B. Compressor performance modelling method based on support vector machine nonlinear regression algorithm. R. Soc. Open Sci. 2020, 7, 191596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Li, Y.; Lai, M.; Xiao, M. Nonlinear structural response prediction based on support vector machines. J. Sound Vib. 2008, 311, 886–897. [Google Scholar] [CrossRef]
- Das, S.; Choudhury, S. Evaluation of effective stiffness of RC column sections by support vector regression approach. Neural Comput. Appl. 2020, 32, 6997–7007. [Google Scholar] [CrossRef]
- A’kif, A.F.; Mohammad, A.; Abdel, R.A.S.; Saad, A.A.; Hani, A.A.; Rida, A.A. Spatial mapping of groundwater springs potentiality using grid search-based and genetic algorithm-based support vector regression. Geocarto Int. 2020, 1716396. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Liang, F.; Yang, Y.; Xu, X.; Dong, J. GSPSO-LRF-ELM: Grid Search and Particle Swarm Optimization-Based Local Receptive Field-Enabled Extreme Learning Machine for Surface Defects Detection and Classification on the Magnetic Tiles. Discret. Dyn. Nat. Soc. 2020, 4565769. [Google Scholar] [CrossRef]
- Xu, Q.; Yan, X.; Grantz, A.D.; Xue, X.; Sun, Y.; Lammers, S.P.; Wang, Z.; Cheng, Q. Improving estimation of evapotranspiration during soil freeze-thaw cycles by incorporating a freezing stress index and a coupled heat and water transfer model into the FAO Penman-Monteith model. Agric. For. Meteorol. 2020, 281, 107847. [Google Scholar] [CrossRef]
- Autchara, P.; Supaporn, K.H.; Jaroon, J. Anodic Stripping Voltammetry with a Dynamic Flow-through Sequential Extraction Method for Fractionation Study of Cadmium and Lead in Soil. Soil Sediment Contam. Int. J. 2020, 29, 650–664. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Terán, E.J.; Montes, M.L.; Rodríguez, C.; Martino, L.; Quiroga, M.; Landa, R.; Torres Sánchez, R.M.; Díaz Pace, D.M. Assessment of sorption capability of montmorillonite clay for lead removal from water using laser–induced breakdown spectroscopy and atomic absorption spectroscopy. Microchem. J. 2019, 144, 159–165. [Google Scholar] [CrossRef]
- Deng, X.; Lü, L.; Li, H.; Luo, F. The adsorption properties of Pb(II) and Cd(II) on functionalized graphene prepared by electrolysis method. J. Hazard. Mater. 2010, 183, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yu, X.; Gao, C.; Jiang, Y.; Han, D.; Liu, J.; Huang, X. Non-conductive nanomaterial enhanced electrochemical response in stripping voltammetry: The use of nanostructured magnesium silicate hollow spheres for heavy metal ions detection. Anal. Chim. Acta 2013, 790, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kadara, R.O.; Tothill, I.E. Stripping chronopotentiometric measurements of Lead(II) and Cadmium(II) in soils extracts and wastewaters using a bismuth film screen-printed electrode assembly. Anal. Bioanal. Chem. 2004, 378, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Kadara, R.O.; Tothill, I.E. Resolving the copper interference effect on the stripping chronopotentiometric response of Lead(II) obtained at bismuth film screen-printed electrode. Talanta 2005, 66, 1089–1093. [Google Scholar] [CrossRef]
- Hu, Z.; Seliskar, C.J.; Heineman, W.R. PAN-incorporated nafion-modifified spectroscopic graphite electrodes for voltammetric stripping determination of lead. Anal. Chim. Acta 1998, 369, 93–101. [Google Scholar] [CrossRef]
- Arrigan, D.W.M.; Svehla, G.; Alderman, J.; Lane, W.A. Ionophore/ionomer films on glassy carbon electrodes for accumulation voltammetry. Investigation of a Lead(II) ionophore. Analyst 1994, 119, 287–292. [Google Scholar] [CrossRef]
- Lin, W.; Zhai, W.; Yan, Y.; Liu, Y. Highly sensitive Pb2+ sensor based on rod-like poly-tyrosine/Bi modified glassy carbon electrode combined with electrodeposition to eliminate Cu2+ interference. Microchem. J. 2020, 160, 105664. [Google Scholar] [CrossRef]
- Hwang, G.H.; Han, W.K.; Park, J.S.; Kang, S.G. An electrochemical sensor based on the reduction of screen-printed bismuth oxide for the determination of trace lead and cadmium. Sens. Actuators B Chem. 2008, 135, 309–316. [Google Scholar] [CrossRef]
- Gupta, S.; Pandotra, P.; Gupta, A.P.; Dhar, J.K.; Sharma, G.; Ram, G.; Husain, M.K.; Bedi, Y.S. Volatile (As and Hg) and non-volatile (Pb and Cd) toxic heavy metals analysis in rhizome of Zingiber officinale collected from different locations of North Western Himalayas by Atomic Absorption Spectroscopy. Food Chem. Toxicol. 2010, 48, 2966–2971. [Google Scholar] [CrossRef]
- Kamal, G.; Yadollah, N.; Omid, O.; Ammar, M. Sulfur-nanoparticle-based method for separation and preconcentration of some heavy metals in marine samples prior to flame atomic absorption spectrometry determination. Talanta 2011, 85, 763–769. [Google Scholar] [CrossRef]
- Maczuga, M.; Economou, A.; Bobrowski, A.; Prodromidis, M.I. Novel screen-printed antimony and tin voltammetric sensors for anodic stripping detection of Pb(II) and Cd(II). Electrochim. Acta 2013, 114, 758–765. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Goddard, N.G.; Fielden, P.R.; Baldock, S.J. Determination of Pb(II) by sequential injection/stripping analysis at all-plastic electrochemical fluid cells with integrated composite electrodes. Talanta 2016, 153, 170–176. [Google Scholar] [CrossRef] [PubMed]

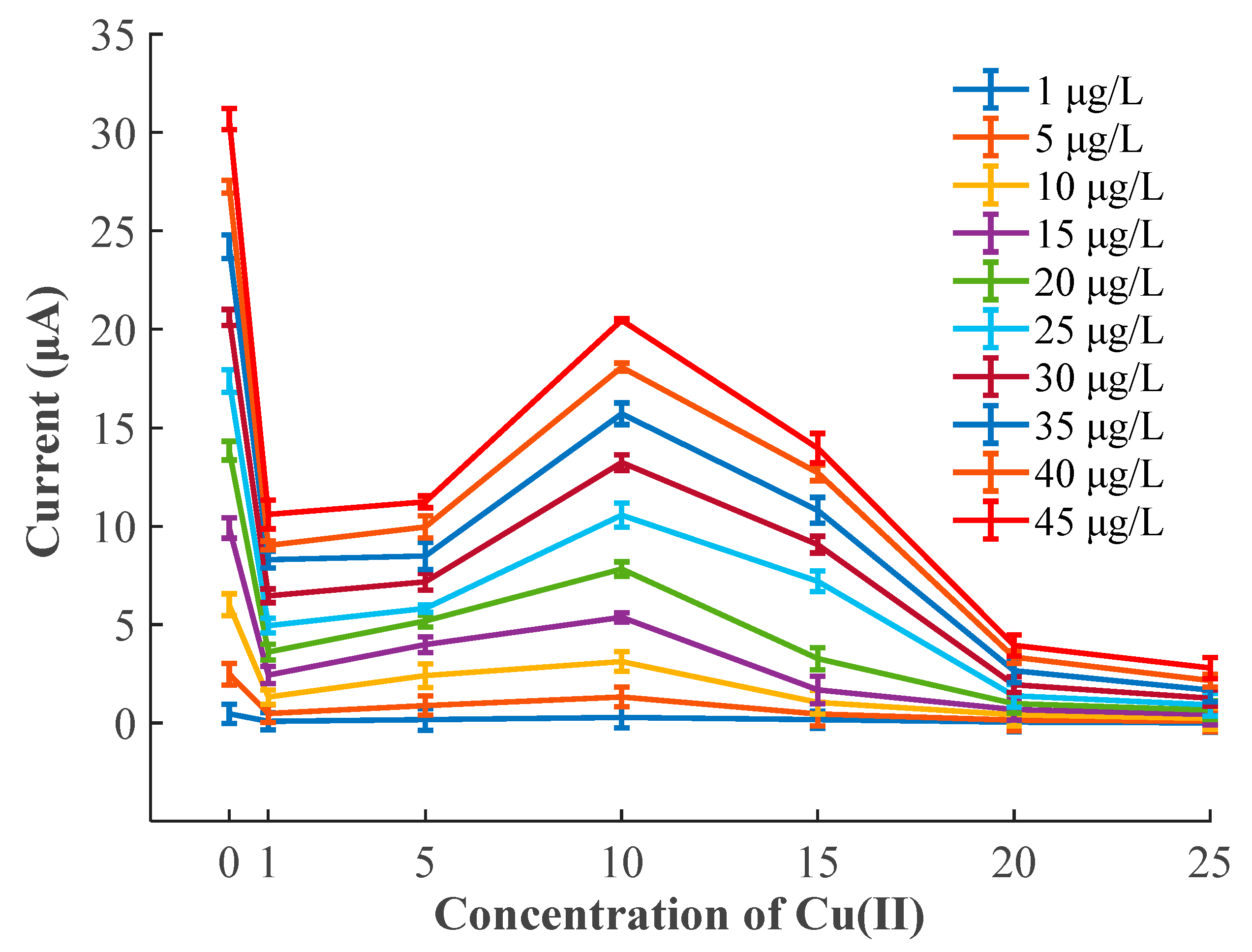
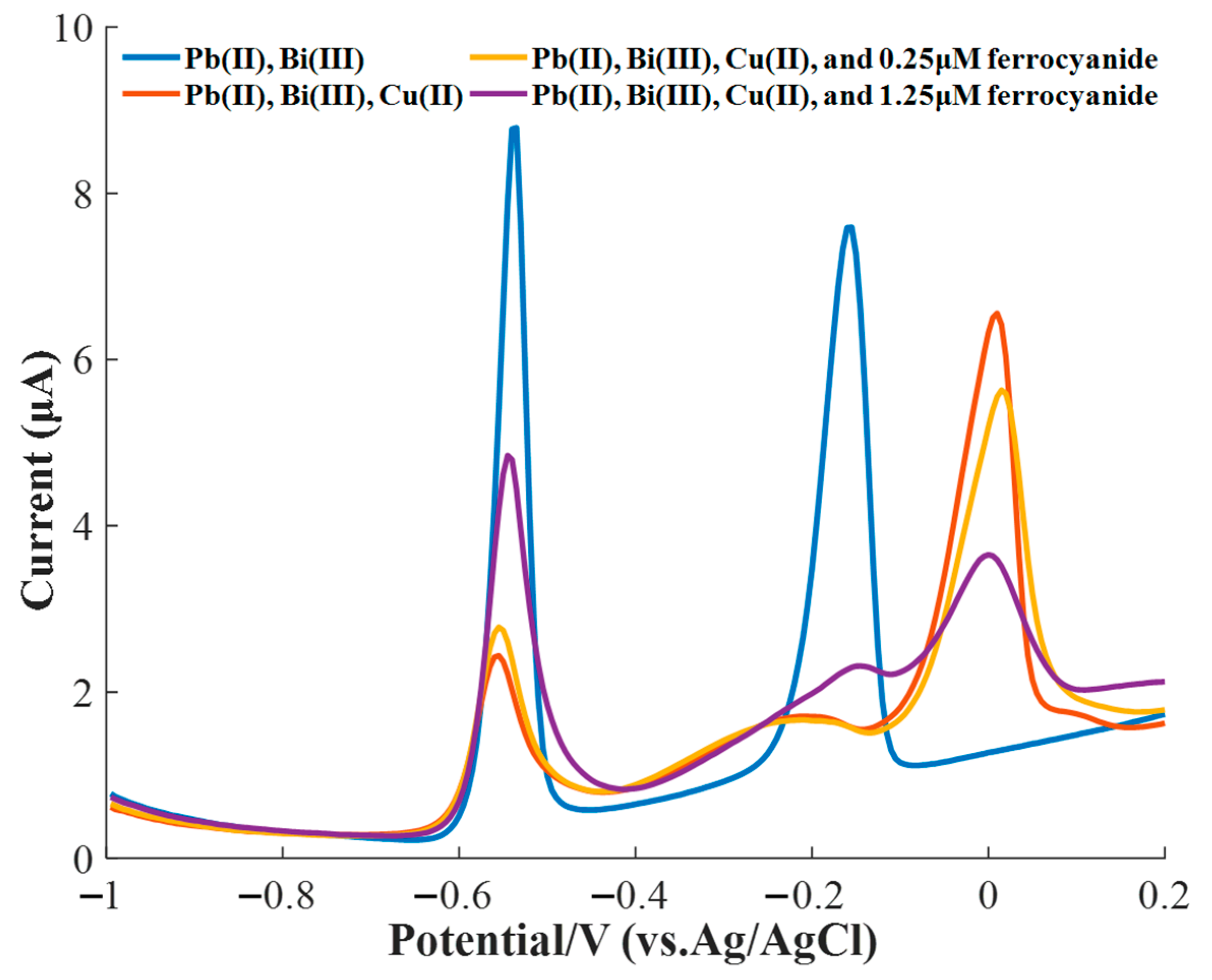

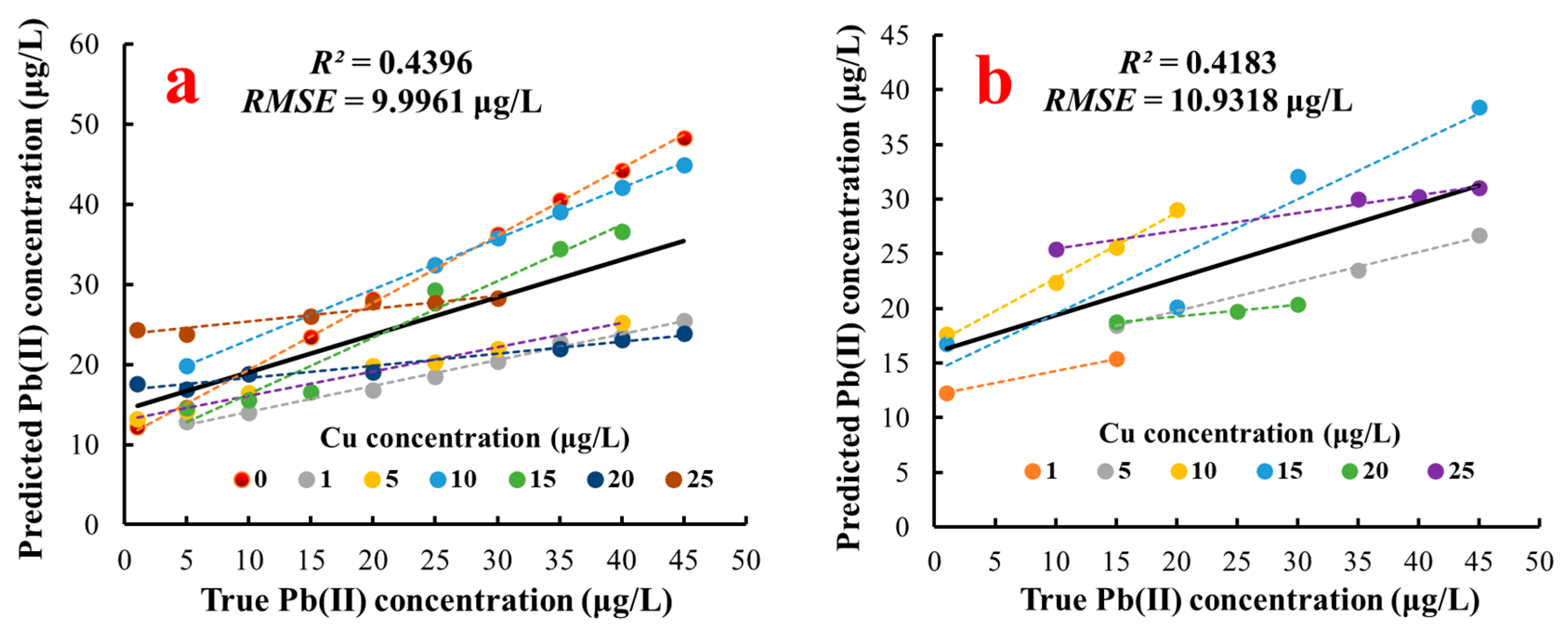
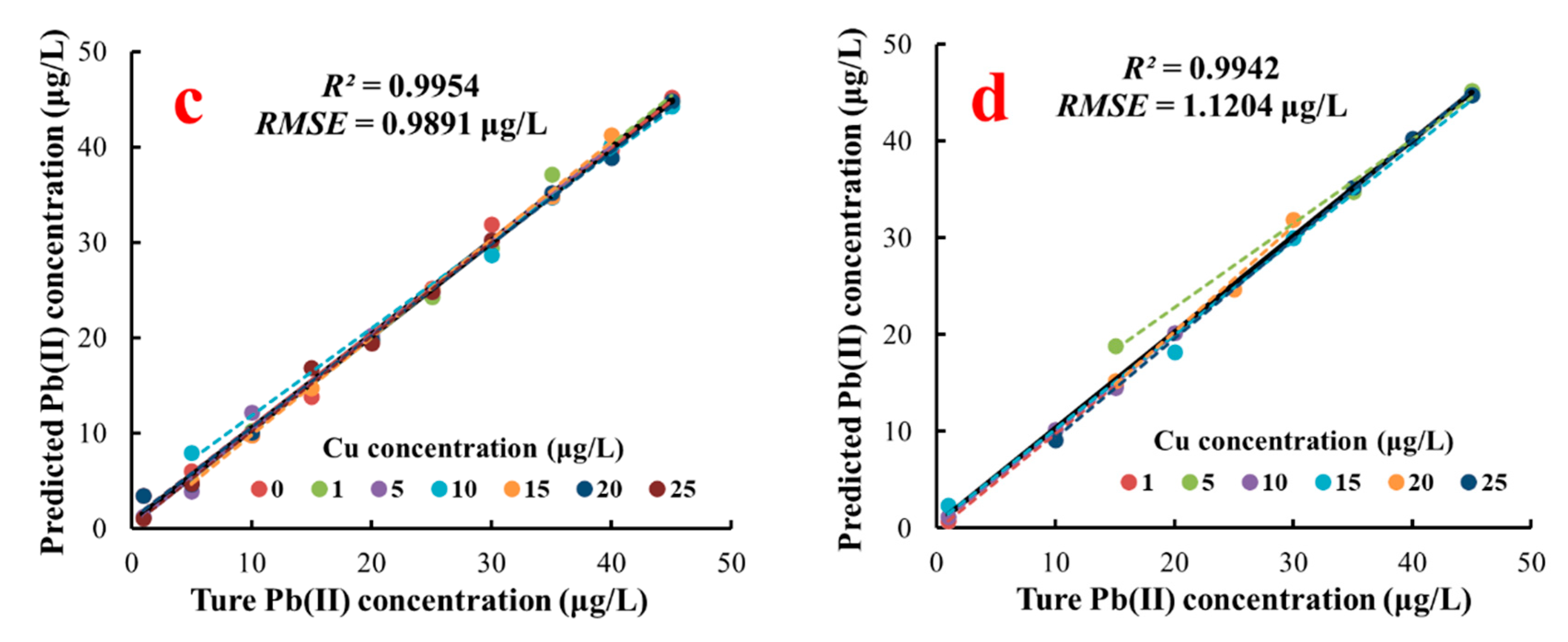
| Concentration of Cu(II) (μg/L) | Calibration Unitary Linear Model of Pb(II) | R2 | p-Value | |||
|---|---|---|---|---|---|---|
| Slope | Intercept | |||||
| Coefficients | Standard Error | Coefficients | Standard Error | |||
| 0 | 0.7011 | 0.0160 | −0.5645 | 0.2974 | 0.9989 | 9.56 × 10−6 |
| 1 | 0.2484 | 0.0095 | −0.9076 | 0.2542 | 0.9884 | 5.01 × 10−6 |
| 5 | 0.2497 | 0.0054 | −0.1332 | 0.1435 | 0.9963 | 5.10 × 10−6 |
| 10 | 0.4766 | 0.0111 | −1.1908 | 0.2961 | 0.9957 | 9.5 × 10−7 |
| 15 | 0.3496 | 0.0259 | −1.8937 | 0.6900 | 0.9581 | 8.57 × 10−5 |
| 20 | 0.0896 | 0.0069 | −0.4743 | 0.1857 | 0.9540 | 1.25 × 10−5 |
| 25 | 0.0608 | 0.005 | −0.3550 | 0.1427 | 0.9417 | 3.23 × 10−5 |
| Model | Training Dataset | Test Dataset | ||||
|---|---|---|---|---|---|---|
| R2 | RMSE (μg/L) | RSD | R2 | RMSE (μg/L) | RSD | |
| Multiple linear regression | 0.4396 | 9.9961 | 165.7286% | 0.4183 | 10.9318 | 230.3635% |
| SVR | 0.9954 | 0.9891 | 7.1973% | 0.9942 | 1.1204 | 7.4282% |
| Electrode | RSD | Eliminating Method | Reference |
|---|---|---|---|
| GC/GQDs-NF/GCE 1 | 35.10% | Adding 0.1 mM ferrocyanide | [12] |
| (BiO)2CO3@SWCNT-Nafion/GCE 2 | 20.54% | Adding 0.1 mM ferrocyanide | [13] |
| SWCNTs-Nafion/IL/SPE 3 | 27.26% | Adding 0.1 mM ferrocyanide | [22] |
| Bi/SPE 4 | 45.00% | Optimizing the added concentration of ferricyanide | [45] |
| Bi/p-Tyr/GCE 5 | 49.91% | Electrodeposition of Cu2+ for 35 min prior to detection of Pb2+ | [48] |
| Bi2O3/GCE 6 | 20.00% | Adding 0.1 mM ferrocyanide | [49] |
| Bi-film/GCE 7 | 7.19% | Combining with support vector regression | This work |
| Sample Number | Added (μg/L) | Stripping Peak Current (μA) | Detected Concentration (μg/L) | Recovery (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pb2+ | Cu2+ | SAM | SVR | AAS | SAM | SVR | AAS | ||
| 1 | - | 0.88 | 0.55 | 2.81 | 5.42 | 5.54 | - | - | - |
| 5 | 2.40 | 0.72 | 7.75 | 10.66 | 10.78 | 98.80 | 104.80 | 104.80 | |
| 10 | 3.99 | 0.77 | 12.84 | 14.54 | 14.86 | 100.30 | 91.20 | 93.20 | |
| 2 | - | 5.37 | 2.82 | 10.23 | 15.55 | 15.85 | - | - | - |
| 10 | 10.56 | 3.06 | 20.17 | 24.78 | 25.72 | 99.40 | 92.30 | 98.70 | |
| 15 | 13.23 | 3.13 | 24.98 | 30.52 | 30.97 | 98.33 | 99.80 | 100.80 | |
| 3 | - | 0.42 | 2.47 | 5.25 | 9.77 | 9.95 | - | - | - |
| 15 | 1.38 | 2.39 | 20.07 | 25.04 | 25.18 | 98.80 | 101.80 | 101.53 | |
| 20 | 1.95 | 2.40 | 25.07 | 30.23 | 30.31 | 99.10 | 102.30 | 101.80 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Zhao, G.; Liu, G. Coupling Square Wave Anodic Stripping Voltammetry with Support Vector Regression to Detect the Concentration of Lead in Soil under the Interference of Copper Accurately. Sensors 2020, 20, 6792. https://doi.org/10.3390/s20236792
Liu N, Zhao G, Liu G. Coupling Square Wave Anodic Stripping Voltammetry with Support Vector Regression to Detect the Concentration of Lead in Soil under the Interference of Copper Accurately. Sensors. 2020; 20(23):6792. https://doi.org/10.3390/s20236792
Chicago/Turabian StyleLiu, Ning, Guo Zhao, and Gang Liu. 2020. "Coupling Square Wave Anodic Stripping Voltammetry with Support Vector Regression to Detect the Concentration of Lead in Soil under the Interference of Copper Accurately" Sensors 20, no. 23: 6792. https://doi.org/10.3390/s20236792





