An Optical Urate Biosensor Based on Urate Oxidase and Long-Lifetime Metalloporphyrins
Abstract
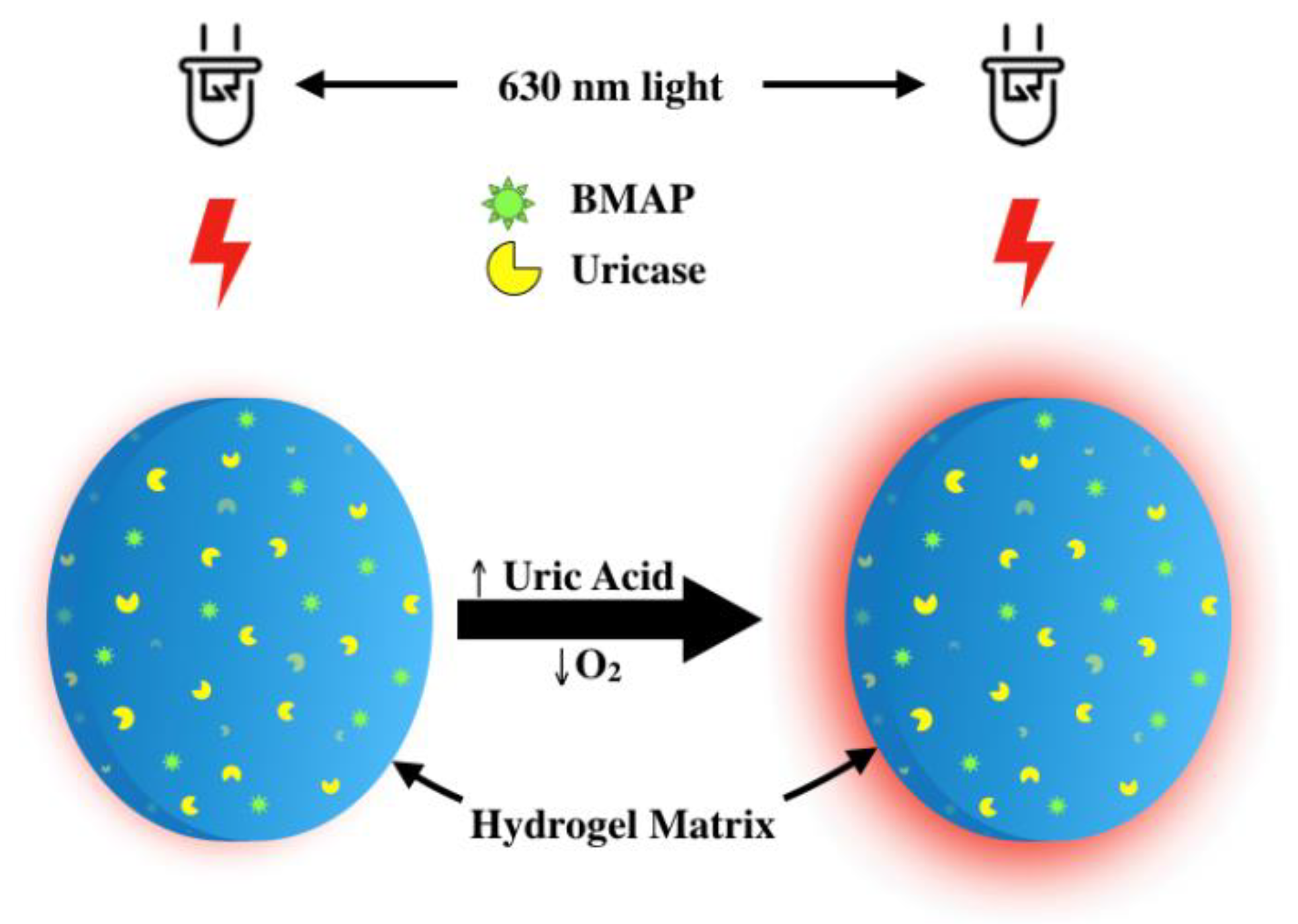
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instrumentation
2.3. Hydrogel Selection
2.4. Hydrogel Fabrication
2.5. Swelling Ratio
2.6. Oxygen Response
2.7. Optical Measurements
2.8. Urate Response
2.9. Selectivity
2.10. Storage Stability
3. Results and Discussion
3.1. Swelling Ratio
3.2. Absorbance and Emission Spectra of Urate Biosensors
3.3. Effect of Oxygen Concentration on Biosensor Response
3.4. Effect of Urate on Phosphorescence Lifetime
3.5. Selectivity
3.6. Storage Stability Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Ghaemi-Oskouie, F.; Shi, Y. The Role of Uric Acid as an Endogenous Danger Signal in Immunity and Inflammation. Curr. Rheumato. Rep. 2011, 13, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Martillo, M.A.; Nazzal, L.; Crittenden, D.B. The crystallization of monosodium urate. Curr Rheumatol Rep. 2014, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Fam, A.G. Gout, diet, and the insulin resistance syndrome. J. Rheumatol. 2002, 29, 1350. [Google Scholar] [PubMed]
- Ragab, G.; Elshahaly, M.; Bardin, T. Gout: An old disease in new perspective—A review. J. Adv. Res. 2017, 8, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Cleophas, M.C.; Joosten, L.A.; Stamp, L.K.; Dalbeth, N.; Woodward, O.M.; Merriman, T.R. ABCG2 polymorphisms in gout: Insights into disease susceptibility and treatment approaches. Pharmgenomics Pers. Med. 2017, 10, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Stiburkova, B.; Pavelcova, K.; Pavlikova, M.; Ješina, P.; Pavelka, K. The impact of dysfunctional variants of ABCG2 on hyperuricemia and gout in pediatric-onset patients. Arthritis Res. Ther. 2019, 21, 77. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-J.; Tseng, C.-C.; Yen, J.-H.; Chang, J.-G.; Chou, W.-C.; Chu, H.-W.; Chang, S.-J.; Liao, W.-T. ABCG2 contributes to the development of gout and hyperuricemia in a genome-wide association study. Sci. Rep. 2018, 8, 3137. [Google Scholar] [CrossRef]
- Choi, H.K.; Mount, D.B.; Reginato, A.M. Pathogenesis of gout. Ann. Internal Med. 2005, 143, 499–516. [Google Scholar] [CrossRef]
- Keenan, R.T. Limitations of the Current Standards of Care for Treating Gout and Crystal Deposition in the Primary Care Setting: A Review. Clin. Ther. 2017, 39, 430–441. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.D.; Pinto, A.B. Clinical manifestations and treatment of gout. Primary Care Update for OB/GYNS 2003, 10, 19–23. [Google Scholar] [CrossRef]
- Pittman, J.R.; Bross, M.H. Diagnosis and management of gout. Am. family phy. 1999, 59, 1799. [Google Scholar]
- Wallace, S.L.; Robinson, H.; Masi, A.T.; Decker, J.L.; Mccarty, D.J.; Yü, T.s.f. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977, 20, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, A.; Morlock, R.; Becker, M.A. A revised estimate of the burden of illness of gout. Curr. Ther. Res. Clin. Exp. 2013, 75, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Edwards, N.L.; Sundy, J.S.; Forsythe, A.; Blume, S.; Pan, F.; Becker, M.A. Work productivity loss due to flares in patients with chronic gout refractory to conventional therapy. J. Med. Econ. 2011, 14, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Paraskos, J.; Berke, Z.; Cook, J.; Miner, J.N.; Braddock, M.; Platt, A.; Hughes, G. An analytical comparison between point-of-care uric acid testing meters. Expert Rev. Mol. Diagnostics 2016, 16, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Lehto, S. Serum uric acid is a strong predictor of stroke in patients with non–insulin-dependent diabetes mellitus. Stroke 1998, 29, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, S.K.; Dipu, M.T.; Mehra, U.R.; Singh, P.; Verma, A.K.; Ramgaokar, J.S. Improved HPLC method for the simultaneous determination of allantoin, uric acid and creatinine in cattle urine. J. Chromatogr. B 2006, 832, 134–137. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Lu, W.; Liao, H.; Liao, F. Uricase based methods for determination of uric acid in serum. Microchim. Acta 2009, 164, 1–6. [Google Scholar] [CrossRef]
- Galbán, J.; Andreu, Y.; Almenara, M.J.; de Marcos, S.; Castillo, J.R. Direct determination of uric acid in serum by a fluorometric-enzymatic method based on uricase. Talanta 2001, 54, 847–854. [Google Scholar] [CrossRef]
- Unruh, R.M.; Roberts, J.R.; Nichols, S.P.; Gamsey, S.; Wisniewski, N.A.; McShane, M.J. Preclinical Evaluation of Poly(HEMA-co-acrylamide) Hydrogels Encapsulating Glucose Oxidase and Palladium Benzoporphyrin as Fully Implantable Glucose Sensors. J Diabetes Sci Technol 2015, 9, 985–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrus, L.P.; Unruh, R.; Wisniewski, N.A.; McShane, M.J. Characterization of Lactate Sensors Based on Lactate Oxidase and Palladium Benzoporphyrin Immobilized in Hydrogels. Biosensors (Basel) 2015, 5, 398–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayamori, Y.; Katayama, Y.; Urata, T. Nonenzymatic elimination of ascorbic acid in clinical samples. Clin. Biochem. 2000, 33, 25–29. [Google Scholar] [CrossRef]
- Schrenkhammer, P.; Wolfbeis, O.S. Fully reversible optical biosensors for uric acid using oxygen transduction. Biosens. Bioelectron. 2008, 24, 994–999. [Google Scholar] [CrossRef]
- Staudinger, C.; Borisov, S.M. Long-wavelength analyte-sensitive luminescent probes and optical (bio)sensors. Methods Appli. Fluoresc. 2015, 3, 042005. [Google Scholar] [CrossRef]
- Imran, M.; Ramzan, M.; Qureshi, K.A.; Khan, A.M.; Tariq, M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Quaranta, M.; Borisov, S.M.; Klimant, I. Indicators for optical oxygen sensors. Bioanal. Rev. 2012, 4, 115–157. [Google Scholar] [CrossRef] [Green Version]
- Vinogradov, S.A.; Wilson, D.F. Metallotetrabenzoporphyrins. New phosphorescent probes for oxygen measurements. J. Chem. Soc., Perkin Trans. 2 1995. [Google Scholar] [CrossRef]
- Niedermair, F.; Borisov, S.M.; Zenkl, G.; Hofmann, O.T.; Weber, H.; Saf, R.; Klimant, I. Tunable Phosphorescent NIR Oxygen Indicators Based on Mixed Benzo- and Naphthoporphyrin Complexes. Inorg. Chem. 2010, 49, 9333–9342. [Google Scholar] [CrossRef]
- Borisov, S.M.; Nuss, G.; Haas, W.; Saf, R.; Schmuck, M.; Klimant, I. New NIR-emitting complexes of platinum(II) and palladium(II) with fluorinated benzoporphyrins. J. Photochem. Photobiol. A 2009, 201, 128–135. [Google Scholar] [CrossRef]
- Peppas, N.A.; Moynihan, H.J.; Lucht, L.M. The structure of highly crosslinked poly(2-hydroxyethyl methacrylate) hydrogels. J. Biomed. Mat. Res. 1985, 19, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Macret, M.; Hild, G. Hydroxyalkyl methacrylates: Hydrogel formation based on the radical copolymerization of 2-hydroxyethylmethacrylate and 2,3-dihydroxypropylmethacrylate. Polymer 1982, 23, 748–753. [Google Scholar] [CrossRef]
- Dušek, K.; Janáček, J. Hydrophilic gels based on copolymers of 2-hydroxyethyl methacrylate with methacrylamide and acrylamide. J. Appl. Polym. Sci. 1975, 19, 3061–3075. [Google Scholar] [CrossRef]
- Montheard, J.-P.; Chatzopoulos, M.; Chappard, D. 2-Hydroxyethyl Methacrylate (HEMA): Chemical Properties and Applications in Biomedical Fields. J. Macromol. Sci. Part C 1992, 32, 1–34. [Google Scholar] [CrossRef]
- Lin, D.C.; Yurke, B.; Langrana, N.A. Mechanical properties of a reversible, DNA-crosslinked polyacrylamide hydrogel. J. Biomech. Eng. 2004, 126, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.R.; Park, J.; Helton, K.; Wisniewski, N.; McShane, M.J. Biofouling of polymer hydrogel materials and its effect on diffusion and enzyme-based luminescent glucose sensor functional characteristics. J Diabetes Sci. Technol. 2012, 6, 1267–1275. [Google Scholar] [CrossRef] [Green Version]
- Ratner, B.D.; Hoffman, A.S. Synthetic Hydrogels for Biomedical Applications. In Hydrogels for Medical and Related Applications; American Chemical Society: Washington, DC, USA, 1976; pp. 1–36. [Google Scholar]
- Rapado, M.; Peniche, C. Synthesis and characterization of pH and temperature responsive poly(2-hydroxyethyl methacrylate-co-acrylamide) hydrogels. Polímeros 2015, 25, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Unruh, R.M. Copolymer hydrogels as fully implantable optical biosensors: Investigating design paradigms to achieve long-term preclinical function. Ph.D Thesis, Texas A & M University, College Station, TX, USA, 9 September 2017. [Google Scholar]
- Yasuda, K.; Okajima, K.; Kamide, K. Study on Alkaline Hydrolysis of Polyacrylamide by 13C NMR. Polym. J. 1988, 20, 1101–1107. [Google Scholar] [CrossRef] [Green Version]
- Bocourt, M.; Bada, N.; Acosta, N.; Bucio, E.; Peniche, C. Synthesis and characterization of novel pH-sensitive chitosan-poly(acrylamide-co-itaconic acid) hydrogels. Polym. Int. 2014, 63, 1715–1723. [Google Scholar] [CrossRef]
- Lin, M.Z.; McKeown, M.R.; Ng, H.-L.; Aguilera, T.A.; Shaner, N.C.; Campbell, R.E.; Adams, S.R.; Gross, L.A.; Ma, W.; Alber, T.; et al. Autofluorescent Proteins with Excitation in the Optical Window for Intravital Imaging in Mammals. Chem. Bio. 2009, 16, 1169–1179. [Google Scholar] [CrossRef] [Green Version]
- Siu, Y.P.; Leung, K.T.; Tong, M.K.; Kwan, T.H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am. J. Kidney Dis. 2006, 47, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Güemes, M.; Rahman, S.A.; Hussain, K. What is a normal blood glucose? Arch. Dis. Child. 2016, 101, 569. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, C.G. Uricase purification and properties. Biochem. J. 1939, 33, 1901–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machida, Y.; Nakanishi, T. Purification and Properties of Uricase from Enterobacter cloacae. Agric. Biol. Chem. 1980, 44, 2811–2815. [Google Scholar] [CrossRef]
- Liu, J.; Li, G.X.; Liu, H.; Zhou, X. Purification and Properties of Uricase from Candida Sp. and Its Application in Uric Acid Analysis in Serum. Ann. N.Y. Acad. Sci. 1995, 750, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, F.; Rossi, M.; Schmidell, W.; Ninow, J.L. Determination of Oxygen Solubility in Liquid Media. ISRN Chem. Eng. 2012, 2012, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Su, L.; Manuzzi, D.; de los Monteros, H.V.E.; Jia, W.; Huo, D.; Hou, C.; Lei, Y. Ultrasensitive and selective non-enzymatic glucose detection using copper nanowires. Biosens. Bioelectron. 2012, 31, 426–432. [Google Scholar] [CrossRef]
- Kulkarni, T.; Slaughter, G. Application of semipermeable membranes in glucose biosensing. Membranes 2016, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.; Fiore, V. Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef] [Green Version]
- Mariani, A.M.; Natoli, M.E.; Kofinas, P. Enzymatic activity preservation and protection through entrapment within degradable hydrogels. Biotechnol. Bioeng. 2013, 110, 2994–3002. [Google Scholar] [CrossRef]
- Teles, F.R.R.; Fonseca, L.P. Applications of polymers for biomolecule immobilization in electrochemical biosensors. Mater. Sci. Eng., C 2008, 28, 1530–1543. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Yuwen, L.-X.; Peng, L.-J. Parameters affecting the performance of immobilized enzyme. J. Chem. 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.M.; Reyes, C.; Lopez, G.P. Common causes of glucose oxidase instability in in vivo biosensing: A brief review. J. diabetes sci. Tech. 2013, 7, 1030–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grootveld, M.; Halliwell, B. Measurement of allantoin and uric acid in human body fluids. A potential index of free-radical reactions in vivo? Biochem. J. 1987, 243, 803–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kono, H.; Chen, C.-J.; Ontiveros, F.; Rock, K.L. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J. Clin. Investig. 2010, 120, 1939–1949. [Google Scholar] [CrossRef]





| Hydrogel Composition | Swelling Ratio |
|---|---|
| 50:50 poly(HEMA-co-AAm) | 249 ± 9.81a |
| 75:25 poly(HEMA-co-AAm) | 131 ± 4.32b |
| 90:10 poly(HEMA-co-AAm) | 100 ± 6.19c |
| Composition | Ksv (% O2)–1 |
|---|---|
| 50:50 poly(HEMA-co-AAm) | 0.32 ± 0.06 |
| 75:25 poly(HEMA-co-AAm) | 0.29 ± 0.02 |
| 90:10 poly(HEMA-co-AAm) | 0.27 ± 0.01 |
| Composition | LOD (mg/dL) | Sensitivity µs/(mg/dL) |
|---|---|---|
| 50:50 poly(HEMA-co-AAm) | 2.28 ± 1.23 | 3.71 ± 0.17 |
| 75:25 poly(HEMA-co-AAm) | 4.01 ± 1.18 | 2.92 ± 0.36 |
| 90:10 poly(HEMA-co-AAm) | 3.13 ± 0.72 | 2.46 ± 0.09 |
| Concentration (mg/dL) | Percent Change | |
|---|---|---|
| Urate | 5 | 28.92 ± 2.10 |
| Ascorbic Acid | 1.8 | 1.17 ± 0.19 |
| Glucose | 90 | 3.15 ± 2.29 |
| Sucrose | 2.7 | 5.14 ± 3.25 |
| Fructose | 0.15 | 0.99 ± 0.61 |
| Urea | 36 | 0.45 ± 0.13 |
| Allantoin | 0.25 | 1.14 ± 0.61 |
| Creatine | 2.6 | 0.73 ± 0.51 |
| Creatinine | 0.9 | 0.63 ± 0.33 |
| Acetaminophen | 0.9 | 0.48 ± 0.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falohun, T.; McShane, M.J. An Optical Urate Biosensor Based on Urate Oxidase and Long-Lifetime Metalloporphyrins. Sensors 2020, 20, 959. https://doi.org/10.3390/s20040959
Falohun T, McShane MJ. An Optical Urate Biosensor Based on Urate Oxidase and Long-Lifetime Metalloporphyrins. Sensors. 2020; 20(4):959. https://doi.org/10.3390/s20040959
Chicago/Turabian StyleFalohun, Tokunbo, and Michael J. McShane. 2020. "An Optical Urate Biosensor Based on Urate Oxidase and Long-Lifetime Metalloporphyrins" Sensors 20, no. 4: 959. https://doi.org/10.3390/s20040959




