Electrochemical Sensor Based on a Carbon Veil Modified by Phytosynthesized Gold Nanoparticles for Determination of Ascorbic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
2.3. Procedures
2.3.1. Synthesis and Characterization of Gold Nanoparticles
2.3.2. Manufacturing of the Sensor (Au-gr/CVE)
2.3.3. Electrochemical Measurements
2.4. Statistical Analysis and Data Treatment
3. Results
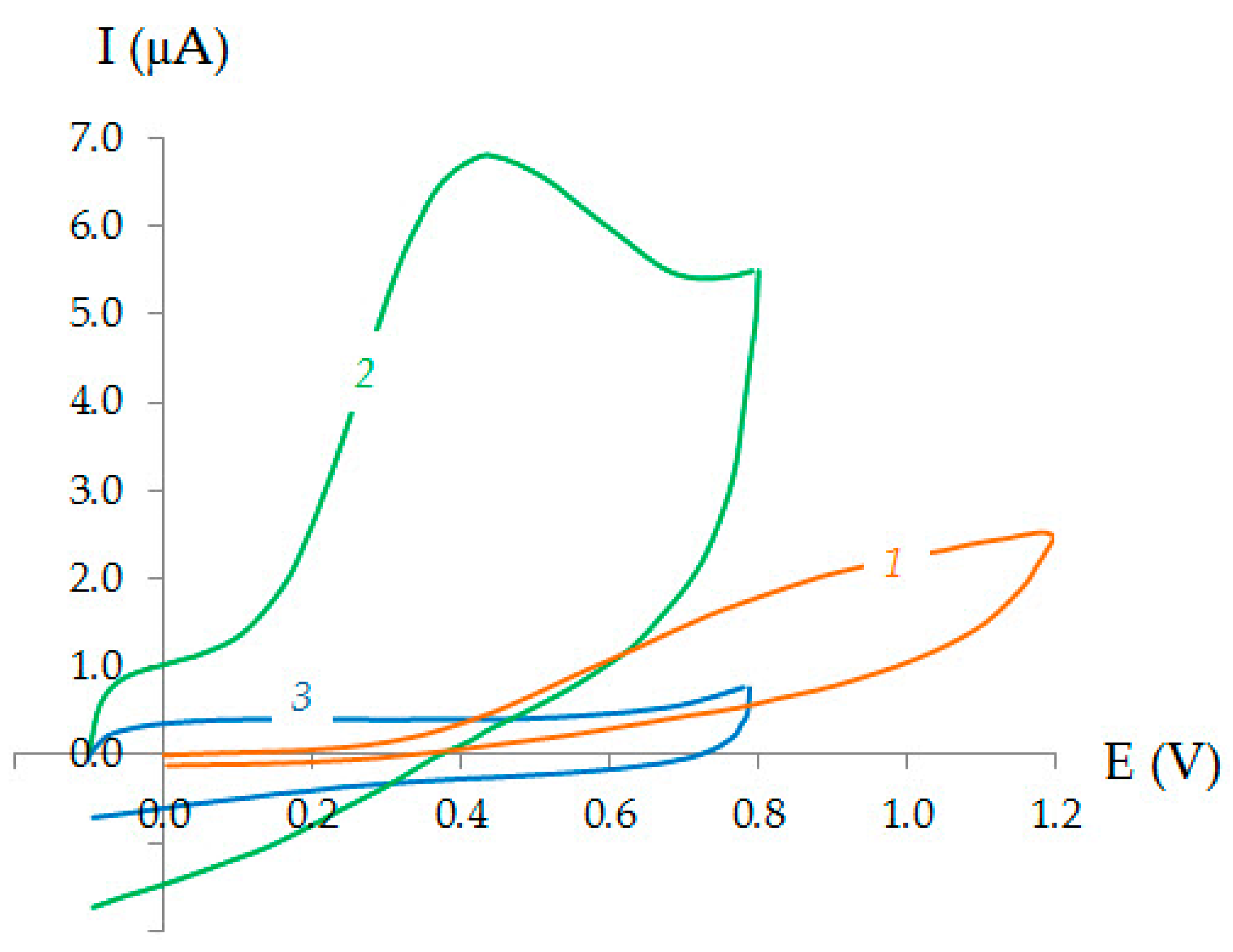
3.1. Electrochemical Behavior of Ascorbic Acid
3.2. Characterization of CVE and Au-gr/CVE
3.3. Electrooxidation of Ascorbic Acid on Au-gr/CVE
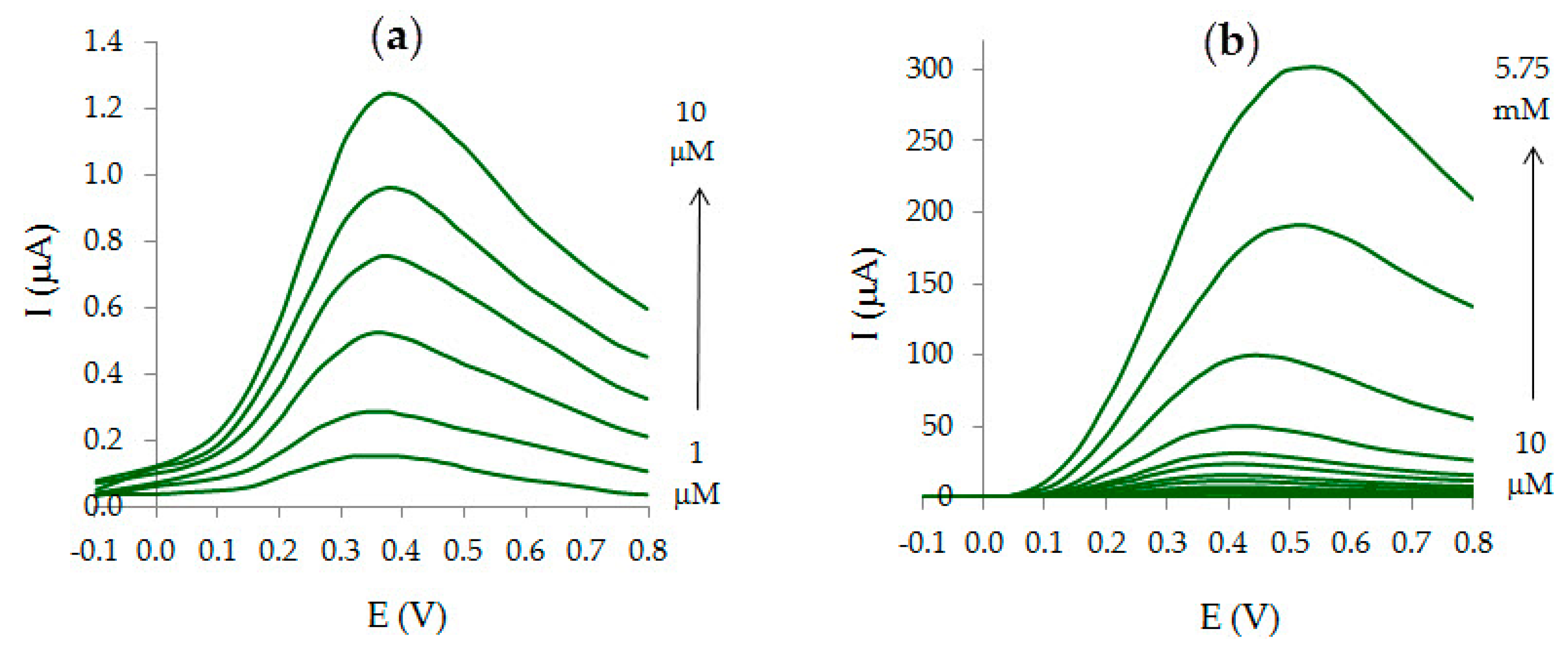
3.4. Analytic Characteristics of Au-gr/CVE
3.5. Determination of Ascorbic Acid in Fruit Juices
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brainina, K.; Tarasov, A.; Khamzina, E.; Stozhko, N.; Vidrevich, M. Contact hybrid potentiometric method for on-site and in situ estimation of the antioxidant activity of fruits and vegetables. Food Chem. 2020, 309, 125703. [Google Scholar] [CrossRef] [PubMed]
- Al Majidi, M.I.H.; Al-Qubury, H.Y. Determination of vitamin C (ascorbic acid) contents in various fruit and vegetable by UV-spectrophotometry and titration methods. J. Chem. Pharm. Sci. 2016, 9, 2972–2974. [Google Scholar]
- Da Silva, T.L.; Aguiar-Oliveira, E.; Mazalli, M.R.; Kamimura, E.S.; Maldonado, R.R. Comparison between titrimetric and spectrophotometric methods for quantification of vitamin C. Food Chem. 2017, 224, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Salkic, M.; Selimovic, A. Spectrophotometric determination of L-ascorbic acid in pharmaceuticals based on its oxidation by potassium peroxymonosulfate and hydrogen peroxide. Croat. Chem. Acta 2015, 88, 73–79. [Google Scholar] [CrossRef]
- Fatin Najwa, R.; Azrina, A. Comparison of vitamin C content in citrus fruits by titration and high performance liquid chromatography (HPLC) methods. Int. Food Res. J. 2017, 24, 726–733. [Google Scholar]
- Cunha-Santos, E.C.E.; Viganó, J.; Neves, D.A.; Martinez, J.; Godoy, H.T. Vitamin C in camu-camu: Evaluation of extraction and analytical methods. Food Res. Int. 2019, 115, 160–166. [Google Scholar] [CrossRef]
- Zuo, R.; Zhou, S.; Zuo, Y.; Deng, Y. Determination of creatinine, uric and ascorbic acid in bovine milk and orange juice by hydrophilic interaction HPLC. Food Chem. 2015, 182, 242–245. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, P.; Wang, X.; Chen, W.; Wu, L.; Wang, Q. Determination of vitamine C in fruits and vegetables by ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Chin. J. Chromatogr. 2016, 34, 1048–1054. [Google Scholar] [CrossRef]
- Kong, W.; Wu, D.; Li, G.; Chen, X.; Gong, P.; Sun, Z.; Chen, G.; Xia, L.; You, J.; Wu, Y. A facile carbon dots based fluorescent probe for ultrasensitive detection of ascorbic acid in biological fluids via non-oxidation reduction strategy. Talanta 2017, 165, 677–684. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Serban, A.I.; Fafaneata, C. Electrochemical methods for ascorbic acid determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Skrovankova, S.; Mlcek, J.; Sochor, J.; Baron, M.; Kynicky, J.; Jurikova, T. Determination of ascorbic acid by electrochemical techniques and other methods. Int. J. Electrochem. Sci. 2015, 10, 2421–2431. [Google Scholar]
- Chang, S.K.; Ismail, A.; Daud, Z.A.M. Ascorbic acid: Properties, determination and uses. In Encyclopedia of Food and Health, 1st ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Wang, H.; Xiao, L.-G.; Chu, X.-F.; Chi, Y.-D.; Yang, X.-T. Rational design of gold nanoparticle/graphene hybrids for simultaneous electrochemical determination of ascorbic acid, dopamine and uric acid. Chin. J. Anal. Chem. 2016, 44, e1617–e1625. [Google Scholar] [CrossRef]
- Ghanbari, K.; Hajian, A. Electrochemical characterization of Au/ZnO/PPy/RGO nanocomposite and its application for simultaneous determination of ascorbic acid, epinephrine, and uric acid. J. Electroanal. Chem. 2017, 801, 466–479. [Google Scholar] [CrossRef]
- Ji, D.Z.; Liu, Z.X.; Liu, L.; Low, S.S.; Lu, Y.L.; Yu, X.J.; Zhu, L.; Li, C.D.; Liu, Q.J. Smartphone-based integrated voltammetry system for simultaneous detection of ascorbic acid, dopamine, and uric acid with graphene and gold nanoparticles modified screen-printed electrodes. Biosens. Bioelectron. 2018, 119, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Scremin, J.; Barbosa, E.C.M.; Salamanca-Neto, C.A.R.; Camargo, P.H.C.; Sartori, E.R. Amperometric determination of ascorbic acid with a glassy carbon electrode modified with TiO2-gold nanoparticles integrated into carbon nanotubes. Microchim. Acta 2018, 185, 251. [Google Scholar] [CrossRef]
- Stozhko, N.Y.; Malakhova, N.A.; Byzov, I.V.; Brainina, K.Z. Electrodes in Stripping Voltammetry: From a Macro- to a Micro- and Nano-Structured Surface. J. Anal. Chem. 2009, 64, 1148–1157. [Google Scholar] [CrossRef]
- Kuang, H.Y.; He, J.H.; Xu, Q.; Song, Z.R. Determination of Ascorbic Acid Based on Gold Nanoparticles-L-Alanine/GCE. Adv. Mater. Res. 2011, 214, 498–502. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, F.; Xu, S.; Xia, Y.; Huang, W.; Li, Z. Fabrication of nanoflower-like dendritic Au and polyaniline composite nanosheets at gas/liquid interface for electrocatalytic oxidation and sensing of ascorbic acid. Electrochem. Commun. 2013, 30, 46–50. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Bukharinova, M.; Vikulova, E. Nanomaterials: Electrochemical properties and application in sensors. Phys. Sci. Rev. 2019, 3, 8050. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Galperin, L.G.; Bukharinova, M.A.; Stozhko, N.Y. Mathematical modeling and experimental study of electrode processes. J. Solid State Electrochem. 2015, 19, 599–606. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Stozhko, N.Y.; Bukharinova, M.A.; Galperin, L.G.; Vidrevich, M.B.; Murzakaev, A.M. Mathematical modeling and experimental data of the oxidation of ascorbic acid on electrodes modified by nanoparticles. J. Solid State Electrochem. 2016, 20, 2323–2330. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Stozhko, N.Y.; Shalygina, Z.V. Surface Microreliefs and Voltage–Current Characteristics of Gold Electrodes and Modified Thick-Film Graphite-Containing Electrodes. J. Anal. Chem. 2004, 59, 753–759. [Google Scholar] [CrossRef]
- Stozhko, N.; Bukharinova, M.; Galperin, L.; Brainina, K. Nanostructured Sensor Based on Gold Nanoparticles and Nafion for Determination of Uric Acid. Biosensors 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stozhko, N.Y.; Bukharinova, M.A.; Khamzina, E.I.; Tarasov, A.V.; Vidrevich, M.B.; Brainina, K.Z. The Effect of the Antioxidant Activity of Plant Extracts on the Properties of Gold Nanoparticles. Nanomaterials 2019, 9, 1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timoszyk, A. A review of the biological synthesis of gold nanoparticles using fruit extracts: Scientific potential and application. Bull. Mater. Sci. 2018, 41, 154. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, K.S.; Husen, A. Recent advances in plant-mediated engineered gold nanoparticles and their application in biological system. J. Trace Elem. Med. Biol. 2017, 40, 10–23. [Google Scholar] [CrossRef]
- Shin, D.; Shen, C.; Sanghadasa, M. Breathable 3D supercapacitors based on activated carbon fiber veil. Adv. Mater. Technol. 2018, 3, 1800209. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Microbial fuel cells based on carbon veil electrodes: Stack configuration and scalability. Int. J. Energy Res. 2008, 32, 1228–1240. [Google Scholar] [CrossRef]
- Banerjee, R.; Bevilacqua, N.; Mohseninia, A.; Wiedemann, B.; Wilhelm, F.; Scholta, J.; Zeis, R. Carbon felt electrodes for redox flow battery: Impact of compression on transport on properties. J. Energy Storage 2019, 26, 100997. [Google Scholar] [CrossRef]
- Duarte, K.D.Z.; Frattini, D.; Kwon, Y. High performance yeast-based microbial fuels cells by surfacant-mediated gold nanoparticles grown atop a carbon felt anode. Appl. Energy 2019, 256, 113912. [Google Scholar] [CrossRef]
- Tools4FRP. Available online: https://www.tools4frp.com/carbon-fibre-veils/ (accessed on 3 December 2019).
- Zhang, X.F.; Li, D.; Liu, K.; Tong, J.; Yi, X.S. Flexible grapheme-coated carbon fober veil/polydimethylsiloxane mats as electrothermal materials with rapid responsiveness. Int. J. Lightweight Mater. Manuf. 2019, 2, 241–249. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Gu, Y.; Gong, Y.; Liang, J.; Wang, S.; Zhang, Z. Resistance heating forming process besed on carbon fiber veil for continuous glass fiber reinforced polypropylene. J. Reinf. Plast. Compos. 2018, 37, 366–380. [Google Scholar] [CrossRef]
- Wei, W.; Wu, S.-G. Study of electrooxidation behavior of nitrite on gold nanoparticles/graphitizing carbon felt electrode and its analytical application. Chin. J. Anal. Chem. 2019, 47, e19014–e19020. [Google Scholar] [CrossRef]
- Kuntolaksono, S.; Matsuura, H. Coulometric analysis of nitrite using electrochemically activated carbon felt electrode. Sens. Mater. 2019, 31, 1215–1224. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, J.; Tricard, S.; Yu, L.; Fang, J. A sensitive and selective electrochemical sensor based on N, P-doped molybdenum carbide@carbon/Prussian blue/graphite felt composite electrode for the detection dopamine. Anal. Chim. Acta 2020, 1094, 80–89. [Google Scholar] [CrossRef]
- Manjubaashini, N.; Sephra, P.J.; Nehru, K.; Sivakumar, M.; Thangadurai, T.D. Electrochemical determination of ATP at rhodamine6G capped gold a nanoparticles modified carbon felt electrode at pH 7.2. Sens. Actuators B 2019, 281, 1054–1062. [Google Scholar] [CrossRef]
- Davies, T.J. Anodic stripping voltammetry with graphite felt electrodes for the trace analysis of silver. Analyst 2016, 141, 4742–4748. [Google Scholar] [CrossRef] [Green Version]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Bukharinova, M.; Khamzina, E.; Vidrevich, M. Potentiometric method of plant microsuspensions antioxidant activity determination. Food Chem. 2019, 278, 653–658. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Galperin, L.G.; Vikulova, E.V.; Stozhko, N.Y.; Murzakaev, A.M.; Timoshenkova, O.R.; Kotov, Y.A. Gold nanoparticles electrooxidation: Comparison of theory and experiment. J. Solid State Electrochem. 2011, 15, 1049–1056. [Google Scholar] [CrossRef]
- Burns, D.T.; Danzer, K.; Townshend, A. Use of the terms “recovery” and “apparent recovery” in analytical procedures (IUPAC Recommendations 2002). Pure Appl. Chem. 2003, 74, 2201–2205. [Google Scholar] [CrossRef]
- Fotouhi, L.; Fatollahzadeh, M.; Heravi, M.M. Electrochemical Behavior and Voltammetric Determination of Sulfaguanidine at a Glassy Carbon Electrode Modified With a Multi-Walled Carbon Nanotube. Int. J. Electrochem. Sci. 2012, 7, 3919–3928. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons Inc: Hoboken, NJ, USA, 2001. [Google Scholar]
- Jayant, I. Gowda Sharanappa, T. Nandibewoor Electrochemical behavior of paclitaxel and its determination at glassy carbon electrode. Asian J. Pharm. Sci. 2014, 9, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Eisele, T.A.; Drake, S.R. The partial compositional characteristics of apple juice from 175 apple varieties. J. Food Compos. Anal. 2005, 18, 213–221. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Negulescu, G.P.; Pisoschi, A. Determination of ascorbic acid content of some fruit juices and wine by voltammetry performed at Pt and carbon paste electrodes. Molecules 2011, 16, 1349. [Google Scholar] [CrossRef] [PubMed]
- Haile Reda, A.; Guesh, F. Determination of Ascorbic Acid in Citrus Sinensis and Ananas Comosus Using Poly(3, 4-Ethylenedioxythiophene) Modified Glassy Carbon Electrode. Am. J. Appl. Chem. 2016, 4, 1–7. [Google Scholar] [CrossRef]
- Wonsawat, W. Determination of Vitamin C (ascorbic acid) in orange juices product. Int. J. Mater. Metall. Eng. 2014, 8, 6. [Google Scholar]
- Bitew, Z.; Amare, M. Electrochemical determination of ascorbic acid in pharmaceutical tablets using carbon paste electrode. Org. Med. Chem. Int. J. 2019, 8, 555749. [Google Scholar] [CrossRef]
- Kong, Y.; Shan, X.; Ma, J.; Chen, M.; Chen, Z. A novel voltammetric sensor for ascorbic acid based on molecularly imprinted poly (o-phenylenediamine-co-o-aminophenol). Anal. Chim. Acta 2014, 809, 54–60. [Google Scholar] [CrossRef]
- Samseya, J.; Srinivasan, R.; Chang, Y.-T.; Tsao, C.-W.; Vasantha, V.S. Fabrication and characterization of high performance polypyrrole modified microarray sensor for ascorbic acid determination. Anal. Chim. Acta 2013, 793, 11–18. [Google Scholar] [CrossRef]
- Abraha, T.; Sergawie, A. Assessment of some selected beverages and fresh edible vegetables as nutritional source of vitamin C (ascorbic acid) by cyclic and square wave voltammetry. Int. J. Sci. Eng. Investig. 2014, 3, 39–49. [Google Scholar]
- Nezamzaden, A.; Amini, M.K.; Faghihian, H. Square-wave voltammetric determination of ascorbic acid based on its electrocatalytic oxidation at zeolite-modified carbon-paste electrode. Int. J. Electrochem. Sci. 2007, 2, 583–594. [Google Scholar]
- Tadese, A.; Subramanian, P.A.; Woldu, A. Electrochemical determination and comparison of ascorbic acid in freshly prepared and bottled fruit juices: A cyclic voltammetric study. J. Chem. Pharm. Res. 2014, 6, 880–888. [Google Scholar]
- State Standard 24556–89. Products of fruits and vegetables processing. In Methods for Determination of Vitamin C; Izdatelstvo Standartov: Moscow, Russian, 2003. [Google Scholar]
- Brainina, K.Z.; Ivanova, A.V.; Sharafutdinova, E.N.; Lozovskaya, E.L.; Shkarina, E.I. Potentiometry as a method of antioxidant activity investigation. Talanta 2007, 71, 13–18. [Google Scholar] [CrossRef]










| Method | Parameter | Au-gr | Au-cit |
|---|---|---|---|
| TEM | Shape | Spherical | Spherical |
| d, nm | 14 ± 7 [25] | 13 and 38 (up to 10 %) [42] | |
| UV-Vis-spectrophotometry | d, nm | 10 ± 1 [25] | 20 [42] |
| LS Voltammetry | Im (Au), µA | 31 ± 5 | 27 ± 4 |
| Em (Au), V | 0.851 ± 0.002 | 0.877 ± 0.011 | |
| Em (AA), V | 0.420 ± 0.006 | 0.442 ± 0.007 |
| Interfering Substance | Concentration of Interfering Substance (CIS), mM | CIS: CAA | AA Response Change, % |
|---|---|---|---|
| Glucose | 1 | 100 | −9.5 |
| Sucrose | 5 | 500 | +4.2 |
| Fructose | 6 | 600 | +4.8 |
| Citric acid | 6 | 600 | −0.5 |
| Tartaric acid | 8 | 800 | −1.6 |
| Malic acid | 10 | 1000 | −0.5 |
| Sensor | LOD, μM | LR, μM | Technique | Object | Ref. |
|---|---|---|---|---|---|
| TiO2-Aunps-MWCNT-DHP/GCE | 1.20 | 5–51 | Am | pharmaceutical and orange juice samples | [16] |
| Aunps-PAN/GCE | 8.20 | 10–12000 | Am | medicine vitamin C tablets | [19] |
| Aunps-ZnO-PPy-RGO/GCE | 0.16 | 2–950 | DPV | vitamin C tablets | [14] |
| Aunps-L-Alanine/GCE | 10.00 | 12–160 | CV | – | [18] |
| Pt-electrode | 87.00 | 310–20000 | DPV | fruit juices, wine | [48] |
| CPE | 20.00 | 70–20000 | DPV | – | [48] |
| PEDOT/GCE | 23.30 | 50–90 | SWV | orange and pineapple juices | [49] |
| SPCE | 1360.00 | 0–10000 | CV | packed orange juice sample | [50] |
| CPE | 1.76 | 10–100 | SWV | pharmaceutical tablets | [51] |
| PoPDoAP/GCE | 36.40 | 100–1000 | DPV | vitamin C tablet and orange juices | [52] |
| Ppy/Au-MA | 5.00 | 10–2200 | SWV | lemon juice and celin tablet chewable | [53] |
| GCE | 11.50 | 8–80 | CV, SWV | beverages and fresh edible vegetables | [54] |
| Fe(III)-Y zeolite/CPE | 0.02 | 0.4–1200 | SWV | citrus juices | [55] |
| CPE | 22.10 | - | CV | fruit juices | [56] |
| Au-gr/CVE | 0.05 | 1–5750 | AV | fruit juice | [this work] |
| Sample | Found in the Sample, mM | Added, mM | Found in the Sample with Additive, mM | Found Additive, mM | R, % |
|---|---|---|---|---|---|
| Cherry-apple juice | 0.81 ± 0.06 | 1.94 | 2.96 ± 0.21 | 2.15 ± 0.23 | 111 |
| Apple juice for children | 0.47 ± 0.01 | 0.97 | 1.43 ± 0.03 | 0.96 ± 0.02 | 99 |
| Apple juice | 0.16 ± 0.01 | 0.25 | 0.418 ± 0.003 | 0.26 ± 0.01 | 104 |
| Apple nectar clarified. | 0.09 ± 0.01 | 0.12 | 0.21 ± 0.02 | 0.120 ± 0.003 | 100 |
| Samples | Found Using Au-gr/CVE | RSD, % | Found by Potentiometric Titration | RSD, % | F-Test | t-Test |
|---|---|---|---|---|---|---|
| Cherry-apple juice | 0.81 ± 0.06 | 6.8 | 0.76 ± 0.07 | 8.7 | 1.43 | 0.67 |
| Apple juice for children | 0.47 ± 0.01 | 2.2 | 0.42 ± 0.03 | 7.6 | 9.00 | 1.82 |
| Apple juice | 0.16 ± 0.01 | 5.0 | 0.16 ± 0.01 | 4.5 | 1.18 | 0.49 |
| Apple nectar clarified | 0.09 ± 0.01 | 6.5 | 0.09 ± 0.01 | 9.0 | 1.97 | 0.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brainina, K.Z.; Bukharinova, M.A.; Stozhko, N.Y.; Sokolkov, S.V.; Tarasov, A.V.; Vidrevich, M.B. Electrochemical Sensor Based on a Carbon Veil Modified by Phytosynthesized Gold Nanoparticles for Determination of Ascorbic Acid. Sensors 2020, 20, 1800. https://doi.org/10.3390/s20061800
Brainina KZ, Bukharinova MA, Stozhko NY, Sokolkov SV, Tarasov AV, Vidrevich MB. Electrochemical Sensor Based on a Carbon Veil Modified by Phytosynthesized Gold Nanoparticles for Determination of Ascorbic Acid. Sensors. 2020; 20(6):1800. https://doi.org/10.3390/s20061800
Chicago/Turabian StyleBrainina, Khiena Z., Maria A. Bukharinova, Natalia Yu. Stozhko, Sergey V. Sokolkov, Aleksey V. Tarasov, and Marina B. Vidrevich. 2020. "Electrochemical Sensor Based on a Carbon Veil Modified by Phytosynthesized Gold Nanoparticles for Determination of Ascorbic Acid" Sensors 20, no. 6: 1800. https://doi.org/10.3390/s20061800







