Inline Reticulorumen pH as an Indicator of Cows Reproduction and Health Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Experimental Design
2.2. Measurements
2.3. Animals and Experimental Condition
2.4. Data Analysis and Statistics
3. Results
3.1. Reticulorumen pH as an Indicator of Reproduction Status of Cows
3.2. Reticulorumen pH as an Indicator of Health Status in Cows
3.3. Correlations of Reticulorumen pH with Indicators from Automatic Milking System (AMS)
4. Discussion
4.1. Reticulorumen pH as an Indicator of Cow Reproduction Success
4.2. Reticulorumen pH and Health Status of Cows
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| °C | Celsius |
| ADF | Acid detergent fiber |
| AMS | Automatic milking system |
| BCS | Body condition score |
| BE (ecf) | base excess in extracellular fluid |
| BW | Body weight |
| Ca | Calcium |
| Chco3 | Bicarbonate |
| cHgb | Hemoglobin concentration |
| CL | Chlorides |
| CP | Crude protein |
| d | Days |
| DM | Dry matter |
| EC | Electrical milk conductivity |
| F/P | Milk fat-protein ratio |
| Fat | Milk fat |
| HCT | Hematocrit |
| h | Hours |
| K | Potassium |
| Kg | Kilogram |
| Kg | Kilograms |
| Lac | Lactate |
| Mcal/kg | mega calories per kilogram |
| min/d | Minutes per day |
| mS/cm | Milisiemens per centimeter |
| MY | Milk yield |
| Na | Sodium |
| NDF | Neutral detergent fiber |
| NEL | Neto energy for lactation |
| NFC | Nonfiber carbohydrates |
| PCO2 | Partial carbon dioxide pressure |
| pH | Hydrogen potential |
| PLF | Precision livestock farming |
| RRpH | Reticulorumen pH |
| RT | Rumination time |
| SARA | Subclinical acidosis |
| SCC | Somatic cell count |
| TCO2 | Total carbon dioxide carbon |
| TMR | Total mix ration |
| tousd/mL | Thousand per millilitre |
References
- Brayer, E. Control Apparatus for Milking Machines. US Patent 4,348,984, 4 September 1982. [Google Scholar]
- Wathes, C.M.; Kristensen, H.H.; Aerts, J.M.; Berckmans, D. Is precision livestock farming an engineer’s daydream or nightmare, an animal’s friend or foe, and a farmer’s panacea or pitfall? Comput. Electron. Agric. 2008, 64, 2–10. [Google Scholar] [CrossRef]
- Soriani, N.; Trevisi, E.; Calamari, L. Relationships between rumination time, metabolic conditions, and health status in dairy cows during the transition period. J. Anim. Sci. 2012, 90, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Schmilovitch, Z.; Katz, G.; Maltz, E.; Kutscher, M.I.; Sarig, M.; Halachmi, I.; Hoffman, A.; Egozi, H.; Uner, E. Spectroscopic Fluid Analyzer. US Patent 7,236,237, 26 June 2007. [Google Scholar]
- Halachmi, I.; Guarino, M.; Bewley, J.; Pastell, M. Smart Animal Agriculture: Application of Real-Time Sensors to Improve Animal Well-Being and Production. Annu. Rev. Anim. Biosci. 2019, 7, 403–425. [Google Scholar] [CrossRef] [PubMed]
- AlZahal, O.; Kebreab, E.; France, J.; Froetschel, M.; McBride, B. Ruminal Temperature May Aid in the Detection of Subacute Ruminal Acidosis. J. Dairy Sci. 2008, 91, 202–207. [Google Scholar] [CrossRef]
- Cantor, M.C.; Costa, J.H.C.; Bewley, J.M. Impact of Observed and Controlled Water Intake on Reticulorumen Temperature in Lactating Dairy Cattle. Animals 2018, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Antanaitis, R.; Juozaitienė, V.; Malašauskienė, D.; Televičius, M. Can rumination time and some blood biochemical parameters be used as biomarkers for the diagnosis ofsubclinical acidosis and subclinical ketosis? Vet. Anim. Sci. 2019, 8, 100077. [Google Scholar] [CrossRef]
- Albaaj, A.; Foucras, G.; Raboisson, D. High somatic cell counts and changes in milk fat and protein contents around insemination are negatively associated with conception in dairy cows. Theriogenology 2017, 88, 18–27. [Google Scholar] [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Rutkauskas, A.; Televičius, M.; Stasiulevičiūtė, I. Reticulorumen temperature and pH as indicators of the likelihood of reproductive success. J. Dairy Res. 2018, 85, 23–26. [Google Scholar] [CrossRef]
- AlZahal, O.; AlZahal, H.; Steele, M.; Van Schaik, M.; Kyriazakis, I.; Duffield, T.; McBride, B. The use of a radiotelemetric ruminal bolus to detect body temperature changes in lactating dairy cattle. J. Dairy Sci. 2011, 94, 3568–3574. [Google Scholar] [CrossRef]
- Cooper-Prado, M.J.; Long, N.M.; Wright, E.C.; Goad, C.L.; Wettemann, R.P. Relationship of ruminal temperature with parturition and estrus of beef cows1. J. Anim. Sci. 2011, 89, 1020–1027. [Google Scholar] [CrossRef]
- AlZahal, O.; Steele, M.; Valdes, E.; McBride, B. Technical note: The use of a telemetric system to continuously monitor ruminal temperature and to predict ruminal pH in cattle. J. Dairy Sci. 2009, 92, 5697–5701. [Google Scholar] [CrossRef] [PubMed]
- Antanaitis, R.; Žilaitis, V.; Juozaitiene, V.; Stoškus, R.; Televičius, M. Changes in reticulorumen content temperature and pH according to time of day and yearly seasons. Pol. J. Veter Sci. 2016, 19, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Colman, E.; Fokkink, W.; Craninx, M.; Newbold, J.; De Baets, B.; Fievez, V. Effect of induction of subacute ruminal acidosis on milk fat profile and rumen parameters. J. Dairy Sci. 2010, 93, 4759–4773. [Google Scholar] [CrossRef] [PubMed]
- Danscher, A.M.; Li, S.; Andersen, P.H.; Khafipour, E.; Kristensen, N.B.; Plaizier, J.C. Indicators of induced subacute ruminal acidosis (SARA) in Danish Holstein cows. Acta Veter Scand. 2015, 57, 39. [Google Scholar] [CrossRef] [PubMed]
- Inchaisri, C.; Chanpongsang, S.; Noordhuizen, J.; Hogeveen, H. The association of ruminal pH and some metabolic parameters with conception rate at first artificial insemination in Thai dairy cows. Trop. Anim. Health Prod. 2013, 45, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Suthar, V.; Burfeind, O.; Patel, J.; Dhami, A.; Heuwieser, W. Body temperature around induced estrus in dairy cows. J. Dairy Sci. 2011, 94, 2368–2373. [Google Scholar] [CrossRef]
- AlZahal, O.; Kebreab, E.; France, J.; McBride, B. A Mathematical Approach to Predicting Biological Values from Ruminal pH Measurements. J. Dairy Sci. 2007, 90, 3777–3785. [Google Scholar] [CrossRef]
- Plaizier, J.; Krause, D.; Gozho, G.; McBride, B. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Veter J. 2008, 176, 21–31. [Google Scholar] [CrossRef]
- Schmitz, R.; Schnabel, K.; Von Soosten, D.; Meyer, U.; Hüther, L.; Spiekers, H.; Rehage, J.; Dänicke, S. Changes of ruminal pH, rumination activity and feeding behaviour during early lactation as affected by different energy and fibre concentrations of roughage in pluriparous dairy cows. Arch. Anim. Nutr. 2018, 72, 1–20. [Google Scholar] [CrossRef]
- Pahl, C.; Hartung, E.; Grothmann, A.; Mahlkow-Nerge, K.; Haeussermann, A. Rumination activity of dairy cows in the 24 h before and after calving. J. Dairy Sci. 2014, 97, 6935–6941. [Google Scholar] [CrossRef]
- Khafipour, E.; Li, S.; Plaizier, J.C.; Krause, D.O. Rumen Microbiome Composition Determined Using Two Nutritional Models of Subacute Ruminal Acidosis. Appl. Environ. Microbiol. 2009, 75, 7115–7124. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Ikeda, A.; Tsuchiya, Y.; Ikuta, K.; Murayama, I.; Kanehira, M.; Okada, K.; Mizuguchi, H. Diagnosis of subacute ruminal acidosis (SARA) by continuous reticular pH measurements in cows. Veter Res. Commun. 2012, 36, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, G.; Okine, E.; Kennelly, J. Effects of Substituting Barley Grain with Corn on Ruminal Fermentation Characteristics, Milk Yield, and Milk Composition of Holstein Cows. J. Dairy Sci. 2001, 84, 2760–2769. [Google Scholar] [CrossRef]
- Esmaeili, M.; Khorvash, M.; Ghorbani, G.; Nasrollahi, S.; Saebi, M. Variation of TMR particle size and physical characteristics in commercial Iranian Holstein dairies and effects on eating behaviour, chewing activity, and milk production. Livest. Sci. 2016, 191, 22–28. [Google Scholar] [CrossRef]
- Bergk, N.; Swalve, H.H. Fat-to-protein-ratio in early lactation as an indicator of herdlife for first lactation dairy cows. Züchtungskunde 2011, 83, 89–103. [Google Scholar]
- Rossow, N. Nutzung der Ergebnisse der Milchleistungsprüfung für die Fütterungs- und Stoffwechselkontrolle. Portal-Rind. 2003. [Google Scholar]
- Oetzel, G.R. Clinical aspects of ruminal acidosis in dairy cattle. In Proceedings of the Thirty-Third Annual Conference, American Association of Bovine Practitioners, Rapid City, SD, USA, 21–23 September 2000; pp. 46–53. [Google Scholar]
- Nocek, J.E. Bovine Acidosis: Implications on Laminitis. J. Dairy Sci. 1997, 80, 1005–1028. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Keunen, J.E.; Walton, J.-P.; Duffield, T.F.; McBride, B.W. Effect of subacute ruminal acidosis on in situ digestion of mixed hay in lactating dairy cows. Can. J. Anim. Sci. 2001, 81, 421–423. [Google Scholar] [CrossRef]
- Gozho, G.; Plaizier, J.; Krause, D.; Kennedy, A.; Wittenberg, K. Subacute Ruminal Acidosis Induces Ruminal Lipopolysaccharide Endotoxin Release and Triggers an Inflammatory Response. J. Dairy Sci. 2005, 88, 1399–1403. [Google Scholar] [CrossRef]
- Khafipour, E.; Krause, D.; Plaizier, J. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 2009, 92, 1060–1070. [Google Scholar] [CrossRef]
- Peterse, D.J. [Nutrition as a possible factor in the pathogenesis of ulcers of the sole in cattle (author’s transl)]. Tijdschr. Diergeneeskd. 1979, 104, 966–970. [Google Scholar]
- Owens, F.N.; Secrist, D.S.; Hill, W.J.; Gill, D.R. Acidosis in cattle: A review. J. Anim. Sci. 1998, 76, 275. [Google Scholar] [CrossRef] [PubMed]
- Gianesella, M.; Morgante, M.; Cannizzo, C.; Stefani, A.; Dalvit, P.; Messina, V.; Giudice, E. Subacute Ruminal Acidosis and Evaluation of Blood Gas Analysis in Dairy Cow. Veter Med. Int. 2010, 2010, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gokce, G.; Citil, M.; Gunes, V.; Atalan, G. Effect of time delay and storage temperature on blood gas and acid-base values of bovine venous blood. Res. Veter Sci. 2004, 76, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Vajda, P.; Pinter, A.B.; Harangi, F.; Farkas, A.; Vastyan, A.M.; Oberritter, Z. Metabolic findings after colocystoplasty in children. Urol 2003, 62, 542–546. [Google Scholar] [CrossRef]
- Hamann, J.; Krömker, V. Potential of specific milk composition variables for cow health management. Livest. Prod. Sci. 1997, 48, 201–208. [Google Scholar] [CrossRef]

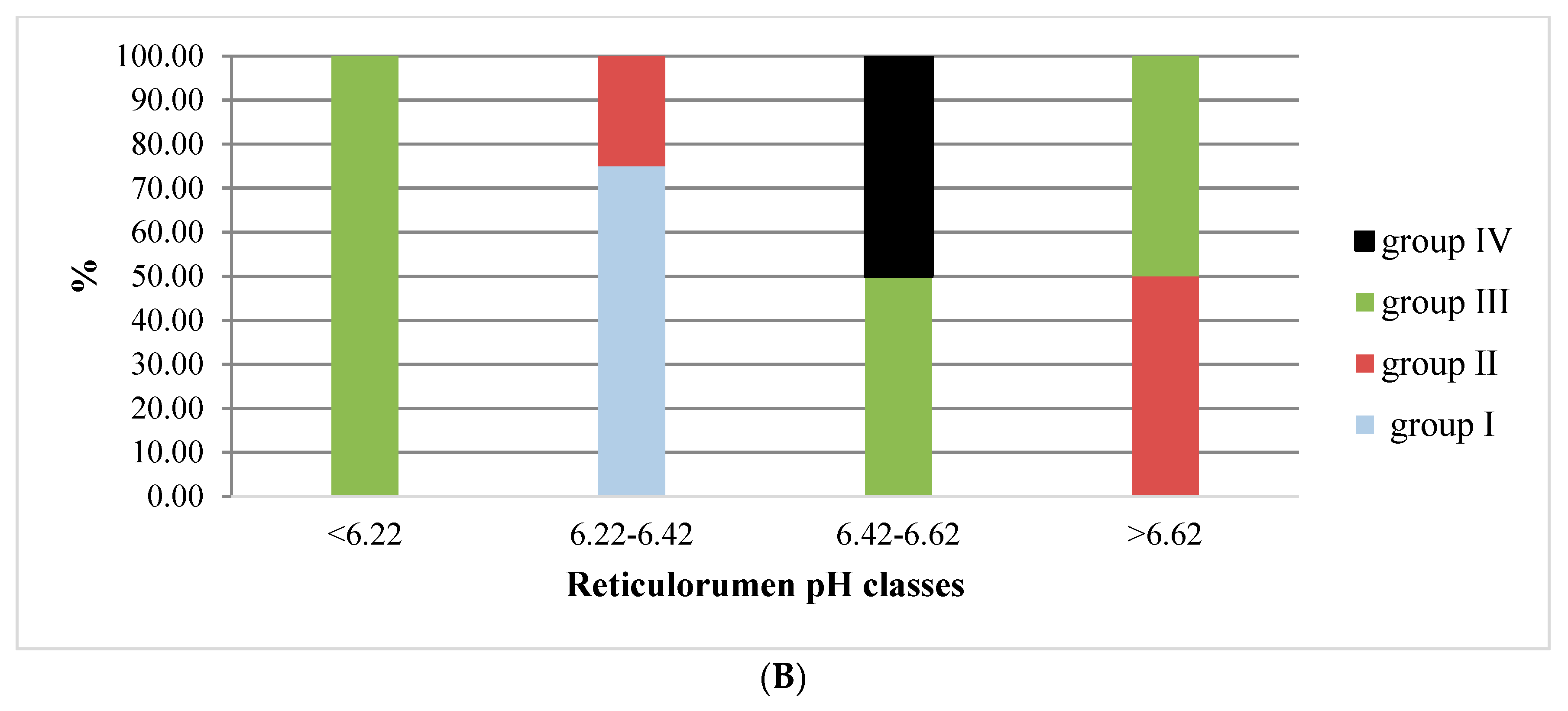

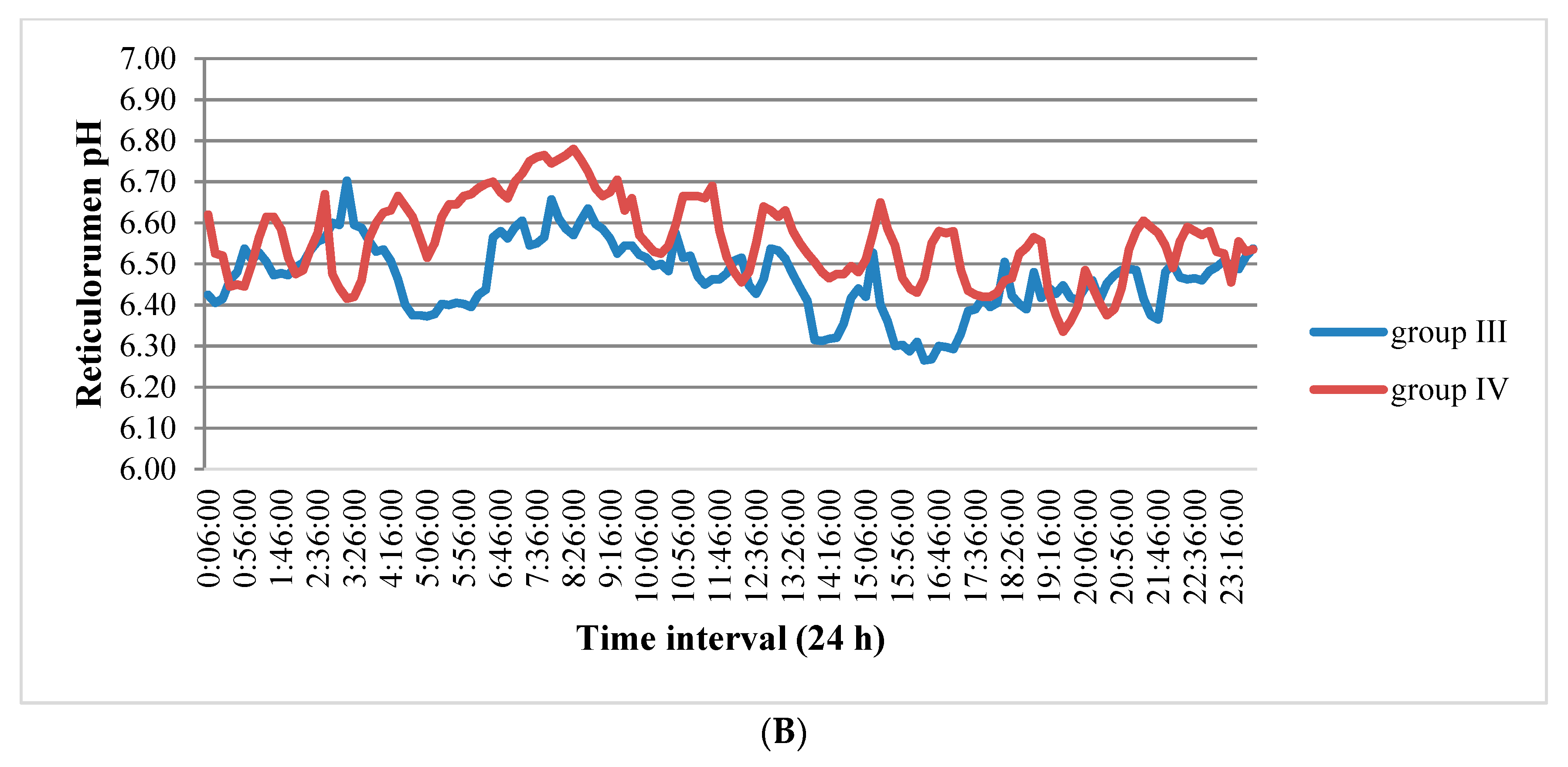


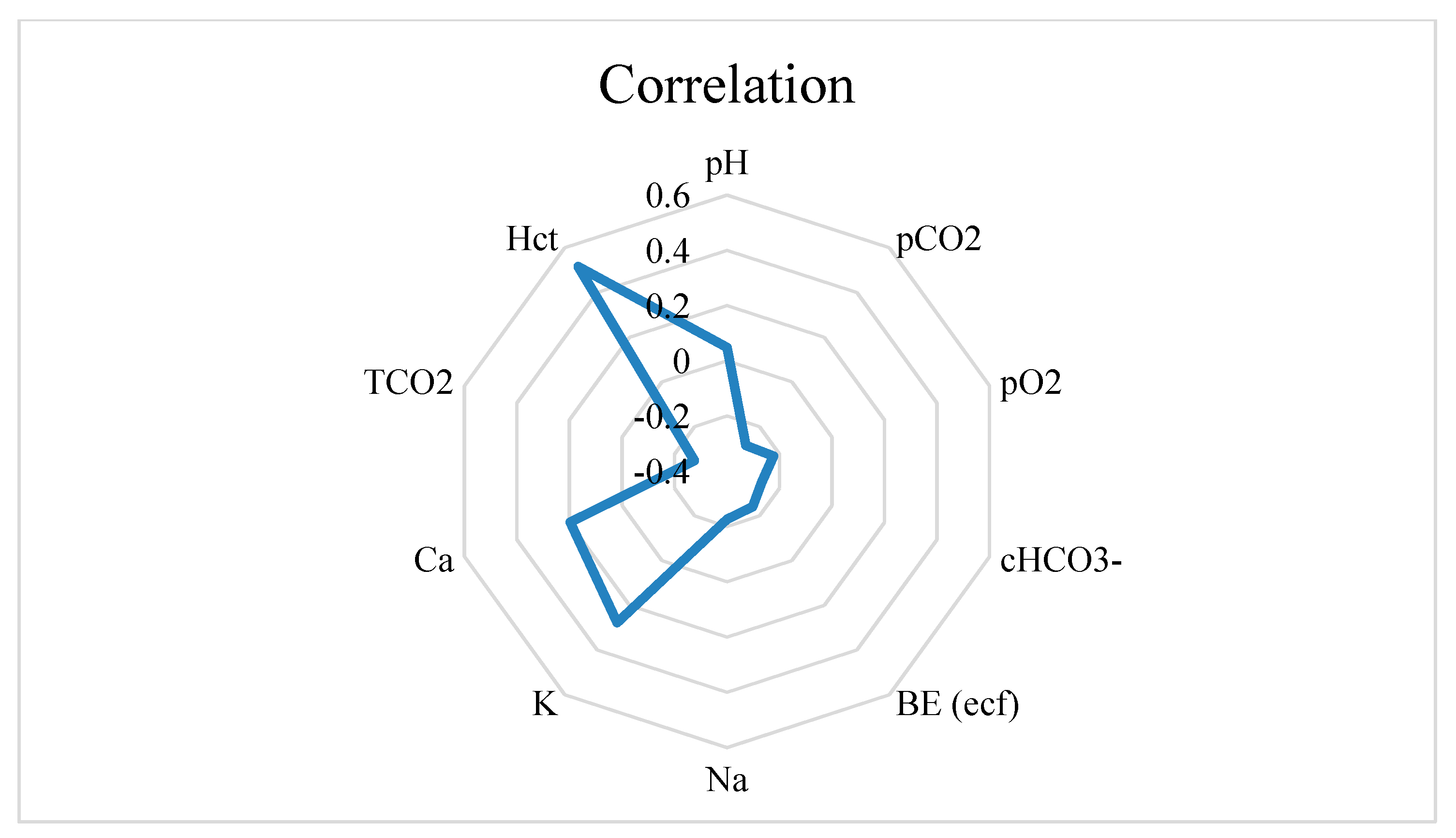
| Group | Days/Status of Reproduction | n | % |
|---|---|---|---|
| I | 15–30 d. postpartum | 35 | 36.8 |
| II | 1–34 d. after insemination | 20 | 21.1 |
| III | 35 d. after insemination (non-pregnant) | 20 | 21.1 |
| IV | 35 d. after insemination (pregnant) | 20 | 21.1 |
| Total | 95 | 100.0 | |
| Reticulorumen pH Class | AMS Parameters (M, SE) | AME Parameters (M, SE) | ||||
|---|---|---|---|---|---|---|
| 1 | Activity (steps/hour) | 10.24 | 1.239 | Fat (%) | 3.58 | 0.187 |
| 2 | 10.30 | 0.506 | 4.58 | 0.076 | ||
| 3 | 8.96 | 0.620 | 3.93 | 0.094 | ||
| 4 | 9.27 | 0.876 | 3.93 | 0.132 | ||
| 1 | Reticulorumen temperature (°C) | 38.78 | 0.078 | Protein (%) | 3.37 | 0.057 |
| 2 | 38.76 | 0.032 | 3.58 | 0.023 | ||
| 3 | 38.79 | 0.039 | 3.43 | 0.028 | ||
| 4 | 38.72 | 0.055 | 3.37 | 0.040 | ||
| 1 | BW (kg) | 756.00 | 61.710 | F/P | 1.06 | 0.048 |
| 2 | 593.67 | 25.193 | 1.28 | 0.020 | ||
| 3 | 630.75 | 30.855 | 1.15 | 0.024 | ||
| 4 | 630.00 | 43.636 | 1.17 | 0.031 | ||
| 1 | RT (min/d) | 487.00 | 24.947 | Lactose (%) | 4.53 | 0.028 |
| 2 | 423.50 | 10.185 | 4.61 | 0.011 | ||
| 3 | 436.75 | 12.474 | 4.59 | 0.014 | ||
| 4 | 478.50 | 17.640 | 4.56 | 0.020 | ||
| 1 | MY (kg/d) | 37.50 | 2.214 | SCC (tousd/mL) | 124.00 | 222.028 |
| 2 | 41.07 | 1.067 | 105.83 | 90.643 | ||
| 3 | 37.13 | 1.307 | 135.25 | 111.014 | ||
| 4 | 49.85 | 1.849 | 95.00 | 156.998 | ||
| Reticulorumen pH Class | Blood Parameters (M, SE) | Blood Parameters (M, SE) | ||||
|---|---|---|---|---|---|---|
| 1 | pH | 7.38 a | 0.016 | Na | 137.00 a | 0.601 |
| 2 | 7.43 b | 0.005 | 137.13 ab | 0.212 | ||
| 3 | 7.42 b | 0.008 | 137.25 ab | 0.3 | ||
| 4 | 7.43 b | 0.011 | 136.00 ac | 0.425 | ||
| 1 | pCO2 | 49.20 a | 2.204 | K | 3.90 a | 0.11 |
| 2 | 45.20 b | 0.779 | 4.10 a | 0.039 | ||
| 3 | 45.13 b | 1.102 | 4.00 a | 0.055 | ||
| 4 | 40.55 a | 1.558 | 4.30 b | 0.078 | ||
| 1 | pO2 | 49.90 a | 19.062 | Ca | 1.24 a | 0.016 |
| 2 | 67.11 a | 6.740 | 1.13 b | 0.006 | ||
| 3 | 61.45 a | 9.531 | 1.14 b | 0.008 | ||
| 4 | 52.00 a | 13.479 | 1.22 a | 0.011 | ||
| 1 | cHCO3- | 29.30 a | 1.288 | TCO2 | 29.20 a | 1.257 |
| 2 | 30.23 ab | 0.455 | 29.90 ab | 0.445 | ||
| 3 | 29.03 a | 0.644 | 28.78 a | 0.629 | ||
| 4 | 27.00 ac | 0.91 | 26.75 ac | 0.889 | ||
| 1 | BE (ecf) | 4.20 a | 1.372 | Hct | 24.00 a | 0.884 |
| 2 | 5.98 ab | 0.485 | 23.75 a | 0.313 | ||
| 3 | 4.48 a | 0.686 | 26.00 b | 0.442 | ||
| 4 | 2.70 ac | 0.97 | 27.00 b | 0.625 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antanaitis, R.; Juozaitienė, V.; Malašauskienė, D.; Televičius, M. Inline Reticulorumen pH as an Indicator of Cows Reproduction and Health Status. Sensors 2020, 20, 1022. https://doi.org/10.3390/s20041022
Antanaitis R, Juozaitienė V, Malašauskienė D, Televičius M. Inline Reticulorumen pH as an Indicator of Cows Reproduction and Health Status. Sensors. 2020; 20(4):1022. https://doi.org/10.3390/s20041022
Chicago/Turabian StyleAntanaitis, Ramūnas, Vida Juozaitienė, Dovilė Malašauskienė, and Mindaugas Televičius. 2020. "Inline Reticulorumen pH as an Indicator of Cows Reproduction and Health Status" Sensors 20, no. 4: 1022. https://doi.org/10.3390/s20041022
APA StyleAntanaitis, R., Juozaitienė, V., Malašauskienė, D., & Televičius, M. (2020). Inline Reticulorumen pH as an Indicator of Cows Reproduction and Health Status. Sensors, 20(4), 1022. https://doi.org/10.3390/s20041022






