Abstract
Sleep during infancy is important for the well-being of both infant and parent. Therefore, there is large interest in characterizing infant sleep with reliable tools, for example by combining actigraphy with 24-h-diaries. However, it is critical to select the right variables to characterize sleep. In a longitudinal investigation, we collected sleep data of 152 infants at ages 3, 6, and 12 months. Using principal component analysis, we identified five underlying sleep composites from 48 commonly-used sleep variables: Sleep Night, Sleep Day, Sleep Activity, Sleep Timing, and Sleep Variability. These composites accurately reflect known sleep dynamics throughout infancy as Sleep Day (representing naps), Sleep Activity (representing sleep efficiency and consolidation), and Sleep Variability (representing day-to-day stability) decrease across infancy, while Sleep Night (representing nighttime sleep) slightly increases, and Sleep Timing becomes earlier as one ages. We uncover interesting dynamics between the sleep composites and demonstrate that infant sleep is not only highly variable between infants but also dynamic within infants across time. Interestingly, Sleep Day is associated with behavioral development and therefore a potential marker for maturation. We recommend either the use of sleep composites or the core representative variables within each sleep composite for more reliable research.
1. Introduction
Why is sleeping the most common behavior of an infant in its first year of life [1]? Sleep fulfills an important function in development. The neurophysiology of sleep is linked to brain maturation, neural reorganization [2,3,4], as well as processes of learning and memory [5,6] (for an overview see [7]). However, aside from the vital importance of sleep for a child, it also affects the quality of infant-parent bonds, as early periods with infant sleep problems have been linked to parental depression and stress [8,9]. Supporting healthy infant sleep can thus improve the wellbeing of the whole family.
Sleep-wake patterns are extensively diversified across infants—and vary to a much greater extent compared to any other period in life [1]. This inter-individual variability makes the establishment of normative age-specific sleep values more difficult. Additionally, sleep is not a one-dimensional construct, but characterized by numerous dimensions of quantity, quality, timing, or consolidation. While sleep undergoes drastic changes across infancy, not all sleep dimensions evolve at the same time or to the same degree. Possibly the most recognizable is the alteration from sleep being distributed throughout the 24-h-day (polyphasic sleep) to one primary sleep phase at nighttime (monophasic sleep)—a transition happening gradually from birth until about 5 years of age [1]. This transition involves a multitude of changes, as it affects not only the timing of sleep, but also its depth [10] and fragmentation [11]. Additionally, sleep quantity, measured as total sleep duration across 24 h, also decreases by ~8 min per month across the first year of life [12]. Notably, alongside the changes in sleep behavior, the neurophysiology of sleep is reorganized and the composition of sleep states change across the first years of life: Rapid eye movement (REM) sleep becomes less predominant and the electrophysiological characteristics typical for adult sleep (sleep spindles, slow waves) emerge [13,14].
Due to the ubiquity of sleep and its importance in early development, it is unsurprising that there is large scientific interest in infant sleep. Researchers use both subjective methods (questionnaires e.g., the Brief Infant Sleep Questionnaire ([BISQ) and 24-h-sleep-wake-diaries [15]) and objective methods (actigraphy [16], videosomnography [17], and polysomnography [18]). Each method has advantages and disadvantages [19]. There is only moderate agreement among the diverse methods, with larger discrepancies between questionnaires vs. objective data than between 24-h-diaries vs. objective data [20,21]. Subjective methods are cost effective and easy to administer to large populations. Yet, they are limited to items parents are aware of (e.g., sleep behavior but not sleep stages) and might be biased by parent perception. Furthermore, the selection of assessment method largely depends on the research question and available resources. Objective methods reduce subjective bias and represent the different dimensions of sleep. Over the past 25 years, the combination of actigraphy with 24-h-diaries has emerged as the preferred method for many infant sleep investigations [22]. Its advantage is the combination of objective and subjective data that allows for the quantification of sleep in large populations and in natural environments, while being cost-effective [22,23]. However, issues remain regarding the standardization of actigraphy, especially in infants and young children [22,24]. However, a remaining issue lies in capturing the dimensions of sleep accurately.
One current issue in investigating infant sleep is the selection of sleep variables. On one hand, there are several possible sleep domains and thus numerous sleep variables that can be calculated. On the other hand, the computation of these sleep variables is not standardized. The current situation leaves researchers to decide which sleep variable and computations to choose [22]. For example, sleep duration is one of the most investigated sleep behaviors in infancy (reported in 82% of studies [12]). However, reports are based on different concepts, such as sleep duration computed across night time only, sleep duration including 24 h, duration of sleep with a split of day/night at a chosen clock time, or with clock times for day/night split that are individually assigned for each infant. This divergence is problematic because it prevents comparability across studies [25]. It is also a likely source for lacking reproducibility. Additionally, researchers might rely on default variables from an automated analysis program, which is dubious if the research question demands more specificity. Using a large number of sleep variables to address the dimensions of sleep will likely increase false positives due to multiple testing [26]. Therefore, one should aim for a reduction of methodological complexity.
A novel and promising approach to handle the complexity of sleep dimensions was recently presented. Based on the data of young children, Staples et al. proposed “sleep composites” that were combined from multiple commonly-used sleep variables. This approach reduces the dependence on single (often overlapping) sleep variables and increases the measurement stability [27]. A total of four sleep composites were discovered in both children and their mothers. These sleep composites contain the key dimensions of sleep: Sleep Duration, reflecting the quantity of sleep during the night; Sleep Timing, reflecting bedtimes and sleep onset times; Sleep Variability, reflecting day-to-day differences in sleep timing and duration; and Sleep Activity, reflecting movements and awakenings during the night. They also found that daytime sleep and sleep latency were separate constructs to these four sleep composites (i.e., loaded on their own composite). The identified sleep composites revealed higher consistency across different assessment timepoints compared to single sleep variables. A higher consistency is important to anchor sleep behaviors as reference in certain age periods, which is crucial, for example, to unravel the influence of early sleep variables on later regulatory, cognitive, or emotional outcomes.
- The goal of this study was to first extend the approach of Staples et al. to an infant dataset and to, secondly, facilitate variable selection for future sleep studies;
- We therefore included 48 single sleep variables, which thoroughly characterize the diverse dimensions of sleep, and then performed a component analysis to identify the core infant sleep composites;
- We then examined the evolution of the sleep composites across repeated assessments throughout the first year of life and also tested for sex differences in the sleep composites. Additionally, we explored the stability of composites as well as the stability of the single sleep variables;
- Finally, to evaluate the relevance of sleep for development and to identify maturational markers, we linked sleep composites to infant behavioral developmental scores.
2. Materials and Methods
2.1. Participants
A total of 152 healthy infants (69 female) in Switzerland participated in a longitudinal study on infant sleep and behavioral development. Of these, a subsample of 50 infants were included in a previous investigation [24]. Caregivers and participants were recruited through maternity wards, midwifes, pediatricians, daycares, letters, social media, personal contacts, and flyers distributed at universities, libraries, supermarkets, schools, family organizations, and community centers. Participants were screened for study eligibility by means of an online questionnaire or telephone interview. Inclusion criteria for infants were good general health, being primarily breastfed at time of inclusion (i.e., inclusion criterium of at least 50% of daily nutrition intake through breastfeeding at the first assessment at the age of 3 months), vaginal birth (no cesarean section), and birth within 37–43 weeks of gestation. Parents were required to have a good knowledge of the German language.
Exclusion criteria for infants were disorders of the central nervous system, acute pediatric disorders, brain damage, chronic diseases, as well as family background of narcolepsy, psychosis, or bipolar disorder. Infants with birth weight below 2500 g, intake of medication affecting the sleep-wake cycle, or antibiotics prior to the first assessment were also excluded.
Ethical approval was obtained from the cantonal ethics committee (BASEC 2016-00730) and study procedures were consistent with the declaration of Helsinki. Written parental consent was obtained after an explanation of the study protocol and before enrollment.
2.2. Experimental Design
We assessed 152 infants longitudinally at the ages 3, 6, and 12 months. We scheduled assessments within a 1-month window around the target age, therefore actual age at the start of assessment was between 2.43–3.39 months, 5.42–6.28 months, and 11.47–12.26 months.
We comprehensively quantified sleep-wake behavior for 11 continuous days. It has previously been suggested that 7 days of recording duration are required to then obtain 5 “complete” and artifact-free days to be included in analysis in children 1–5 years of age [28]. We expected further increased data loss in infants and therefore extended the recording length in our study to 11 days. Ankle actigraphy and a 24-h-diary were simultaneously acquired during each of the three assessments, in alignment with our published recommendations for studying this age group [23]. GENEActiv movement sensors “actigraphs” (Activinsights Ltd., Kimbolton, UK, 43 × 40 × 13 mm, MEMS sensor, 16 g, 30 Hz Frequency recording resolution), which are sensitive to +/− 8 g range at 3.9 mg resolution, were attached to the infant’s left ankle in a modified sock (pocket sewn onto its side) or with a Tyvek paper strap. Parents were instructed to only remove the actigraph for bathing/swimming activities and to document any removal of the actigraph in the 24-h-diary. In the 24-h-diary (adapted from [21]), parents reported in 15-min intervals on infant sleep and external movement occurring during infant sleep, e.g., sleeping in the parents arms, stroller, or baby sling etc. Further recorded parameters included feeding, crying episodes (>15 min), and bedtimes (putting infant to bed in the evening and picking it up from the bed in the morning).
Additionally, in online questionnaires, parents reported information on family background, health, and demographics. Families received small gifts for their participation.
2.3. Behavioral Development
Behavioral developmental status was assessed with the age-appropriate Ages and Stages questionnaire (ASQ) [29]. A Collective Score, represented by the sum of scores across five sub-domains (Communication, Gross Motor, Fine Motor, Problem Solving, and Personal Social), was computed to quantify overall development. Additionally, we analyzed Personal Social and Gross Motor individually because these subscales correlated with the well-validated testing battery Bayley Scales of Infant Development [30] and specifically also because these two sub-domains can indicate developmental delay [29,31]. Participants whose questionnaire was completed later than 1 week after the last day of the corresponding assessment were excluded from analysis and missing data was inputted (Section 2.4.2).
2.4. Sleep Analysis
2.4.1. Sleep–Wake-Behavior
Actigraphy data was processed according to our standard protocols [24]. Binary data were extracted using GENEactiv PC Software (Version 3.1), imported into Matlab (R2016b), and converted to activity counts [32]. The conversion included a 3–11 Hz bandpass filter and signal compression to 15 s bins. Acceleration data from the three movement axes was combined using sum of squares. The signal was then compiled to one data point per minute (analysis resolution). A published algorithm [33] was used to identify infant sleep and wake periods, and a 6-step modification [24] was applied to refine prediction for a better fit with the 24-h-diary. The first step of the modification (distinction between periods of high and low activity) was changed to use a threshold of ‘mean activity * 0.72′. Time periods without actigraphy information (i.e., when the actigraph was not worn) were identified through the 24-h-diary or visual inspection (abrupt periods of no activity). These were, whenever possible, replaced with information on sleep or wake from the 24-h-diary.
2.4.2. Handling of Missing Data
For some infants no sleep data was available for all timepoints: n = 2 at 3 months (study enrollment at later age), n = 4 at 6 months (n = 3 device failure, n = 1 parent withdrew from sleep assessment part of study), and n = 9 at 12 months (n = 2 device failure, n = 3 participant attrition, n = 2 parent withdrew from sleep assessment part of study, n = 1 family moved away, n = 1 chronic sickness). Participants were instructed to collect actigraphy data for the duration of 11 continuous days (i.e., putting actigraph on before bedtime on the first day and removing it after getting up on the last day). Yet sickness and vacation of participants as well as device failure prevented the full 11-day recording in some cases (n = 10 at 3 months, n = 28 at 6 months, n = 28 at 12 months). Furthermore, in some instances the recording period was extended beyond the 11 days (e.g., because the original device was temporarily lost or parents recorded longer, n = 27 at 3 months, n = 23 at 6 months, n = 15 at 12 months). Therefore, recordings with available data for both the actigraphy and 24-h-diary lasted on average 10.76 ± 1.72 days: 11.13 ± 1.17 days at 3 months, 10.60 ± 1.91 at 6 months, and 10.55 ± 1.93 at 12 months. Additionally, single days were excluded if infants were either sick (except for common cold symptoms) or if the actigraph was removed for a longer time duration (1 h for partial-day variables, 3 h for entire-day variables, 5 min for variables relying on movement counts, and for clock time variables (e.g., Sleep Onset) a 30-min time window centered on the clock time of parent-reported infant sleep on- or offset, see Supplementary Table S1). Single days were furthermore excluded if the fit between actigraphy-based data and 24-h-diary was poor (see Supplementary Table S1).
2.4.3. Calculation of Sleep Variables
To capture the multitude of dimensions of infant sleep, we calculated 48 sleep variables of interest, based on previous definitions [22,27,34] (Table 1). A total of 3 valid recording days of actimetry were set as the minimum to compute sleep variables in each participant. We selected 3 valid recording days because acceptable reliability for most sleep variables (>0.70) was reported regarding a majority of sleep variables at an infant age of 12 months [28]. For variability variables, a minimum of 5 valid recording days was required, which was chosen in order to maximize the included data and reliable estimates. All calculated sleep variables (except variability variables which were standard deviations across days) were averaged across all valid recorded days. After calculating sleep variables, additional exclusions were performed: For time zone change of >1 h less than 1 week before the recording (n = 1 at 12 months), for medication affecting sleep (n = 2 at 3 months), and for medical problems (n = 1 at 6 months, n = 2 at 12 months) or psychological trauma experienced (n = 1 at 12 months).

Table 1.
Definition and descriptive statistics at 3, 6, and 12 months of the 48 infant sleep variables based on the 24-h-diary and actigraphy that entered the principal component analysis. Bedtime and Get up Time and their variability variables are based on parent report in their 24-h-diary, all other variables are based on actigraphy (with adjustments from diaries as reported in Section 2.4.1). Clock-time variables are reported in two formats: clock times and minutes per day. Mean ± Standard Deviation (Minimum – Maximum).
2.4.4. Data Imputation
Subsequent analyses were done in R (version 3.5.0) [35] and RStudio (version 1.1.463) [36], with several packages for data handling (tidyr, eeptools, reshape, dplyr, lubridate, phyloseq, VIM, margrittr, chron, kableExtra, knitr, and qwraps2) and plotting (corrplot, ggplot2, lattice, ggfortify, sjPlot, and cowplot) [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. Missing and excluded data were inputted using multiple imputation in the mice package [55] and additional functions from miceadds, MKmisc, and micemd package [56,57,58]. Missing data ranged from 0% to 22.32% per variable. The dataset used for imputation included all sleep variables and several demographic variables (see Appendix A). All numerical variables were predicted using the method “2l.pmm”, using the participant ID as the grouping variable and assessment age (3/6/12 months) as slope. Binary variables were predicted using the method “logreg” and categorical variables were predicted using either “polyreg” or “polyr”. Two-level structure was not included in binary and categorical variable prediction. A total of 100 imputations were run with 100 iterations each using 5 cores (20 imputations per core). Data quality of the imputations were visually controlled with density plots (observed vs. imputed values) and line plots for a convergence of iterations. The reported method and prediction matrix were chosen due to best fit of the density plot.
2.4.5. Sleep Composites
We used an integrative and data-driven approach to congregate the 48 infant sleep variables (such as Total Sleep Time, see Table 1 for full description) to the core composite scores, inspired by an approach in young children [27]. We applied principal component analysis (PCA) with promax rotation (psych package [59]) across all participants and all assessment timepoints. Since we included more variables than Staples et al., we examined the best solution with scree and parallel plots as well as the interpretability of the resulting composites, which suggested a 5-component solution. We removed single sleep variables with absolute factor loadings below 0.512 as recommended for sample sizes exceeding 100 [60]. This led to the exclusion of 14 variables (see Table 2). Additionally, we excluded Sleep Duration 24 h (min, minutes scored ‘Sleep’ across 24 h) for interpretability (details below). In total, 33 variables were included in the final PCA solution, with 3 to 10 single sleep variables assigned to each sleep composite (Table 2).

Table 2.
Single sleep variables and PCA solution with oblique rotation (promax rotation). The numbers in parentheses link the single sleep variables to their explanation in Table 1. Values in bold indicate the strongest loading. A total of 14 variables were excluded due to their low loading (<0.512) on any of the sleep composites. These were (7) Sleep Latency (min), (8) Variability of Sleep Latency (SD), (20) Variability of Sleep Efficiency, (26) Variability of Nocturnal Wake Frequency per Hour (SD), (29) Variability Percent Active Epochs (SD), (31) Variability of Longest Sleep (SD), (35) Variability Nap counter (SD), (37) Variability Sleep after Wake Onset (SD), (39) Variability of Sleep Duration 24 h (SD), (41) Variability Sleep Duration Day (SD), (42) Sleep Duration Night (min), (43) Variability of Sleep Duration Night (SD), (45) Variability % Sleep Duration Night (SD), and (46) Sleep Regularity Index Whole Day (Ratio).
Each subsequent model was run with all 100 imputations of the PCA-derived scores for each participant and sleep composite (unweighted average of the highest loadings). All results were pooled across all 100 models. To evaluate the effects of age and sex, we used linear regression models. With the corrplot package we examined correlations between sleep composites and assessment time points using Spearman correlation coefficients. Bonferroni correction was applied to address multiple comparison issues. To test the stability of effects across development, the range of each infant’s percentiles across all assessment timepoints was evaluated (within-subject stability). The stability of composites vs. single variables was evaluated using paired t-tests.
Associations of sleep composites with behavioral outcomes were identified based on longitudinal multilevel models using the lme4 package and by including participant ID for the intercepts and timepoint as slope. Covariates were exact age and sex, and predictors were the 5 sleep composites. Values were considered outliers if they exceeded 1.5 times the interquartile range below the 1st quartile or above the 3rd quartile. Reported statistics include outliers, but any changes in significance due to exclusions of outliers are mentioned specifically. Significance level was set to below 0.05.
3. Results
3.1. Five Principal Components Express All Infant Sleep Variables: Infant Sleep Composites
We achieved reduction of complexity of infant sleep variables by determining five core sleep composites. The relationship of each of the 48 original sleep variables with the sleep composites is represented as “loadings” (Table 2). The five sleep composites explain a total of 71% of the variances, which yields a diagonal fit of 0.98. This revealed:
- Sleep Activity—Larger values reflect more movements and more awakenings during the night as well as less regularity of awakenings. The most representative (i.e., with highest loading) single variable were Sleep Efficiency (negative) or Longest Nocturnal Wake (positive);
- Sleep Timing—Larger values reflect later clock time of bed times and sleep times. The most representative single variable was Sleep Offset;
- Sleep Night—Larger values reflect longer nighttime sleep opportunity and longer nighttime sleep duration. The most representative single variable was Sleep Period;
- Sleep Day—Larger values reflect longer daytime sleep duration, more daytime naps, and lower regularity in daytime sleep. The most representative variables were Longest Wake (negatively) or Nap Counter (positively);
- Sleep Variability—Larger values reflect higher variability between measurement days (standard deviation) within Sleep Timing and Sleep Night. The most representative single variable was Variability of Sleep Opportunity.
Interestingly 24 h Sleep Duration showed the highest loading on the Sleep Day composite, meaning it was more related to Sleep Day than Sleep Night. This finding demonstrates a tight link between naps and total sleep duration. However, to make the interpretation of the Sleep Day composite easier, we removed this variable from subsequent analyses.
3.2. Sleep Composites Accurately Reflect Sleep Maturation Across Infancy
To ensure that the sleep composites accurately reflect the maturation of sleep patterns in infancy, we examined changes in the sleep composites across age. As expected, Sleep Activity, Sleep Day, Sleep Timing, and Sleep Variability all decreased with age (Sleep Activity t(434.25) = −14.59, p < 0.001, Sleep Day t(413.53) = −25.09, p < 0.001, Sleep Timing t(426.65) = −5.78, p < 0.001, and Sleep Variability t(423.45) = −6.13, p < 0.001). In other words, in comparison to infants at a younger age, older infants showed lower activity at night and woke up less frequently (b = −0.15 per month older), slept less often and also shorter during the day (b = −0.21), went to sleep earlier at night and woke up earlier in the morning (b = −0.07), and were more consistent in their sleep timing and nighttime sleep duration (b = −0.08). Sleep Night on the other hand, slightly increased with age (t(421.30) = 2.59, p = 0.01), indicating that older infants slept more at night (b = 0.03). Therefore, sleep composites capture the sleep maturation in infancy well. Moreover, within the same models we could observe sex differences in Sleep Activity (female vs. male t(431.04) = −3.84, p < 0.001) and Sleep Variability (t(434.79) = −1.88, p = 0.06), yet the latter was significant only after the exclusion of outliers (t(413.77) = −2.21, p = 0.03). Girls showed lower nightly activity and reduced wakings (b = −0.29 for female) and were more consistent in their sleep routine (with outliers b = −0.17/without outliers b = −0.19 for female). No sex differences were detected in the other sleep composites (p > 0.05).
3.3. Strong Correlations between the Sleep Composites
Next, we investigated the interrelationships between the sleep composites. Notably, each sleep composite correlated significantly with all other sleep composites, indicating that while sleep is a multidimensional construct, the different dimensions are tightly intertwined (all p < 0.001; Figure 1). Interestingly, the strongest positive correlation was found between Sleep Activity and Sleep Day (rs = 0.50, p < 0.001). Higher activity at night was associated with more sleep during the day. Surprisingly, this association was stronger than the association of Sleep Activity and Sleep Night. As expected, a strong positive correlation was found between Sleep Timing and Sleep Variability (rs = 0.48, p < 0.001), i.e., the later the sleep timing, the higher the Sleep Variability. Stronger negative correlations were found between Sleep Day and Sleep Night (rs = −0.26, p < 0.001) with infants that slept more during the day, slept less at night. A strong negative association was also found for Sleep Night with Sleep Timing (rs = −0.25, p < 0.001), such that infants with later sleep times had less nighttime sleep. In summary, even though the approach clearly identified five core sleep composites of infant sleep, those composites are nonetheless also correlated with each other.
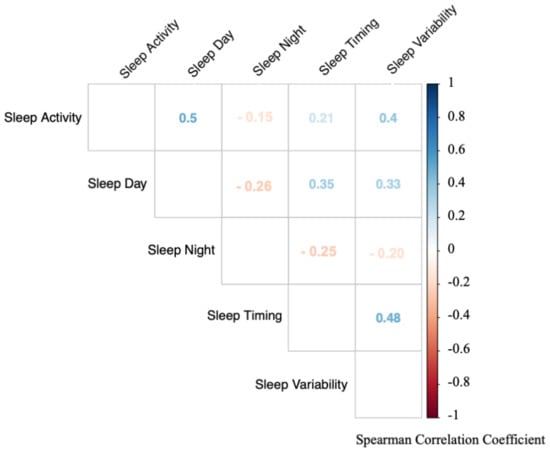
Figure 1.
Correlations between the infant sleep composites based on all assessment timepoints. Each sleep composite is significantly associated with all other composites (all p < 0.001). Colors indicate strength of correlation (red = negative correlations, blue = positive correlations). Numbers indicate spearman correlation coefficient (rs).
3.4. Stability of Sleep Composites
To investigate the stability of sleep composites across the first infant year, we examined correlation coefficients between all assessment time points of each sleep composite (Table 3). Most sleep composites significantly correlated between the adjacent time points (3 vs. 6 or 6 vs. 12 months). Only Sleep Timing also significantly correlated between 3 and 12 months. While Sleep Variability and Sleep Night significantly correlated when outliers were removed, this correlation being low and suggesting no stability (R2 = 0.07). To better understand the dynamics, we calculated the within-subject stability, i.e., consistency of the position of each subject in relation to all other participants. On average, children had a maximum change of 29% for Sleep Timing, 38% for Sleep Night, 43% for Sleep Variability and Sleep Day, and 45% for Sleep Activity from 3–12 months (values from one imputation). This suggests that although most sleep behaviors are stable in the short term, they are dynamic across the first year of infancy.

Table 3.
Spearman Correlation Coefficients (rs) of sleep composites across assessment time points. Significant correlations are presented in bold (Bonferroni corrected p-value below 0.0033). The correlation marked with * are significant upon exclusion of outliers: Sleep Variability rs = 0.26, p = 0.002, Sleep Night rs = 0.26, p = 0.001). Composites are most stable across adjacent time points, but only Sleep Timing is stable across the entire first year.
3.5. Stability of Sleep Composites vs. Single Sleep Variables
Subsequently we tested whether the sleep composites were more stable across the assessment timepoints compared to the stability of single sleep variables, as observed in young children and adults [27]. We used the within-subject stability and compared it between single and composite variables. We tested this within-subject stability in Sleep Timing (the most stable variable) and in Sleep Activity, (the least stable variable). Within-subject stability was also computed for the single sleep variables that loaded the highest and lowest on both sleep composites: Sleep Offset and Bedtime as well as Longest Nocturnal Wake and Percent Active Epochs. An exemplary comparison of one imputation and six random participants is shown in Figure 2. There was no significant difference between the within-subject stability of Sleep Activity and Longest Nocturnal Wake (t(115.69) = −0.17, p = 0.86) nor between the within-subject stability of Sleep Activity and Percent Active Epochs (t(134.03) = 0.10, p = 0.92), indicating no advantage in within-subject stability in the sleep composite as compared to within-subject stability in single sleep variables. In other words, infants showed variable sleep behavior no matter how it was quantified. Similarly, there was no significant difference between the within-subject stability of Sleep Timing and Bedtime (t(124.88) = 0.40, p = 0.69). Contrastingly, Sleep Timing showed higher within-subject stability as compared to Sleep Offset (t(106.64) = 3.20, p = 0.002). Thus, we cannot confirm higher within-subject stability in sleep composites compared to within-subject stability in single sleep variables across the first year of life.
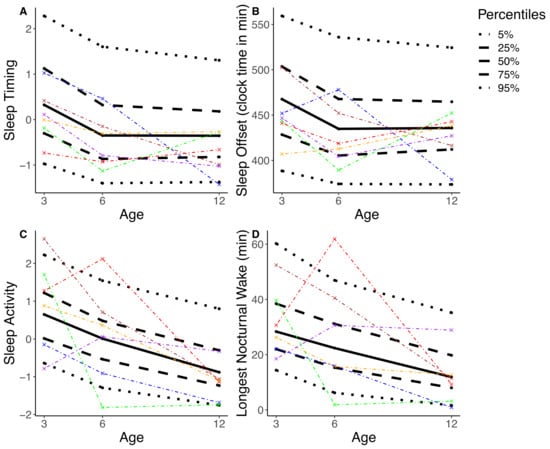
Figure 2.
The percentile distribution is illustrated based on one randomly selected imputation (black; solid line = median, dashed line = interquartile range, dotted line = 90th percentile). A total of six randomly selected participants are represented, each with specific color. (A) shows Sleep Timing, the most stable composite. (B) shows Sleep Offset, the highest loading single variable for Sleep Timing. (C) shows Sleep Activity, the least stable composite. (D) shows Longest Nocturnal Wake, the highest loading single variable for Sleep Activity. Results illustrate that the position of a participant within the percentile distribution fluctuates across the assessments of 3, 6, and 12 months. In other words, e.g., an infant with a comparatively high score on Sleep Activity at 3 months does not necessarily maintain a high score in Sleep Activity at 6 and 12 months.
3.6. Association of Sleep Composite with Behavioral Development
Lastly, we evaluated whether infant sleep composites are linked to behavioral developmental status. Multilevel models across all assessment timepoints revealed a negative link between Sleep Day and ASQ-Collective score (b = −6.65, t(344.65) = −2.22, p = 0.03). No association was observed between behavioral development and other sleep composites (p > 0.05, Table 4). The effect between Sleep Day and Collective Score was more pronounced after reducing the model to only include Sleep Day and control variables (exact age, sex) and no other sleep composites (b = −7.88, t(358.69) = −2.83, p = 0.005). This association suggests that infants with more daytime sleep had lower overall developmental scores. To investigate this finding in more depth we determined whether the effects persisted in the two behavioral sub-scores Personal-social and Gross Motor. This was not the case as no significant effects between the behavioral sub-scores and any of the sleep composites were found (p > 0.05). It is thus likely that the effect of Sleep Day with behavioral developmental is driven by the combination over multiple scales of development.

Table 4.
Associations between sleep composites and behavioral development as quantified by the Ages and Stages questionnaire. Bold font indicates significant associations (p < 0.05). SE = Standard error of measurement.
4. Discussion
In this study we demonstrate that numerous dimensions of infant sleep can be reduced to the five core sleep composites: Sleep Activity, Sleep Timing, Sleep Day, Sleep Night, and Sleep Variability. The sleep composites undergo developmental changes that align with the known maturation of sleep behaviors. We thus recommend the use of sleep composites to reduce variables and to streamline analyses between different lines of research.
Furthermore, both the majority of sleep composites, as well as the single sleep variables show only limited within-subject stability across the first year of infancy, which contrasts with reports in older children. The only notable exception is Sleep Timing, which is stable across the first year of life, and indicates either a parental or infant preference. The lack of within-subject stability can be problematic for studies with a single assessment time-point, because results will vary depending on the assessment time-point. We thus recommend the use of multiple assessment time points, especially when the early sleep behavior is used to predict later cognitive or behavioral outcomes. Interestingly, Sleep Day is associated with behavioral developmental scores, therefore being a potential marker for maturation. Additionally, we report a sex difference in Sleep Activity, with male participants showing more and longer awakenings during the night compared to female infants.
We confirm for the first time the existence of the five infant sleep composites, Sleep Activity, Sleep Timing, Sleep Day, Sleep Night, and Sleep Variability, as previously identified in 2.5–3.5-year-old children [27] and which correspond to the most fundamental dimensions of sleep regulation. We adhered to the same terminology used by Staples et al., except for replacing Sleep Duration with Sleep Night to differentiate it from Sleep Day. To represent sleep in the earliest period of life, we included several variables pertaining to daytime sleep (e.g., number of naps, longest duration of consolidated wake). This confirmed Sleep Day as a construct separate from the other sleep composites. In comparison to Staples et al. our method explains sleep variable variance to a slightly lower extent (71% vs. 82%), which might be caused by the difference in the assessed sleep variables or the different age range (33 vs. 18). Importantly, the proposed sleep composites follow the primary developmental trajectories of the single sleep variables [17,61]. Overall, we conclude that the sleep composites are consistent from infancy to childhood and correspond well to the known core maturation of infant sleep patterns.
The selection of variables can be difficult because of the diversity of sleep variables and computations. Using too many variables can lead to multiple testing problems and increase false positive findings [26]. Thus, using composites to reduce the number of variables facilitates investigations in multi-dimensional research. Our results demonstrate that the resulting sleep composites remain consistent across early development, which aligns with Staples et al., even though different sleep variables were used for computations and even though actigraphy devices differed (GENEActive in our study, MicroMini Motionlogger used by Staples et al.). While in both studies a Sadeh algorithm was used, we used the algorithm specifically developed for infants [33] whereas Staples et al. used the Sadeh algorithm developed on adolescents and adults [62] and then validated in children [28]. Our analysis confirms that all single sleep variables identical to Staples et al., [27] loaded onto the same sleep composite. This strongly supports the use of sleep composites, which has the additional advantage to enhance comparability across studies. Moreover, when computation of sleep composites is not possible, our results can guide the selection of variables. Specifically, it is preferable to select single sleep variables to reflect all sleep composites.
While sleep composites showed some stability across adjacent time periods (3–6 and 6–12 months), the majority of sleep composites did not maintain strong stability across the longest period from 3 to 12 months (except for Sleep Timing). This aligns with a previous report, which examined stability of sleep behaviors from 3 to 42 months [11] and found more stability in sleep duration across shorter time intervals while sleep onset time was very stable. Compared to children, adolescents, and adults, the stability of sleep variables is exceptionally low in infants. In children 3–7 years old, the year-to-year stability was moderate (r = 0.4–0.6) in variables related to Sleep Night and Sleep Timing (even though low stability was noted in Sleep Activity) [63]. Thus, the stability of Sleep Night increases until childhood, while Sleep Timing remains stable and Sleep Activity remains variable until adolescence. A 10-year-long study examining dynamics of sleep duration based on interviews from ages 1 to 10 years reported annual fluctuations, yet overall long-term stability [64]. In adults, year-to-year correlation is high for most sleep measures, especially when derived from several nights (r = 0.48–0.93) [65,66,67,68]. While in older children, the instability of sleep behaviors might be due to measurement imprecisions and therefore is improved by using composites (shown by Staples et al.), it seems that the instability of sleep behaviors in infancy is inherent in the behavior itself. Hence, because variability in infant sleep persists naturally, infant sleep composites are not eliminating this variability. One solution to address this point, specifically when examining later outcomes, is to perform a repeated-measures design, as has been previously suggested by Ednick et al. [69]. If this is not possible, it is important to clarify the age group a finding relates to.
The high within-infant stability of Sleep Timing is notable and we assume that it is largely parent-driven. This is confirmed by the finding that parent’s bedtimes are positively correlated with Sleep Timing (Mother rs = 0.33, p < 0.001 Father rs = 0.24, p < 0.001; exploratory analysis using the reported bedtimes in the Pittsburgh Sleep Quality index). Not surprisingly, parents with later bedtimes also have infants with later sleep timing. However, interestingly, parental bedtimes only explain a small amount of variance in Sleep Timing (Mother partial η2 = 0.05, Father partial η2 = 0.03). Therefore, variance in infant’s sleep remains unexplained by parent’s bedtime preferences. It is unclear whether this variance relates to other parental factors (e.g., cognitions about regular timing of infant sleep), or if the infants themselves already start to demonstrate clock time preference as an early form of infant chronotype. It has previously been reported that infant chronotype may depend on the infant’s sex [70]. However, we did not find any differences in Sleep Timing between boys and girls, which would support this concept.
Intriguingly, we found a difference in Sleep Activity between male and female infants. This is both surprising because most previous studies in infants reported no sex differences [11,71,72] and unsurprisingly, because these differences are well known in adults [73,74,75]. Boys commonly show higher activity levels [76], which could cause more activity during sleep in infant boys. However, one study reported sex differences as young as 2 weeks in electroencephalographic recordings [77]. Therefore, with methods that are sufficiently sensitive, sex differences in sleep behaviors can be detected already very early in life.
A final study goal was to examine if any of the sleep composites mirrors behavioral maturation. Thus, we tested the association between sleep composites and behavioral developmental status. Indeed, infants with more daytime sleep (Sleep Day) had lower ASQ-Collective scores. Our results align with Spruyt et al. who report a negative association between daytime sleep at 12 months with emotional regulation and behavioral maturation [78]. Of relevance might be that the variable Sleep Day shows the largest developmental changes across the first year of life. For example, the infant’s hours asleep during the day as well as the number of naps are dramatically reduced by half in the short period from 3 to 12 months of age. Furthermore, the neurophysiology of daytime sleep also changes with age: 5-year-old children show decreased slow wave activity (a marker of sleep need) during an afternoon nap compared to 2- and 3-year-old children [10], which also suggests that daytime sleep specifically reflects maturation of the central nervous system. When infants are young, napping is important for new memory formation [5,79,80]. When infants get older, their tolerance of longer wake periods increases, which likely also includes their capacity of information acquisition without an immediate nap. Therefore, we hypothesize that a faster decrease in daytime sleep reflects more advanced maturation on a neuronal level. Kurdziel et al. support this theory by demonstrating that naps enhance memory performance in pre-school children only in those children who habitually nap [81] (however see [82]). Therefore, children likely stop to take regular naps when they have developed a physiological tolerance to longer wake periods and when they can retain information without a subsequent nap.
Limitations
We included a comprehensive list of commonly used sleep variables of infants and young children. Since the structuring of factors in the principal component analysis depends on the variables used, the sleep composites identified in this study might not be representative for other investigations that include different single sleep variables. Furthermore, the choice of five factors for the PCA was based on both, data driven criteria, as well as on the interpretability of the resulting sleep composites. It is therefore possible that from a data-driven perspective, more factors would result in a better model fit. However, we prioritized the interpretability of composites so that infant sleep composites can be used for analysis with other datasets. Furthermore, our data are biased toward a higher parental education level (data not shown) and therefore more homogenous than the general population.
5. Conclusions
Our five sleep composites accurately characterized the complex dimensions of infant sleep and reflect known maturational dynamics of infant sleep. To increase comparison across studies, we suggest that researchers use infant sleep composites or, if not possible, single sleep variables with high loadings on the sleep composite of interest. As infant sleep behavior is highly variable both between and within infants, we recommend using multiple assessment time points, especially for testing sleep behaviors as predictors for later cognitive, emotional, or behavioral outcomes. Future experiments may target Sleep Timing as a possible early chronotype and Sleep Day as a maturational marker. Therefore, this study opens up new possibilities to standardize and advance the emerging field of infant sleep research.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-8220/20/24/7188/s1, Table S1: Exclusion Sleep Variables, Table S2: Prediction Matrix. The datasets are available from the corresponding author on reasonable request and pending ethics approval. The analysis scripts are deposited on the Open Science Framework: DOI 10.17605/OSF.IO/6S3PW.
Author Contributions
Conceptualization, S.F.S., S.K., R.H. and M.K.; methodology, S.F.S., S.K., R.H. and M.K.; software, S.F.S.; formal analysis, S.F.S.; investigation, S.F.S., S.K.; resources, S.K., R.H. and M.K.; data curation, S.F.S.; writing—original draft preparation, S.F.S.; writing—review and editing, S.F.S., S.K., R.H. and M.K.; visualization, S.F.S.; supervision, S.K.; project administration, S.F.S.; funding acquisition, S.K., S.F.S., R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Zurich (Clinical Research Priority Program “Sleep and Health”, Forschungskredit FK-18-047, Faculty of Medicine), the Swiss National Science Foundation (PCEFP1-181279, P0ZHP1-178697), Foundation for Research in Science and the Humanities (STWF-17-008), and the Olga Mayenfisch Stiftung.
Acknowledgments
We thank the parents and infants for participating in our study. We would like to thank the students and interns of the Baby Sleep laboratory for their help with data collection: Sina Boschert-Hennrich, Rita Grolimund, Viktoria Gastens, Lara Barblan, Rahel Nicolet, Melanie Auer, Monika Stoller, and Juliane Berger. Additionally, we thank Andjela Markovic, Christophe Mühlematter, Matthieu Beaugrand, and Valeria Jaramillo for reviewing code and general discussion and feedback.
Conflicts of Interest
The authors declare no conflict of interest. M.K. declares to have received advisory fees from Bayer, GSK, Novartis, Astra, Boehringer Ingelheim and Mundipharma outside the submitted work. M.K. is also a board member of the Deep Breath Initiative (DBI), a company that specializes and provides services in the field of exhaled breath analysis. R.H. is a partner of Tosoo AG, a company developing wearables for sleep electrophysiology monitoring and stimulation.
Appendix A
The imputation data set included the participant number (complete), the timepoint of assessment (complete), gender (complete), exact age at assessment (complete or set to assessment timepoint for data points to impute), Collective Score (4.5% missing), Gross Motor (4.5 % missing), and Personal Social (4.5% missing) scores from the Ages and Stages questionnaire [29], gestation age at birth (complete), sleep environment for the baby at night (own room, parent’s room, room shared with sibling; complete for missing data we inferred from the other assessment time points), number of children in family (complete), analysis run for gut microbiota (complete, if there was not gut microbiota data, the run at which the data would have been analyzed was used), probiotics use (yes/no) at 3 (5.2 % missing) and 6 months (3% missing), antibiotics use at 6 (3% missing) and 12 months (6.7% missing, “never”, “unknown time”, “2–4 weeks before assessment”, “<2 weeks before assessment”), 3 alpha diversity measures for the gut microbiota (Shannon, Observed, Chao all 4.1% missing), gut microbiota cluster (4.1% missing), gut microbiota age prediction (4.1% missing), education mother (0.2% missing) and father (2.8% missing, “none”, “apprenticeship”, “high school”, “university”, “PhD”), and bottle and breastfeeding frequency (4.9% missing, 0 for never or rarely breastfed, 1 if breastfed occasionally, regularly or daily). All 48 sleep variables were included, which ranged from 4.9% missing (Bedtime) to 22.3% missing (Variability of Total Sleep Time, Variability of Sleep Efficiency, Variability of Sleep after Wake Onset). The missing data from each variable were predicted by all other variables in the dataset that correlated with the variable with r ≥ 0.1. The parental education and bottle and breastfeeding frequency variables had to be excluded as predictors because inclusion of them lead to inability to specify the model (see prediction matrix in the Supplementary Table S2).
References
- Iglowstein, I.; Jenni, O.G.; Molinari, L.; Largo, R.H. Sleep Duration from Infancy to Adolescence: Reference Values and Generational Trends. Pediatrics 2003, 111, 302–307. [Google Scholar] [CrossRef]
- Kurth, S.; Ringli, M.; Geiger, A.; LeBourgeois, M.; Jenni, O.G.; Huber, R. Mapping of Cortical Activity in the First Two Decades of Life: A High-Density Sleep Electroencephalogram Study. J. Neurosci. 2010, 30, 13211–13219. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, I.; Campbell, I.G. Sleep EEG changes during adolescence: An index of a fundamental brain reorganization. Brain Cogn. 2010, 72, 56–65. [Google Scholar] [CrossRef]
- Cao, J.; Herman, A.B.; West, G.B.; Poe, G.R.; Savage, V.M. Unraveling why we sleep: Quantitative analysis reveals abrupt transition from neural reorganization to repair in early development. Sci. Adv. 2020, 6, eaba0398. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Mölle, M.; Friederici, A.D.; Born, J. Sleep-dependent memory consolidation in infants protects new episodic memories from existing semantic memories. Nat. Commun. 2020, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, A.; Brichet, M.; De Tiège, X.; Peigneux, P.; Urbain, C. The power of children’s sleep—Improved declarative memory consolidation in children compared with adults. Sci. Rep. 2020, 10, 9979. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, M.; Sadeh, A.I. Sleep and development: Introduction to the monograph. Monogr. Soc. Res. Child Dev. 2015, 80, 1–14. [Google Scholar] [CrossRef]
- Lam, P.; Hiscock, H.; Wake, M. Outcomes of infant sleep problems: A longitudinal study of sleep, behavior, and maternal well-being. Pediatrics 2003, 111, e203–e207. [Google Scholar] [CrossRef]
- Martin, J.; Hiscock, H.; Hardy, P.; Davey, B.; Wake, M. Adverse Associations of Infant and Child Sleep Problems and Parent Health: An Australian Population Study. Pediatrics 2007, 119, 947–955. [Google Scholar] [CrossRef]
- Kurth, S.; Lassonde, J.M.; Pierpoint, L.A.; Rusterholz, T.; Jenni, O.G.; McClain, I.J.; Achermann, P.; LeBourgeois, M.K. Development of nap neurophysiology: Preliminary insights into sleep regulation in early childhood. J. Sleep Res. 2016, 25, 646–654. [Google Scholar] [CrossRef]
- Scher, A.; Epstein, R.; Tirosh, E. Stability and changes in sleep regulation: A longitudinal study from 3 months to 3 years. Int. J. Behav. Dev. 2004, 28, 268–274. [Google Scholar] [CrossRef]
- Galland, B.C.; Taylor, B.J.; Elder, D.E.; Herbison, P. Normal sleep patterns in infants and children: A systematic review of observational studies. Sleep Med. Rev. 2012, 16, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Louis, J.; Zhang, J.; Revol, M.; Debilly, G.; Challamel, M. Ontogenesis of nocturnal organization of sleep spindles: A longitudinal study during the first 6 months of life. Electroencephalogr. Clin. Neurophysiol. 1992, 83, 289–296. [Google Scholar] [CrossRef]
- Jenni, O.G.; Borbély, A.A.; Achermann, P. Development of the nocturnal sleep electroencephalogram in human infants. Am. J. Physiol. Integr. Comp. Physiol. 2004, 286, R528–R538. [Google Scholar] [CrossRef] [PubMed]
- Parmelee, A.H. Sleep Patterns in Infancy A Study of One Idant from Birth to Eight Months of Age. Acta Paediatr. 1961, 50, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A.; Lavie, P.; Scher, A.; Tirosh, E.; Epstein, R. Actigraphic home-monitoring sleep-disturbed and control infants and young children: A new method for pediatric assessment of sleep-wake patterns. Pediatrics 1991, 87, 494–499. [Google Scholar]
- Anders, T.F.; Keener, M. Developmental Course of Nighttime Sleep-Wake Patterns in Full-Term and Premature Infants during the First Year of Life. I. Sleep 1985, 8, 173–192. [Google Scholar] [CrossRef]
- Hagne, I. Development of the Sleep EEG in Normal Infants during the First Year of Life. Acta Paediatr. 1972, 61, 25–53. [Google Scholar] [CrossRef]
- Sadeh, A., III. Sleep assessment methods. Monogr. Soc. Res. Child Dev. 2015, 80, 33–48. [Google Scholar] [CrossRef]
- Camerota, M.; Tully, K.P.; Grimes, M.; Gueron-Sela, N.; Propper, C.B. Assessment of infant sleep: How well do multiple methods compare? Sleep 2018, 41. [Google Scholar] [CrossRef]
- Werner, H.; Molinari, L.; Guyer, C.; Jenni, O. Agreement Rates between Actigraphy, Diary, and Questionnaire for Children’s Sleep Patterns. Arch. Pediatr. Adolesc. Med. 2008, 162, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, L.J.; Montgomery-Downs, H.E.; Insana, S.P.; Walsh, C.M. Use of actigraphy for assessment in pediatric sleep research. Sleep Med. Rev. 2012, 16, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Schoch, S.F.; Kurth, S.; Werner, H. Actigraphy in sleep research with infants and young children: Current practices and future benefits of standardized reporting. J. Sleep Res. 2020, e13134. [Google Scholar] [CrossRef]
- Schoch, S.F.; Jenni, O.G.; Kohler, M.; Kurth, S. Actimetry in infant sleep research: An approach to facilitate comparability. Sleep 2019, 42. [Google Scholar] [CrossRef] [PubMed]
- Alamian, A.; Wang, L.; Hall, A.M.; Pitts, M.; Ikekwere, J. Infant sleep problems and childhood overweight: Effects of three definitions of sleep problems. Prev. Med. Rep. 2016, 4, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, J.P. Multiple Hypothesis Testing. Annu. Rev. Psychol. 1995, 46, 561–584. [Google Scholar] [CrossRef]
- Staples, A.D.; Bates, J.E.; Petersen, I.T.; McQuillan, M.E.; Hoyniak, C. Measuring sleep in young children and their mothers: Identifying actigraphic sleep composites. Int. J. Behav. Dev. 2019, 43, 278–285. [Google Scholar] [CrossRef]
- Acebo, C.; Sadeh, A.; Seifer, R.; Tzischinsky, O.; Wolfson, A.R.; Hafer, A.; Carskadon, M.A. Estimating Sleep Patterns with Activity Monitoring in Children and Adolescents: How Many Nights Are Necessary for Reliable Measures? Sleep 1999, 22, 95–103. [Google Scholar] [CrossRef]
- Squires, J.; Potter, L.; Bricker, D. The ASQ User’s Guide for the Ages & Stages Questionnaires: A Parent-Completed, Child-Monitoring System; Paul H Brookes Publishing: Baltimore, MD, USA, 1995; ISBN 978-1-55766-179-1. [Google Scholar]
- Gollenberg, A.L.; Lynch, C.; Jackson, L.W.; McGuinness, B.M.; Msall, M.E. Concurrent validity of the parent-completed Ages and Stages Questionnaires, 2nd Ed. with the Bayley Scales of Infant Development II in a low-risk sample. Child Care Health Dev. 2009, 36, 485–490. [Google Scholar] [CrossRef]
- Valla, L.; Wentzel-Larsen, T.; Hofoss, D.; Slinning, K. Prevalence of suspected developmental delays in early infancy: Results from a regional population-based longitudinal study. BMC Pediatr. 2015, 15, 215. [Google Scholar] [CrossRef]
- Lindert, B.H.W.T.; Van Someren, E.J.W. Sleep Estimates Using Microelectromechanical Systems (MEMS). Sleep 2013, 36, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A.; Acebo, C.; Seifer, R.; Aytur, S.; Carskadon, M.A. Activity-based assessment of sleep-wake patterns during the 1st year of life. Infant Behav. Dev. 1995, 18, 329–337. [Google Scholar] [CrossRef]
- Phillips, A.J.K.; Clerx, W.M.; O’Brien, C.S.; Sano, A.; Barger, L.K.; Picard, R.W.; Lockley, S.W.; Klerman, E.B.; Czeisler, C.A. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci. Rep. 2017, 7, 3216. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; R Studio Inc.: Boston, MA, USA, 2015; Volume 42, p. 14. Available online: http://www.rstudio.com (accessed on 15 December 2020).
- Wickham, H.; Henry, L. Tidyr: Tidy Messy Data. R Package Version 1.02; R Package: Madison, WI, USA, 2019. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84); R Package: Madison, WI, USA, 2017; Available online: https://github.com/taiyun/corrplot (accessed on 15 December 2020).
- Becker, J.P.; Knowles, J.E.; Knowles, M.J.E. Package ‘Eeptools’. Available online: https://cran.r-project.org/web/packages/eeptools/index.html2020 (accessed on 15 December 2020).
- Wickham, H. Reshaping data with the reshape package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Wickham, H.; Francois, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation; R Package: Madison, WI, USA, 2015. [Google Scholar]
- Wickham, H. ggplot2: Elegant graPhics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Grolemund, G.; Wickham, H. Dates and times made easy with lubridate. J. Stat. Softw. 2011, 40, 1–25. [Google Scholar] [CrossRef]
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Kowarik, A.; Templ, M. Imputation with the R Package VIM. J. Stat. Softw. 2016, 74, 1–16. [Google Scholar] [CrossRef]
- Bache, S.M.; Wickham, H. Magrittr: A Forward-Pipe Operator for R; R Package: Madison, WI, USA, 2014. [Google Scholar]
- James, D.; Hornik, K. Chron: Chronological Objects Which Can Handle Dates and Times; R Package: Madison, WI, USA, 2010. [Google Scholar]
- Tang, Y.; Horikoshi, M.; Li, W. Ggfortify: Unified Interface to Visualize Statistical Results of Popular R Packages. R J. 2015, 8, 474–485. [Google Scholar] [CrossRef]
- Lüdecke, D. Sjplot: Data Visualization for Statistics in Social Science; R Package: Madison, WI, USA, 2018. [Google Scholar]
- Zhu, H. KableExtra: Construct Complex Table with ’Kable’ and Pipe Syntax; R Package Version 0.90; R Package: Madison, WI, USA, 2018; Available online: https://cran.r-project.org/web/packages/kableExtra/index.html (accessed on 15 December 2020).
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for “ggplot2”; R Package Version 0.94; R Package: Madison, WI, USA, 2019. [Google Scholar]
- Xie, Y. Knitr: A General-Purpose Tool for Dynamic Report Generation in R; R Package: Madison, WI, USA, 2013. [Google Scholar]
- DeWitt, P. Qwraps2: Quick Wraps 2; R Package Version 0.3.0; R Package: Madison, WI, USA, 2018. [Google Scholar]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations inR. J. Stat. Softw. 2011, 45, 8. [Google Scholar] [CrossRef]
- Robitzsch, A.; Grund, S.; Henke, T.; Robitzsch, M.A. Package ‘Miceadds’; R Package: Madison, WI, USA, 2017. [Google Scholar]
- Audigier, V.; Resche-Rigon, M. Micemd: Multiple Imputation by Chained Equations with Multilevel Data; R Package: Madison, WI, USA, 2017. [Google Scholar]
- Kohl, M. MKmisc: Miscellaneous Functions from M. Kohl; R Package Version 0.94; R Package: Madison, WI, USA, 2013. [Google Scholar]
- Revelle, W.; Revelle, M.W. Package ‘Psych’. Compr. R Arch. Netw. 2015. Available online: https://cran.r-project.org/web/packages/psych/psych.pdf (accessed on 15 December 2020).
- Stevens, J. Applied Multivariate Statistics for the Social Sciences, 2nd ed.; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 1992; ISBN 978-0-8058-1154-4. [Google Scholar]
- Bruni, O.; Baumgartner, E.; Sette, S.; Ancona, M.; Caso, G.; Di Cosimo, M.E.; Mannini, A.; Ometto, M.; Pasquini, A.; Ulliana, A.; et al. Longitudinal Study of Sleep Behavior in Normal Infants during the First Year of Life. J. Clin. Sleep Med. 2014, 10, 1119–1127. [Google Scholar] [CrossRef]
- Sadeh, A.; Sharkey, K.M.; Carskadon, M.A. Activity-Based Sleep-Wake Identification: An Empirical Test of Methodological Issues. Sleep 1994, 17, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Williams, S.M.; Farmer, V.L.; Taylor, B.J. The Stability of Sleep Patterns in Children 3 to 7 Years of Age. J. Pediatr. 2015, 166, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Jenni, O.; Molinari, L.; Caflisch, J.; Largo, R.H. Sleep Duration from Ages 1 to 10 Years: Variability and Stability in Comparison with Growth. Pediatrics 2007, 120, 769. [Google Scholar] [CrossRef] [PubMed]
- Akerstedt, T.; Kecklund, G. Stability of Day and Night Sleep-A Two-Year Follow-Up of EEG Parameters in Three-Shift Workers. Sleep 1991, 14, 507–510. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Knutson, K.; Rathouz, P.J.; Yan, L.L.; Liu, K.; Lauderdale, D.S. Intra-Individual Daily and Yearly Variability in Actigraphically Recorded Sleep Measures: The CARDIA Study. Sleep 2007, 30, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Gaines, J.; Vgontzas, A.N.; Fernandez-Mendoza, J.; Basta, M.; Pejovic, S.; He, F.; Bixler, E.O. Short- and Long-Term Sleep Stability in Insomniacs and Healthy Controls. Sleep 2015, 38, 1727–1734. [Google Scholar] [CrossRef]
- Druiven, S.J.M.; Hovenkamp-Hermelink, J.H.; Knapen, S.E.; Kamphuis, J.; Haarman, B.C.M.; Penninx, B.W.; Antypa, N.; Meesters, Y.; Schoevers, R.; Riese, H. Stability of chronotype over a 7-year follow-up period and its association with severity of depressive and anxiety symptoms. Depress. Anxiety 2020, 37, 466–474. [Google Scholar] [CrossRef]
- Ednick, M.; Cohen, A.P.; McPhail, G.L.; Beebe, D.; Simakajornboon, N.; Amin, R.S. A Review of the Effects of Sleep during the First Year of Life on Cognitive, Psychomotor, and Temperament Development. Sleep 2009, 32, 1449–1458. [Google Scholar] [CrossRef]
- Randler, C.; Faßl, C.; Kalb, N. From Lark to Owl: Developmental changes in morningness-eveningness from new-borns to early adulthood. Sci. Rep. 2017, 7, srep45874. [Google Scholar] [CrossRef]
- Tikotzky, L.; De Marcas, G.; Har-Toov, J.; Dollberg, S.; Bar-Haim, Y.; Sadeh, A. Sleep and physical growth in infants during the first 6 months. J. Sleep Res. 2010, 19, 103–110. [Google Scholar] [CrossRef]
- Sadeh, A.; Flint-Ofir, E.; Tirosh, T.; Tikotzky, L. Infant sleep and parental sleep-related cognitions. J. Fam. Psychol. 2007, 21, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Kurina, L.M.; Thisted, R.A.; Chen, J.-H.; McClintock, M.K.; Waite, L.; Lauderdale, D.S. Actigraphic sleep characteristics among older Americans. Sleep Health 2015, 1, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.F.V.D.; Miedema, H.M.; Tulen, J.H.; Hofman, A.; Neven, A.K.; Tiemeier, H. Sex Differences in Subjective and Actigraphic Sleep Measures: A Population-Based Study of Elderly Persons. Sleep 2009, 32, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Jean-Louis, G.; Mendlowicz, M.V.; Von Gizycki, H.; Zizi, F.; Nunes, J. Assessment of Physical Activity and Sleep by Actigraphy: Examination of Gender Differences. J. Women’s Health Gender Based Med. 1999, 8, 1113–1117. [Google Scholar] [CrossRef]
- Campbell, D.W.; Eaton, W.O. Sex differences in the activity level of infants. Infant Child Dev. 1999, 8, 1–17. [Google Scholar] [CrossRef]
- Bach, V.; Telliez, F.; Leke, A.; Libert, J.-P. Gender-related sleep differences in neonates in thermoneutral and cool environments. J. Sleep Res. 2000, 9, 249–254. [Google Scholar] [CrossRef]
- Spruyt, K.; Aitken, R.J.; So, K.; Charlton, M.; Adamson, T.M.; Horne, R.S. Relationship between sleep/wake patterns, temperament and overall development in term infants over the first year of life. Early Hum. Dev. 2008, 84, 289–296. [Google Scholar] [CrossRef]
- Hupbach, A.; Gomez, R.L.; Bootzin, R.R.; Nadel, L. Nap-dependent learning in infants. Dev. Sci. 2009, 12, 1007–1012. [Google Scholar] [CrossRef]
- Seehagen, S.; Konrad, C.; Herbert, J.S.; Schneider, S. Timely sleep facilitates declarative memory consolidation in infants. Proc. Natl. Acad. Sci. USA 2015, 112, 1625–1629. [Google Scholar] [CrossRef]
- Kurdziel, L.; Duclos, K.; Spencer, R.M.C. Sleep spindles in midday naps enhance learning in preschool children. Proc. Natl. Acad. Sci. USA 2013, 110, 17267–17272. [Google Scholar] [CrossRef]
- Lukowski, A.F.; Milojevich, H.M. Sleeping like a baby: Examining relations between habitual infant sleep, recall memory, and generalization across cues at 10 months. Infant Behav. Dev. 2013, 36, 369–376. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).