Rational Design of Molecularly Imprinted Polymers Using Quaternary Ammonium Cations for Glyphosate Detection
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
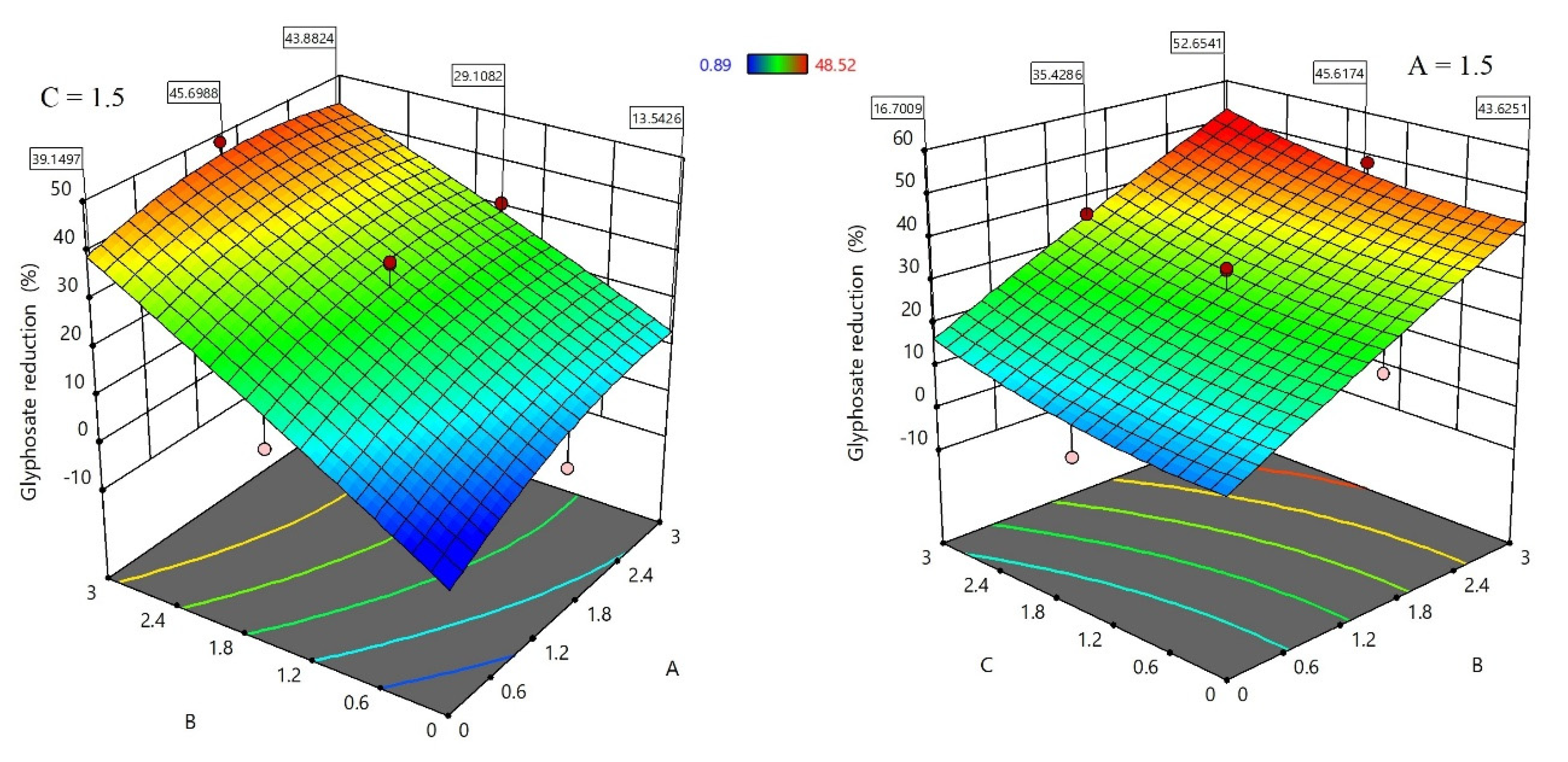
3.1. Functional Monomers and the Glyphosate-Functional Monomers Ratio Optimization Using Design of Experiment (DOE)
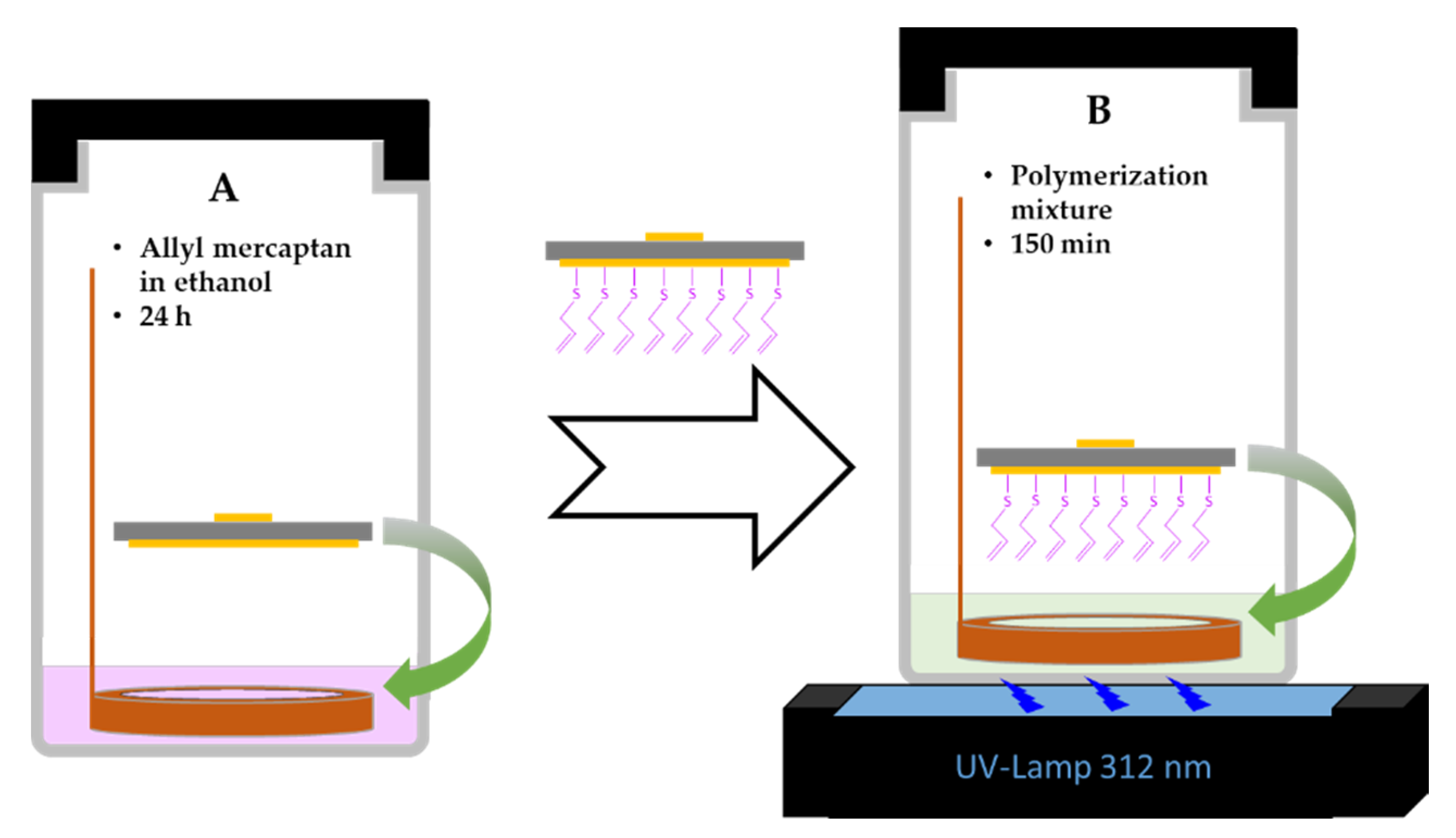
3.2. Polymerization and Characterization
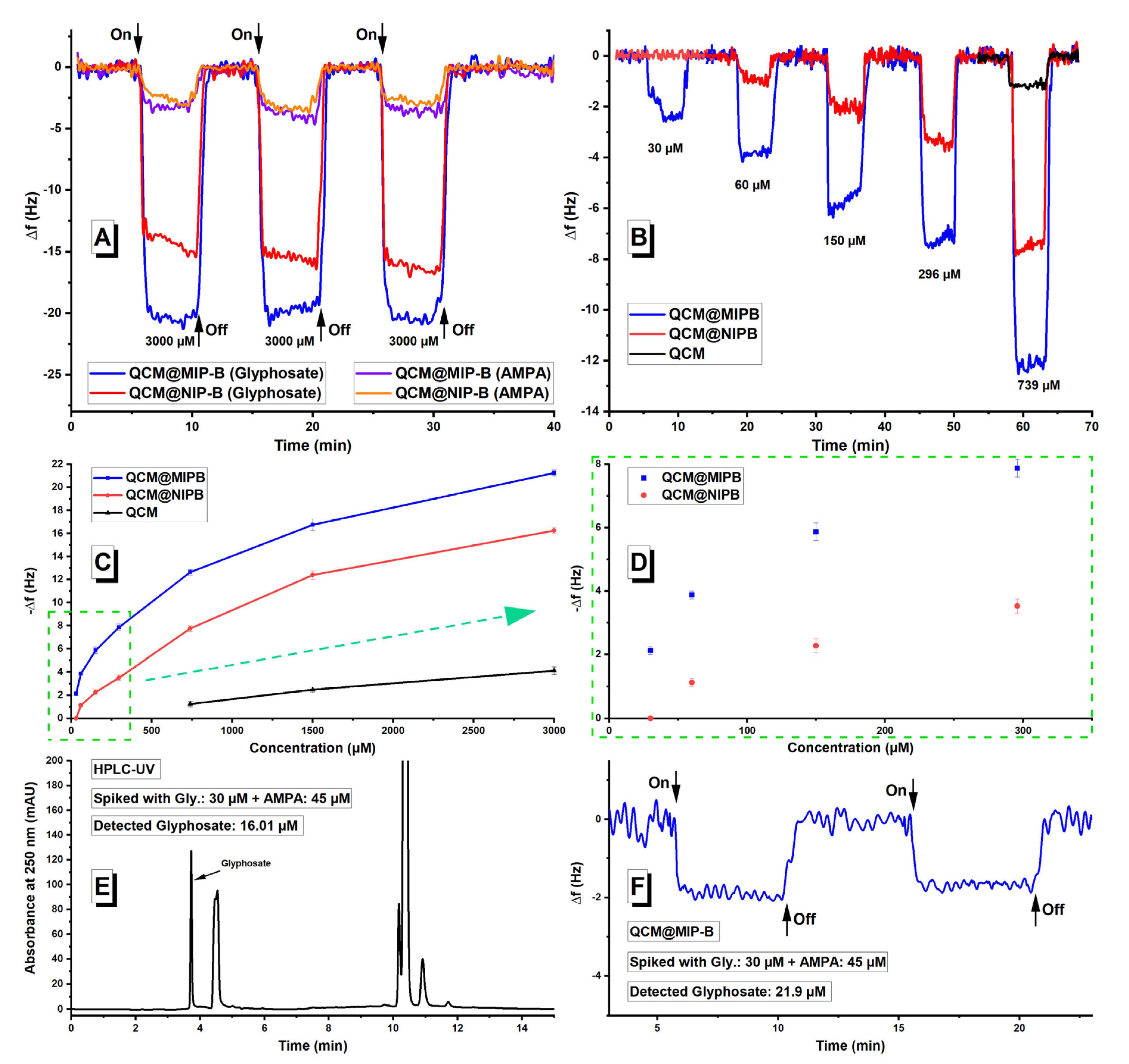
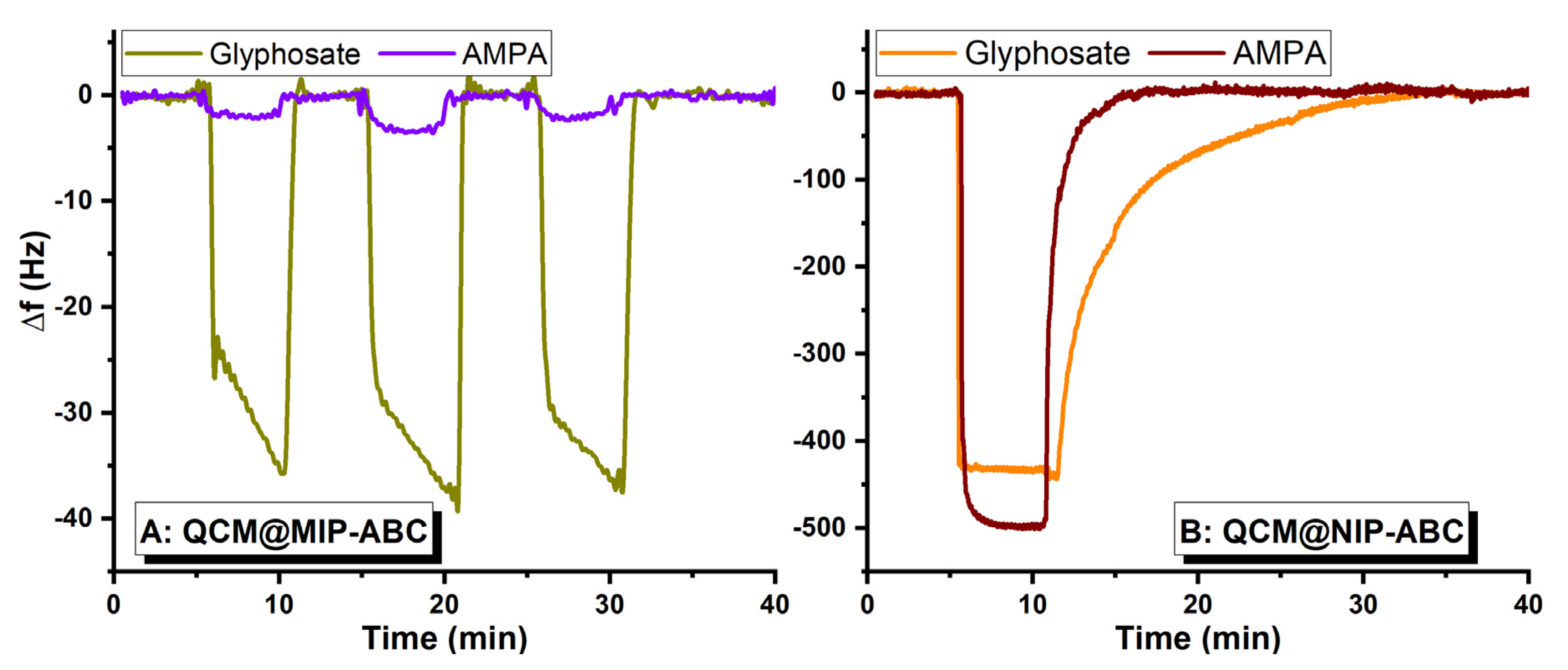
3.3. Investigation of Sensor Application of Designed Polymers Using QCM Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huhn, C. More and enhanced glyphosate analysis is needed. Anal. Bioanal. Chem. 2018, 410, 3041–3045. [Google Scholar] [CrossRef] [PubMed]
- Noori, J.S.; Dimaki, M.; Mortensen, J.; Svendsen, W.E. Detection of Glyphosate in Drinking Water: A Fast and Direct Detection Method without Sample Pretreatment. Sensors 2018, 18, 2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Wang, L.; Shen, C.; Wang, C.; Hu, X.; Wang, G. An electrochemical sensor on the hierarchically porous Cu-BTC MOF platform for glyphosate determination. Sens. Actuators B Chem. 2019, 283, 487–494. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Lin, Y.-S.; Xiao, G.-T.; Chiu, T.-C.; Hu, C.-C. A highly selective and sensitive nanosensor for the detection of glyphosate. Talanta 2016, 161, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yang, K.-L. Development of an oligopeptide functionalized surface plasmon resonance biosensor for online detection of glyphosate. Anal. Chem. 2013, 85, 5727–5733. [Google Scholar] [CrossRef] [PubMed]
- Khenifi, A.; Derriche, Z.; Forano, C.; Prevot, V.; Mousty, C.; Scavetta, E.; Ballarin, B.; Guadagnini, L.; Tonelli, D. Glyphosate and glufosinate detection at electrogenerated NiAl-LDH thin films. Anal. Chim. Acta 2009, 654, 97–102. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, X.; Lu, Y.; Li, H.; Xu, X. Performance of CuAl-LDH/Gr Nanocomposite-Based Electrochemical Sensor with Regard to Trace Glyphosate Detection in Water. Sensors 2020, 20, 4146. [Google Scholar] [CrossRef]
- Do, M.H.; Dubreuil, B.; Peydecastaing, J.; Vaca-Medina, G.; Nhu-Trang, T.-T.; Jaffrezic-Renault, N.; Behra, P. Chitosan-Based Nanocomposites for Glyphosate Detection Using Surface Plasmon Resonance Sensor. Sensors 2020, 20, 5942. [Google Scholar] [CrossRef]
- González-Martínez, M.A.; Brun, E.M.; Puchades, R.; Maquieira, A.; Ramsey, K.; Rubio, F. Glyphosate immunosensor. Application for water and soil analysis. Anal. Chem. 2005, 77, 4219–4227. [Google Scholar] [CrossRef]
- Bettazzi, F.; Romero Natale, A.; Torres, E.; Palchetti, I. Glyphosate Determination by Coupling an Immuno-Magnetic Assay with Electrochemical Sensors. Sensors 2018, 18, 2965. [Google Scholar] [CrossRef] [Green Version]
- Wulff, G. Molecular Imprinting in Cross-Linked Materials with the Aid of Molecular Templates—A Way towards Artificial Antibodies. Angewandte Chemie International Edition in English. Angew. Chem. 1995, 34, 1812–1832. [Google Scholar] [CrossRef]
- Beyazit, S.; Tse Sum Bui, B.; Haupt, K.; Gonzato, C. Molecularly imprinted polymer nanomaterials and nanocomposites by controlled/living radical polymerization. Prog. Polym. Sci. 2016, 62, 1–21. [Google Scholar] [CrossRef]
- Zarejousheghani, M.; Lorenz, W.; Vanninen, P.; Alizadeh, T.; Cämmerer, M.; Borsdorf, H. Molecularly Imprinted Polymer Materials as Selective Recognition Sorbents for Explosives: A Review. Polymers 2019, 11, 888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haupt, K.; Medina Rangel, P.X.; Bui, B.T.S. Molecularly Imprinted Polymers: Antibody Mimics for Bioimaging and Therapy. Chem. Rev. 2020, 120, 9554–9582. [Google Scholar] [CrossRef]
- Chianella, I.; Guerreiro, A.; Moczko, E.; Caygill, J.S.; Piletska, E.V.; de Vargas Sansalvador, I.M.P.; Whitcombe, M.J.; Piletsky, S.A. Direct replacement of antibodies with molecularly imprinted polymer nanoparticles in ELISA--development of a novel assay for vancomycin. Anal. Chem. 2013, 85, 8462–8468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esen, C.; Czulak, J.; Cowen, T.; Piletska, E.; Piletsky, S.A. Highly Efficient Abiotic Assay Formats for Methyl Parathion: Molecularly Imprinted Polymer Nanoparticle Assay as an Alternative to Enzyme-Linked Immunosorbent Assay. Anal. Chem. 2019, 91, 958–964. [Google Scholar] [CrossRef]
- Canfarotta, F.; Poma, A.; Guerreiro, A.; Piletsky, S. Solid-phase synthesis of molecularly imprinted nanoparticles. Nat. Protoc. 2016, 11, 443–455. [Google Scholar] [CrossRef]
- Medina Rangel, P.X.; Laclef, S.; Xu, J.; Panagiotopoulou, M.; Kovensky, J.; Tse Sum Bui, B.; Haupt, K. Solid-phase synthesis of molecularly imprinted polymer nanolabels: Affinity tools for cellular bioimaging of glycans. Sci. Rep. 2019, 9, 3923. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Ambrosini, S.; Tamahkar, E.; Rossi, C.; Haupt, K.; Tse Sum Bui, B. Toward a Universal Method for Preparing Molecularly Imprinted Polymer Nanoparticles with Antibody-like Affinity for Proteins. Biomacromolecules 2016, 17, 345–353. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Alcacer, A.; Bausells, J.; Jaffrezic-Renault, N.; Zine, N.; Errachid, A. Experimental Study and Mathematical Modeling of a Glyphosate Impedimetric Microsensor Based on Molecularly Imprinted Chitosan Film. Chemosensors 2020, 8, 104. [Google Scholar] [CrossRef]
- Do, M.H.; Florea, A.; Farre, C.; Bonhomme, A.; Bessueille, F.; Vocanson, F.; Tran-Thi, N.-T.; Jaffrezic-Renault, N. Molecularly imprinted polymer-based electrochemical sensor for the sensitive detection of glyphosate herbicide. Int. J. Environ. Anal. Chem. 2015, 95, 1489–1501. [Google Scholar] [CrossRef]
- Mazouz, Z.; Rahali, S.; Fourati, N.; Zerrouki, C.; Aloui, N.; Seydou, M.; Yaakoubi, N.; Chehimi, M.M.; Othmane, A.; Kalfat, R. Highly Selective Polypyrrole MIP-Based Gravimetric and Electrochemical Sensors for Picomolar Detection of Glyphosate. Sensors 2017, 17, 2586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, B.B.; Jauhari, D.; Tiwari, M.P. Doubly imprinted polymer nanofilm-modified electrochemical sensor for ultra-trace simultaneous analysis of glyphosate and glufosinate. Biosens. Bioelectron. 2014, 59, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Wu, K.; Zhang, L.; Ge, S.; Yu, J. A molecularly imprinted polypyrrole for ultrasensitive voltammetric determination of glyphosate. Microchim. Acta 2017, 184, 1959–1967. [Google Scholar] [CrossRef]
- Zhang, C.; She, Y.; Li, T.; Zhao, F.; Jin, M.; Guo, Y.; Zheng, L.; Wang, S.; Jin, F.; Shao, H.; et al. A highly selective electrochemical sensor based on molecularly imprinted polypyrrole-modified gold electrode for the determination of glyphosate in cucumber and tap water. Anal. Bioanal. Chem. 2017, 409, 7133–7144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yan, M.; Zhang, C.; Peng, R.; Ma, D.; Yu, J. Determination of glyphosate in foodstuff by one novel chemiluminescence-molecular imprinting sensor. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1482–1486. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Abroa-Nemeir, I.; Ben Halima, H.; Gallardo-Gonzalez, J.; El Alami El Hassani, N.; Alcacer, A.; Bausells, J.; Jaffrezic-Renault, N.; et al. Electrochemical impedance spectroscopy determination of glyphosate using a molecularly imprinted chitosan. Sens. Actuators B Chem. 2020, 309, 127753. [Google Scholar] [CrossRef]
- da Mata, K.; Corazza, M.Z.; de Oliveira, F.M.; de Toffoli, A.L.; Teixeira Tarley, C.R.; Moreira, A.B. Synthesis and characterization of cross-linked molecularly imprinted polyacrylamide for the extraction/preconcentration of glyphosate and aminomethylphosphonic acid from water samples. React. Funct. Polym. 2014, 83, 76–83. [Google Scholar] [CrossRef]
- Gomez-Caballero, A.; Diaz-Diaz, G.; Bengoetxea, O.; Quintela, A.; Unceta, N.; Goicolea, M.A.; Barrio, R.J. Water compatible stir-bar devices imprinted with underivatised glyphosate for selective sample clean-up. J. Chromatogr. A 2016, 1451, 23–32. [Google Scholar] [CrossRef]
- Puzio, K.; Claude, B.; Amalric, L.; Berho, C.; Grellet, E.; Bayoudh, S.; Nehmé, R.; Morin, P. Molecularly imprinted polymer dedicated to the extraction of glyphosate in natural waters. J. Chromatogr. A 2014, 1361, 1–8. [Google Scholar] [CrossRef]
- Wulff, G. Fourty years of molecular imprinting in synthetic polymers: Origin, features and perspectives. Microchim. Acta 2013, 180, 1359–1370. [Google Scholar] [CrossRef]
- Zarejousheghani, M. Towards in-Field Sample-Preparation and Detection: Development of New Sample Preparation Formats Using Molecularly Imprinted Polymers for the Combination with Field-Deployable Detectors. Ph.D. Thesis, Martin-Luther-Universität Halle-Wittenberg, Halle, Germany, 2019. [Google Scholar]
- Zarejousheghani, M.; Fiedler, P.; Möder, M.; Borsdorf, H. Selective mixed-bed solid phase extraction of atrazine herbicide from environmental water samples using molecularly imprinted polymer. Talanta 2014, 129, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zarejousheghani, M.; Möder, M.; Borsdorf, H. A new strategy for synthesis of an in-tube molecularly imprinted polymer-solid phase microextraction device: Selective off-line extraction of 4-nitrophenol as an example of priority pollutants from environmental water samples. Anal. Chim. Acta 2013, 798, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Yan, M. (Ed.) Molecularly Imprinted Materials: Science and Technology; CRC PRESS: Boca Raton, FL, USA, 2020; ISBN 0367578190. [Google Scholar]
- Zarejousheghani, M.; Schrader, S.; Möder, M.; Mayer, T.; Borsdorf, H. Negative electrospray ionization ion mobility spectrometry combined with paper-based molecular imprinted polymer disks: A novel approach for rapid target screening of trace organic compounds in water samples. Talanta 2018, 190, 47–54. [Google Scholar] [CrossRef]
- Zarejousheghani, M.; Schrader, S.; Möder, M.; Schmidt, M.; Borsdorf, H. A new strategy for accelerated extraction of target compounds using molecularly imprinted polymer particles embedded in a paper-based disk. J. Mol. Recognit. 2018, 31. [Google Scholar] [CrossRef] [Green Version]
- Zarejousheghani, M.; Schrader, S.; Möder, M.; Lorenz, P.; Borsdorf, H. Ion-exchange molecularly imprinted polymer for the extraction of negatively charged acesulfame from wastewater samples. J. Chromatogr. A 2015, 1411, 23–33. [Google Scholar] [CrossRef]
- Le Goff, N.; Fomba, I.; Prost, E.; Merlier, F.; Haupt, K.; Duma, L.; Fayeulle, A.; Falcimaigne-Cordin, A. Renewable Plant Oil-Based Molecularly Imprinted Polymers as Biopesticide Delivery Systems. Acs Sustain. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Diltemiz, S.E.; Hür, D.; Keçili, R.; Ersöz, A.; Say, R. New synthesis method for 4-MAPBA monomer and using for the recognition of IgM and mannose with MIP-based QCM sensors. Analyst 2013, 138, 1558–1563. [Google Scholar] [CrossRef]
- Ghanem, A.; Bados, P.; Kerhoas, L.; Dubroca, J.; Einhorn, J. Glyphosate and AMPA analysis in sewage sludge by LC-ESI-MS/MS after FMOC derivatization on strong anion-exchange resin as solid support. Anal. Chem. 2007, 79, 3794–3801. [Google Scholar] [CrossRef]
- Daskal, Y.; Tauchnitz, T.; Güth, F.; Dittrich, R.; Joseph, Y. Assembly Behavior of Organically Interlinked Gold Nanoparticle Composite Films: A Quartz Crystal Microbalance Investigation. Langmuir 2017, 33, 11869–11877. [Google Scholar] [CrossRef]
- Sprankle, P.; Meggitt, W.F.; Penner, D. Adsorption, Mobility, and Microbial Degradation of Glyphosate in the Soil. Weed Sci. 1975, 23, 229–234. [Google Scholar] [CrossRef]
- Chieng, B.; Ibrahim, N.; Yunus, W.; Hussein, M. Poly(lactic acid)/Poly(ethylene glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers 2014, 6, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Louette, P.; Bodino, F.; Pireaux, J.-J. Poly(methyl methacrylate) (PMMA) XPS Reference Core Level and Energy Loss Spectra. Surf. Sci. Spectra 2005, 12, 69–73. [Google Scholar] [CrossRef]
- Wang, H.; Chen, M.; Jin, C.; Niu, B.; Jiang, S.; Li, X.; Jiang, S. Antibacterial 2-(Methacryloyloxy) ethyl Trimethylammonium Chloride Functionalized Reduced Graphene Oxide/Poly(ethylene-co-vinyl alcohol) Multilayer Barrier Film for Food Packaging. J. Agric. Food Chem. 2018, 66, 732–739. [Google Scholar] [CrossRef]
- Umpleby, R.J.; Baxter, S.C.; Rampey, A.M.; Rushton, G.T.; Chen, Y.; Shimizu, K.D. Characterization of the heterogeneous binding site affinity distributions in molecularly imprinted polymers. J. Chromatogr. B 2004, 804, 141–149. [Google Scholar] [CrossRef]
- Sigmund, G.; Gharasoo, M.; Hüffer, T.; Hofmann, T. Deep Learning Neural Network Approach for Predicting the Sorption of Ionizable and Polar Organic Pollutants to a Wide Range of Carbonaceous Materials. Environ. Sci. Technol. 2020, 54, 4583–4591. [Google Scholar] [CrossRef] [Green Version]
- Mertens, M.; Höss, S.; Neumann, G.; Afzal, J.; Reichenbecher, W. Glyphosate, a chelating agent-relevant for ecological risk assessment? Environ. Sci. Pollut. Res. Int. 2018, 25, 5298–5317. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, J.; Shea, K.J. Tuning the Protein Corona of Hydrogel Nanoparticles: The Synthesis of Abiotic Protein and Peptide Affinity Reagents. Acc. Chem. Res. 2016, 49, 1200–1210. [Google Scholar] [CrossRef]
- Hoshino, Y.; Koide, H.; Furuya, K.; Haberaecker, W.W.; Lee, S.-H.; Kodama, T.; Kanazawa, H.; Oku, N.; Shea, K.J. The rational design of a synthetic polymer nanoparticle that neutralizes a toxic peptide in vivo. PNAS 2012, 109, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Yonamine, Y.; Hoshino, Y.; Shea, K.J. ELISA-mimic screen for synthetic polymer nanoparticles with high affinity to target proteins. Biomacromolecules 2012, 13, 2952–2957. [Google Scholar] [CrossRef] [Green Version]
- Yonamine, Y.; Yoshimatsu, K.; Lee, S.-H.; Hoshino, Y.; Okahata, Y.; Shea, K.J. Polymer nanoparticle-protein interface. Evaluation of the contribution of positively charged functional groups to protein affinity. Acs Appl. Mater. Interfaces 2013, 5, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Lieberzeit, P.; Glanznig, G.; Jenik, M.; Sylwia Gazda-Miarecka, S.; Dickert, F.; Leidl, A. Softlithography in Chemical Sensing—Analytes from Molecules to Cells. Sensors 2005, 5, 509–518. [Google Scholar] [CrossRef] [Green Version]





| Factors | Notation | Units | Levels | |||
|---|---|---|---|---|---|---|
| (3-Acrylamidopropyl)trimethylammonium chloride | 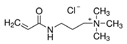 | A | mmol | 0 | 1.5 | 3 |
| [2-(Acryloyloxy)ethyl]trimethylammonium chloride |  | B | mmol | 0 | 1.5 | 3 |
| Diallyldimethylammonium chloride | 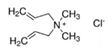 | C | mmol | 0 | 1.5 | 3 |
| Glyphosate |  | D | mmol | 1 | 1 | 1 |
| No. | A | B | C | Found Glyphosate (µg) | SD | Glyphosate-Reduction (%) |
|---|---|---|---|---|---|---|
| 1 | 3 | 0 | 3 | 14.52 | 0.84 | 23.57 |
| 2 | 3 | 1.5 | 1.5 | 13.12 | 2.14 | 30.94 |
| 3 | 0 | 1.5 | 1.5 | 16.77 | 2.58 | 11.73 |
| 4 | 0 | 0 | 3 | 18.35 | 0.48 | 3.42 |
| 5 | 0 | 3 | 3 | 9.78 | 1.87 | 48.52 |
| 6 | 3 | 3 | 0 | 10.81 | 1.39 | 43.10 |
| 7 | 1.5 | 1.5 | 3 | 12.18 | 1.46 | 35.89 |
| 8 | 3 | 0 | 0 | 17.02 | 0.40 | 10.42 |
| 9 | 0 | 3 | 0 | 12.20 | 1.32 | 35.78 |
| 10 | 1.5 | 1.5 | 0 | 15.06 | 1.15 | 20.73 |
| 11 | 0 | 0 | 0 | 18.83 | 0.60 | 0.89 |
| 12 | 1.5 | 1.5 | 1.5 | 12.78 | 1.54 | 32.73 |
| 13 | 1.5 | 1.5 | 1.5 | 12.68 | 1.72 | 33.26 |
| 14 | 1.5 | 0 | 1.5 | 18.66 | 1.07 | 1.780 |
| 15 | 3 | 3 | 3 | 10.19 | 2.35 | 46.36 |
| 16 | 1.5 | 3 | 1.5 | 9.83 | 1.80 | 48.26 |
| Source | 1 DF | 2 SS | 3 MS | F-Value | p-Value | 4 Cont.% |
|---|---|---|---|---|---|---|
| Model | 9 | 3957.3 | 439.7 | 9.88 | 0.0057 | 93.68 |
| A | 1 | 292.14 | 292.14 | 6.56 | 0.0428 | 6.91 |
| B | 1 | 3310.22 | 3310.22 | 74.36 | 0.0001 | 78.36 |
| C | 1 | 219.4 | 219.4 | 4.93 | 0.0682 | 5.19 |
| AB | 1 | 75.15 | 75.15 | 1.69 | 0.2415 | 1.78 |
| AC | 1 | 0.1624 | 0.1624 | 0.0036 | 0.9538 | 0 |
| BC | 1 | 0.0128 | 0.0128 | 0.0003 | 0.987 | 0 |
| A2 | 1 | 48.32 | 48.32 | 1.09 | 0.3376 | 1.14 |
| B2 | 1 | 0.9366 | 0.9366 | 0.021 | 0.8894 | 0.02 |
| C2 | 1 | 19.13 | 19.13 | 0.4298 | 0.5364 | 0.45 |
| Residual | 6 | 267.09 | 44.51 | 6.32 | ||
| Lack of Fit | 5 | 266.95 | 53.39 | 380.13 | 0.0389 | 6.32 |
| Pure Error | 1 | 0.1405 | 0.1405 | 0 | ||
| Total | 15 | 4224.39 | 100 |
| Glyphosate (g) | A (g) | B (g) | C (g) | Porogen 1 (µL) | EGDMA 2 (µL) | AIBN 3 (mg) | |
|---|---|---|---|---|---|---|---|
| MIP-B | 0.0102 | - | 0.0477 | - | 2834 | 226 | 10 |
| NIP-B | - | - | 0.0440 | - | 2834 | 226 | 10 |
| MIP-ABC | 0.0106 | 0.0336 | 0.0453 | 0.0285 | 2834 | 226 | 10 |
| NIP-ABC | - | 0.0320 | 0.0456 | 0.0293 | 2834 | 226 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarejousheghani, M.; Jaafar, A.; Wollmerstaedt, H.; Rahimi, P.; Borsdorf, H.; Zimmermann, S.; Joseph, Y. Rational Design of Molecularly Imprinted Polymers Using Quaternary Ammonium Cations for Glyphosate Detection. Sensors 2021, 21, 296. https://doi.org/10.3390/s21010296
Zarejousheghani M, Jaafar A, Wollmerstaedt H, Rahimi P, Borsdorf H, Zimmermann S, Joseph Y. Rational Design of Molecularly Imprinted Polymers Using Quaternary Ammonium Cations for Glyphosate Detection. Sensors. 2021; 21(1):296. https://doi.org/10.3390/s21010296
Chicago/Turabian StyleZarejousheghani, Mashaalah, Alaa Jaafar, Hendrik Wollmerstaedt, Parvaneh Rahimi, Helko Borsdorf, Stefan Zimmermann, and Yvonne Joseph. 2021. "Rational Design of Molecularly Imprinted Polymers Using Quaternary Ammonium Cations for Glyphosate Detection" Sensors 21, no. 1: 296. https://doi.org/10.3390/s21010296






