Modeling the Conductivity Response to NO2 Gas of Films Based on MWCNT Networks
Abstract
:1. Introduction
2. Model Derivation
2.1. Theoretical Derivation
2.2. Model Calibration Procedure (Parameters Estimation)
3. Materials and Methods
3.1. Materials
3.2. Sensing Film Preparation
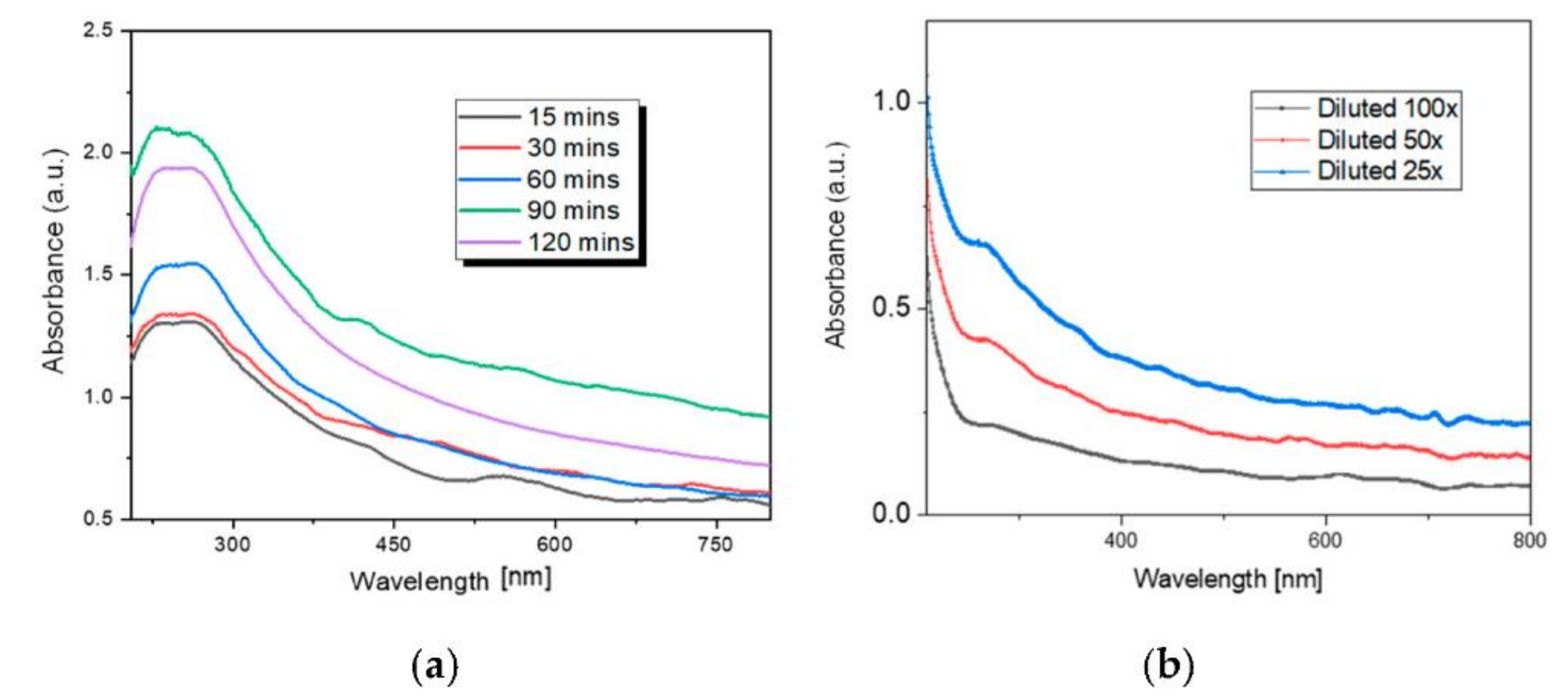
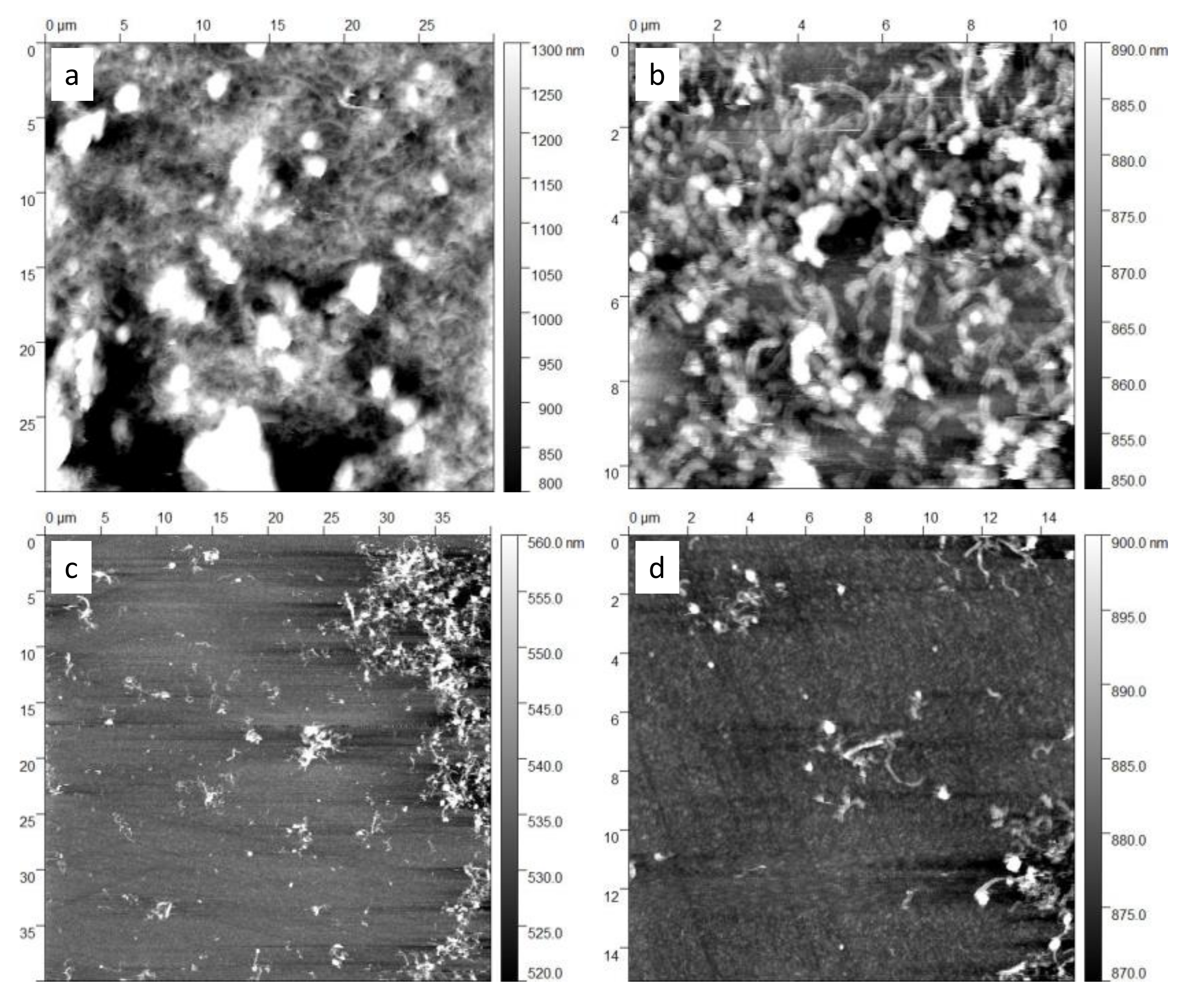
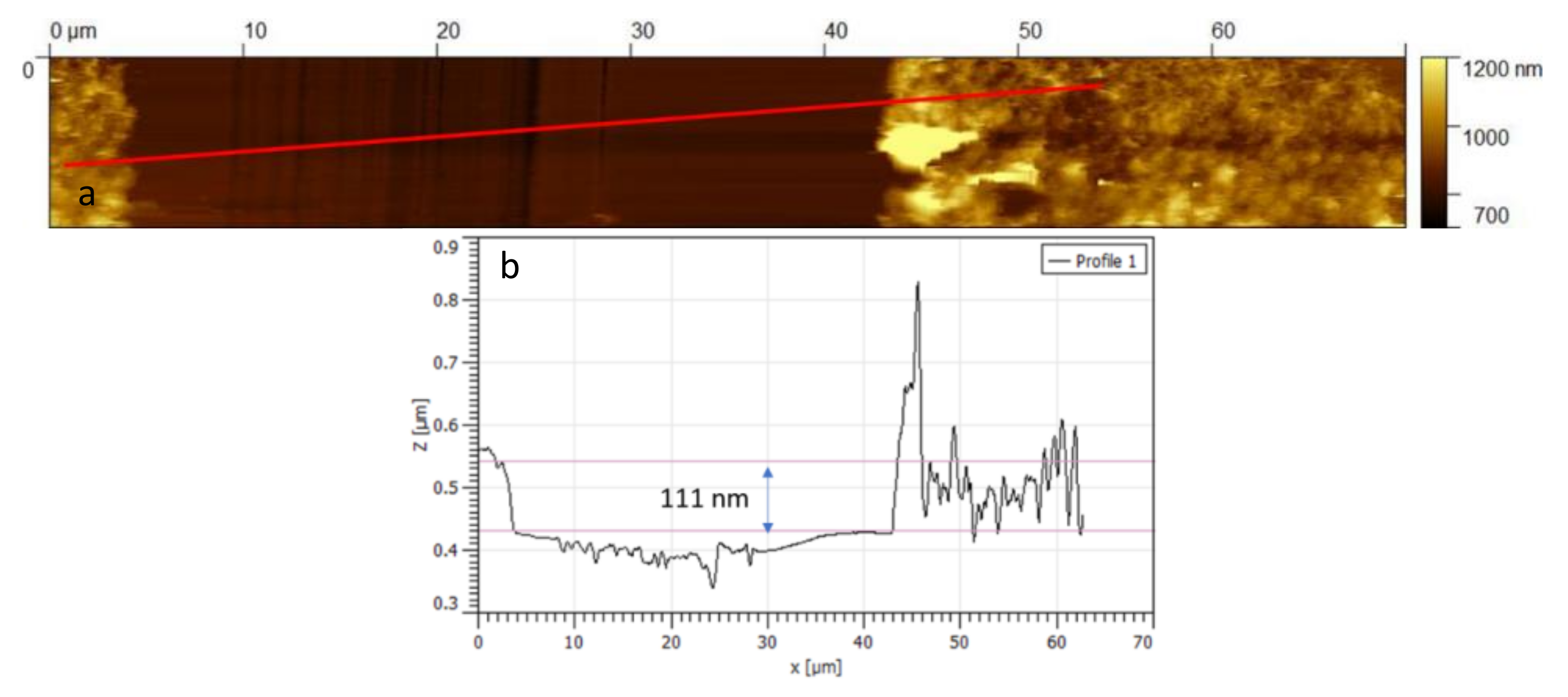
3.3. Sensing Material Characterization
3.4. Impedance Characterization
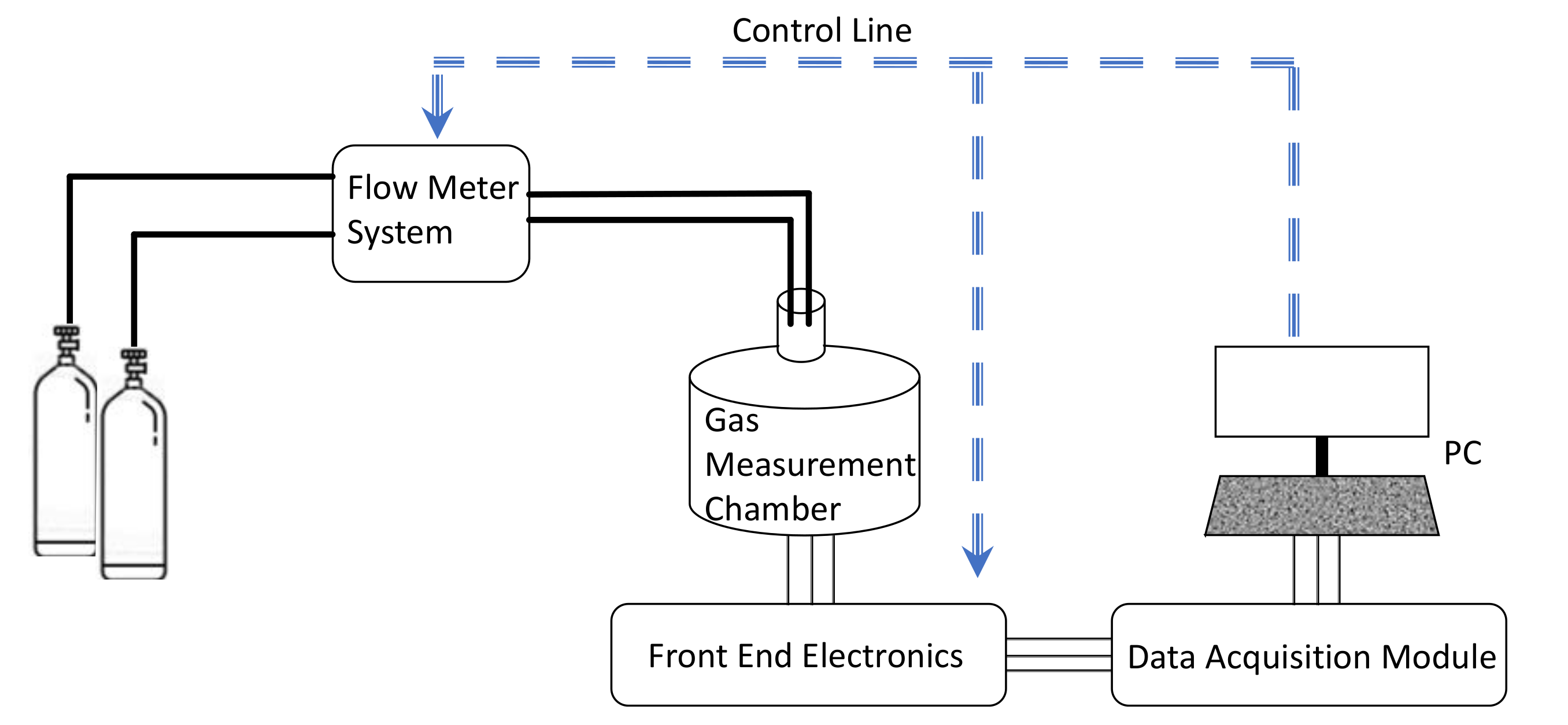
3.5. Gas Sensing Properties Characterization System
4. Characterization Results
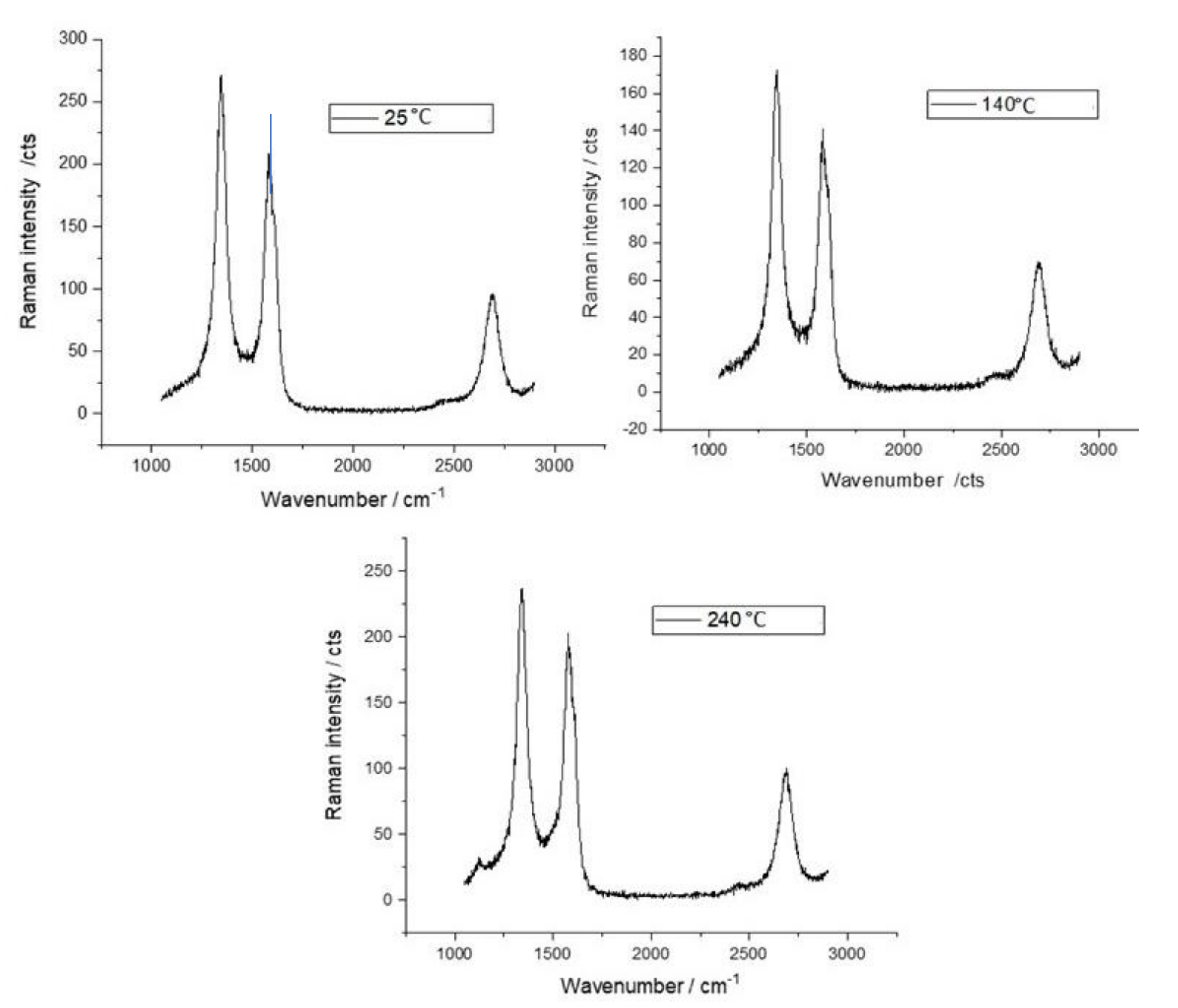
4.1. Material Characterization
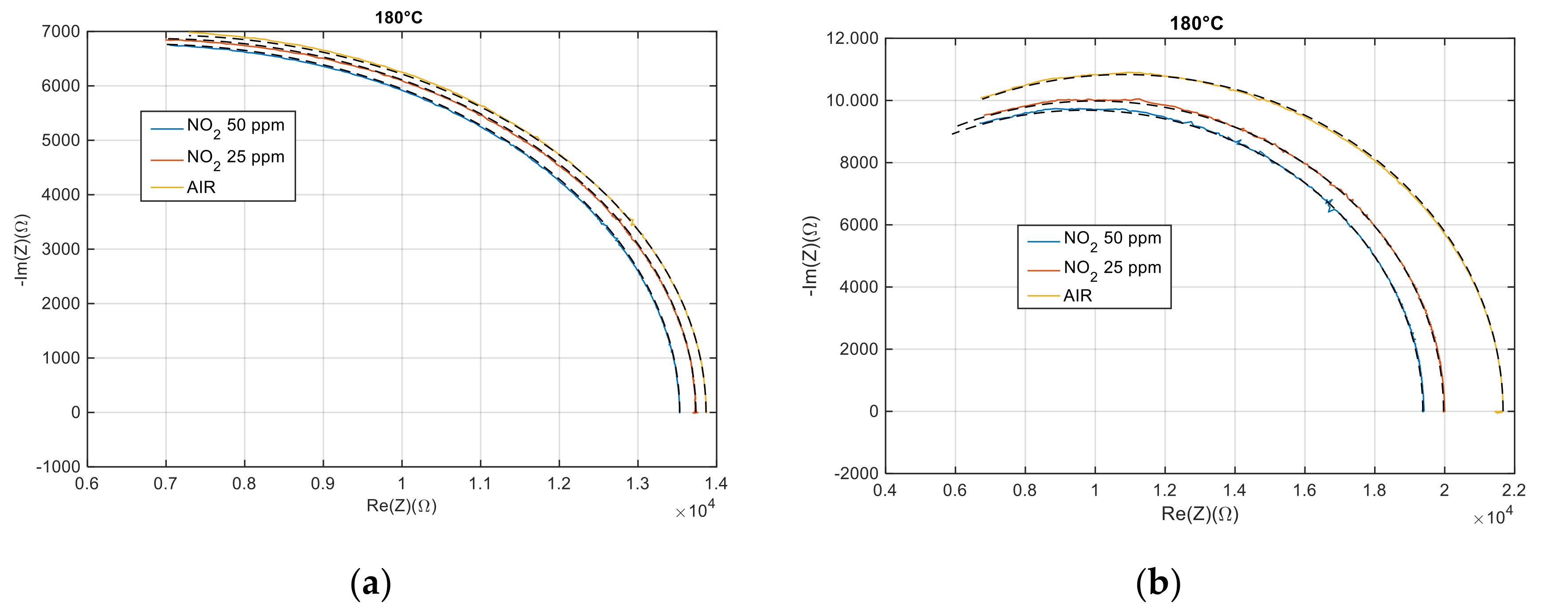
4.2. Electrical Characterization
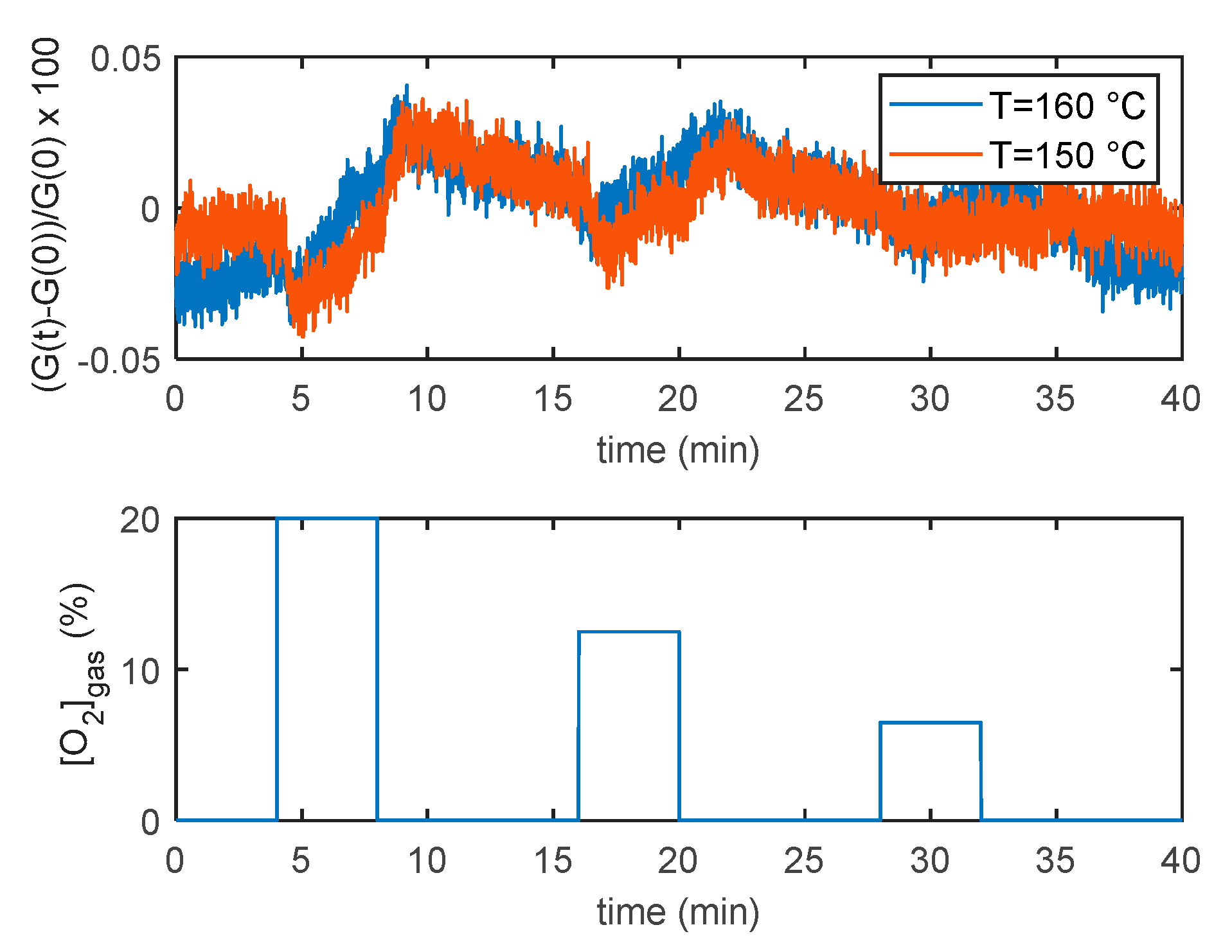
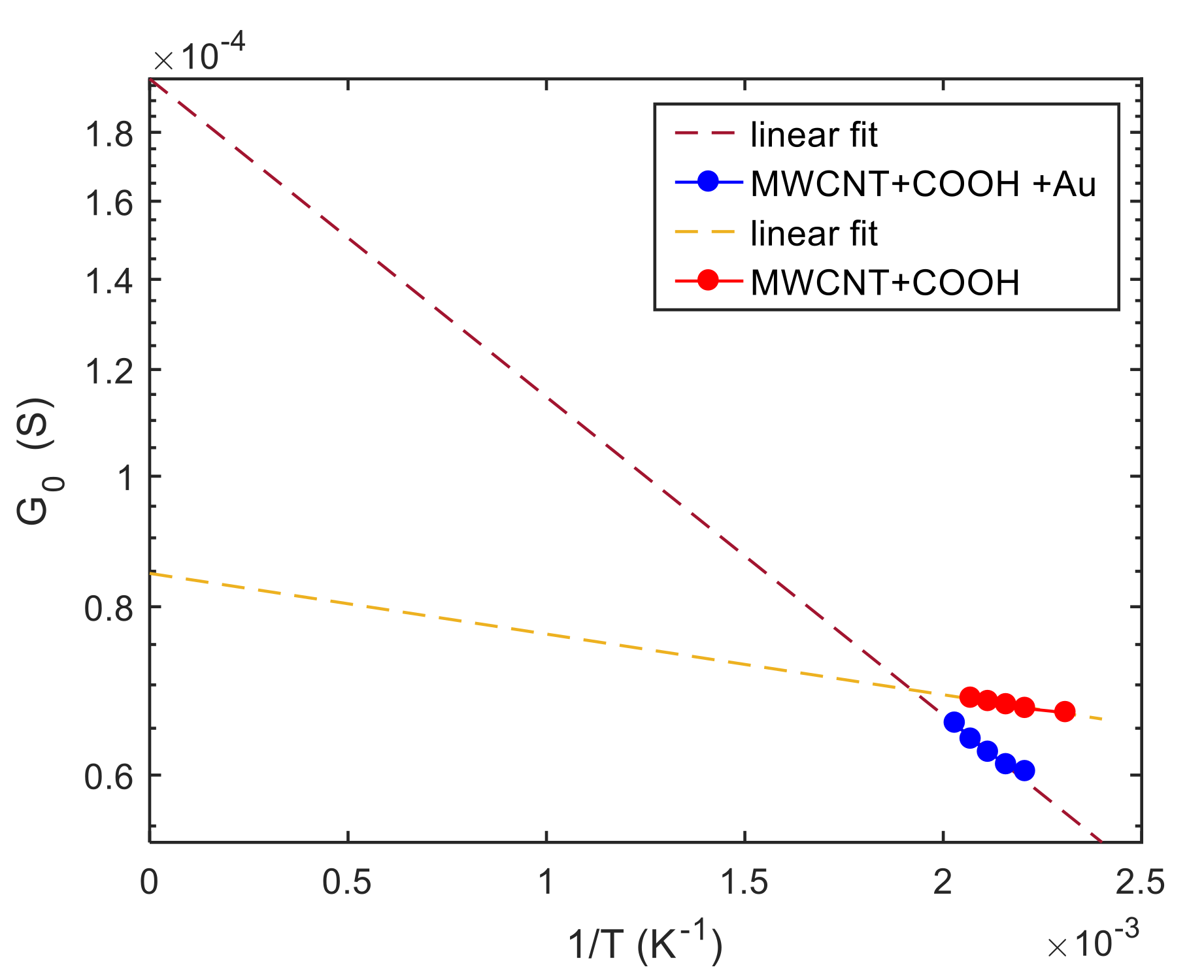
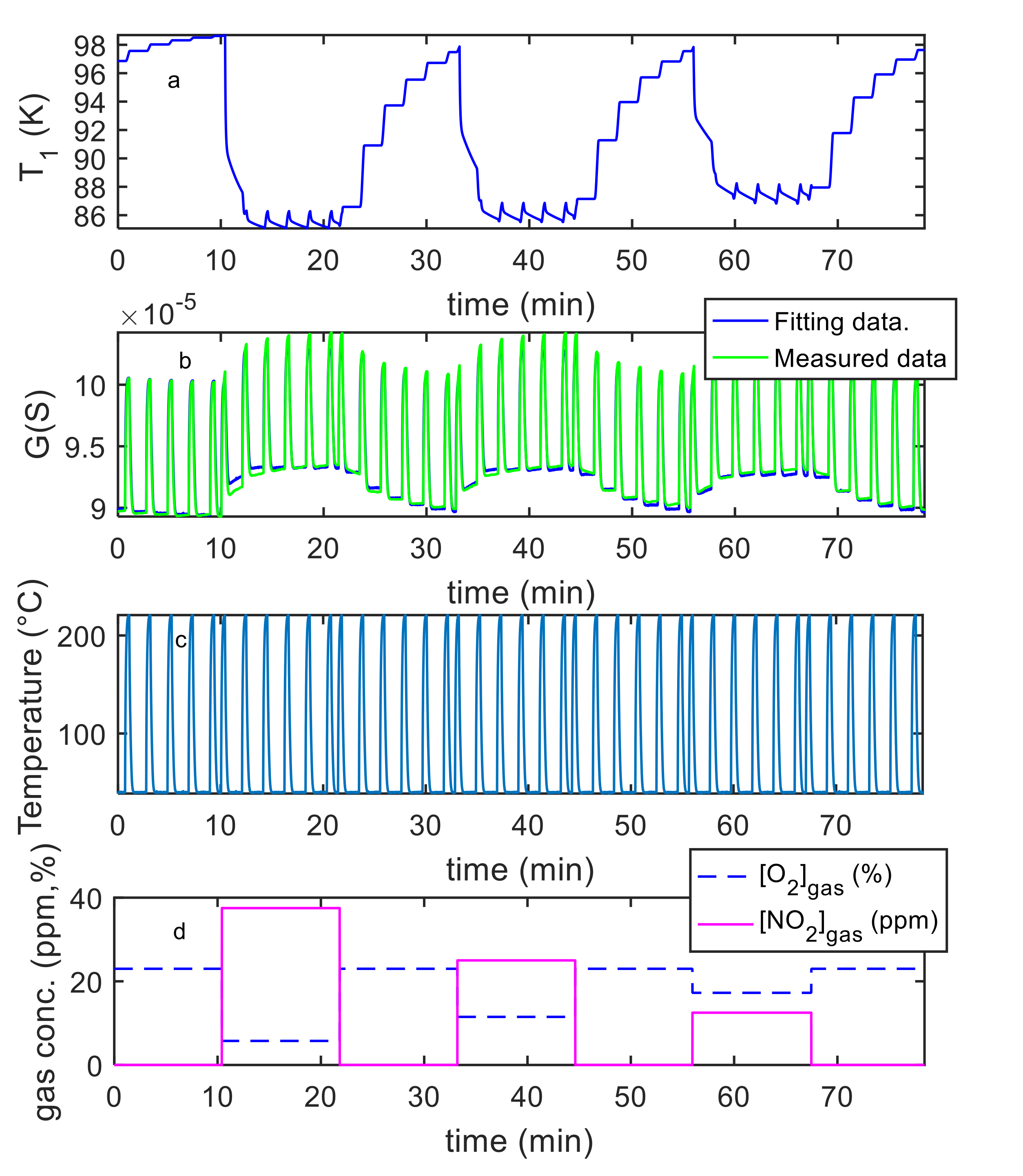
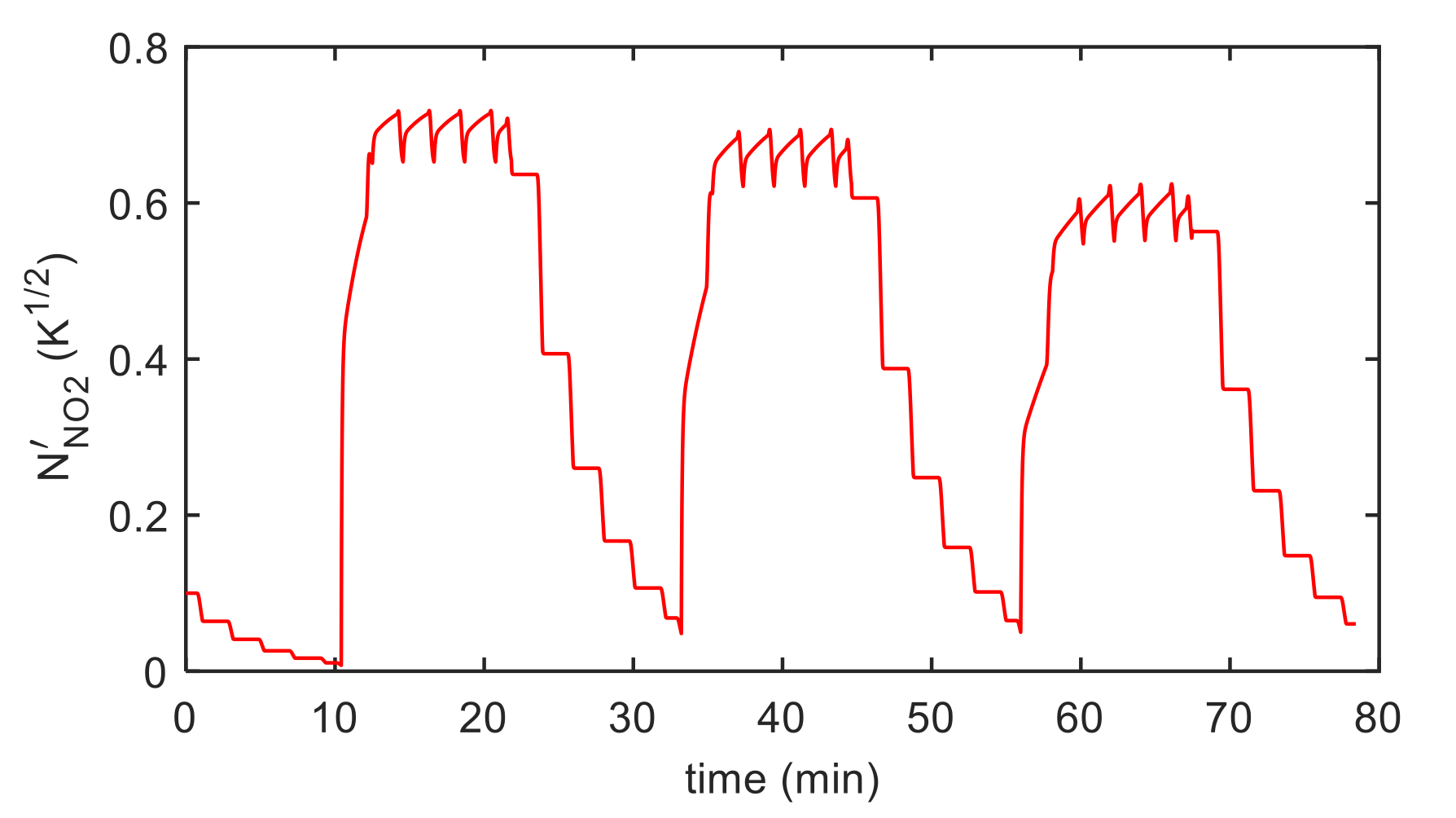
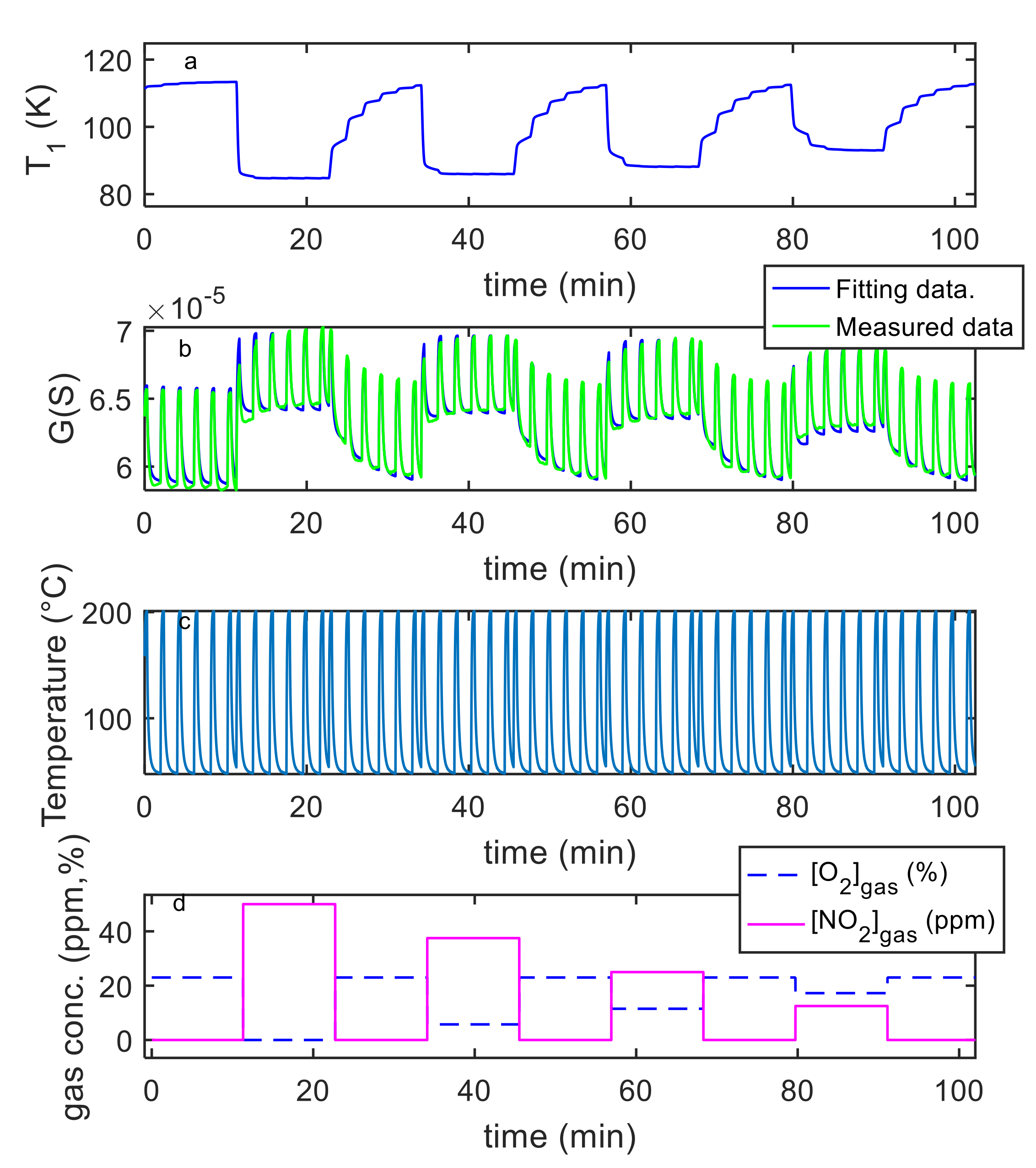
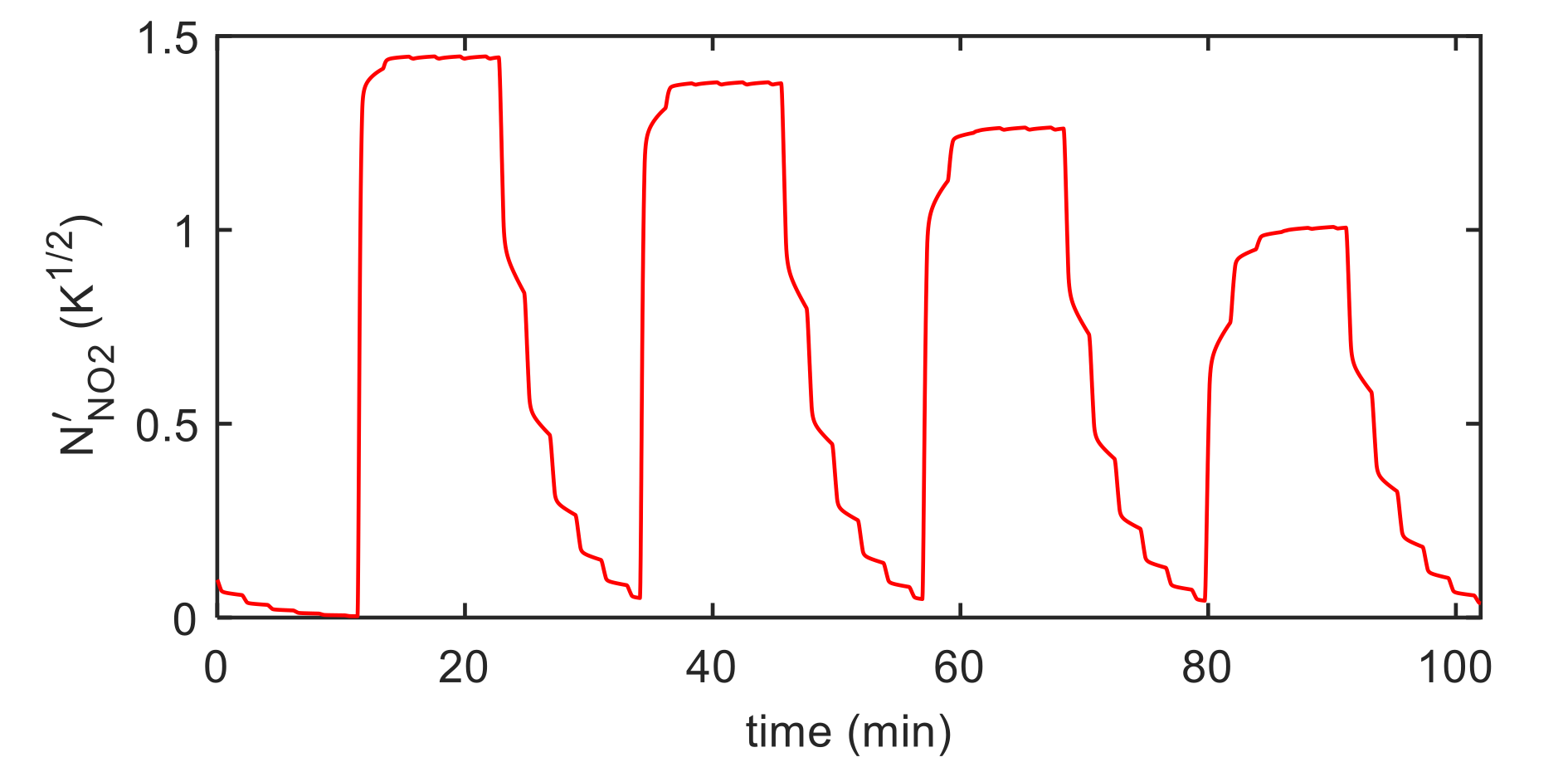
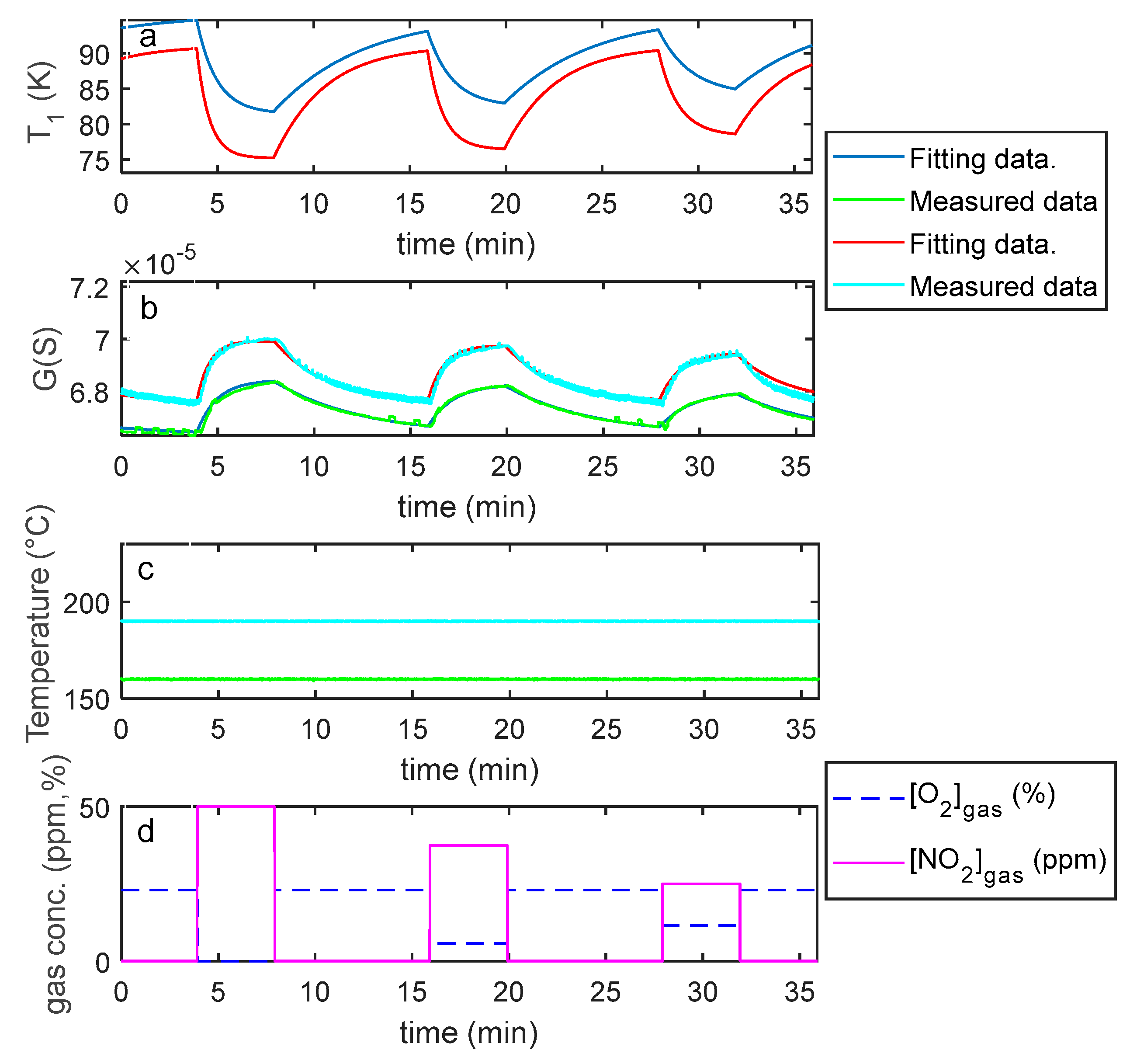
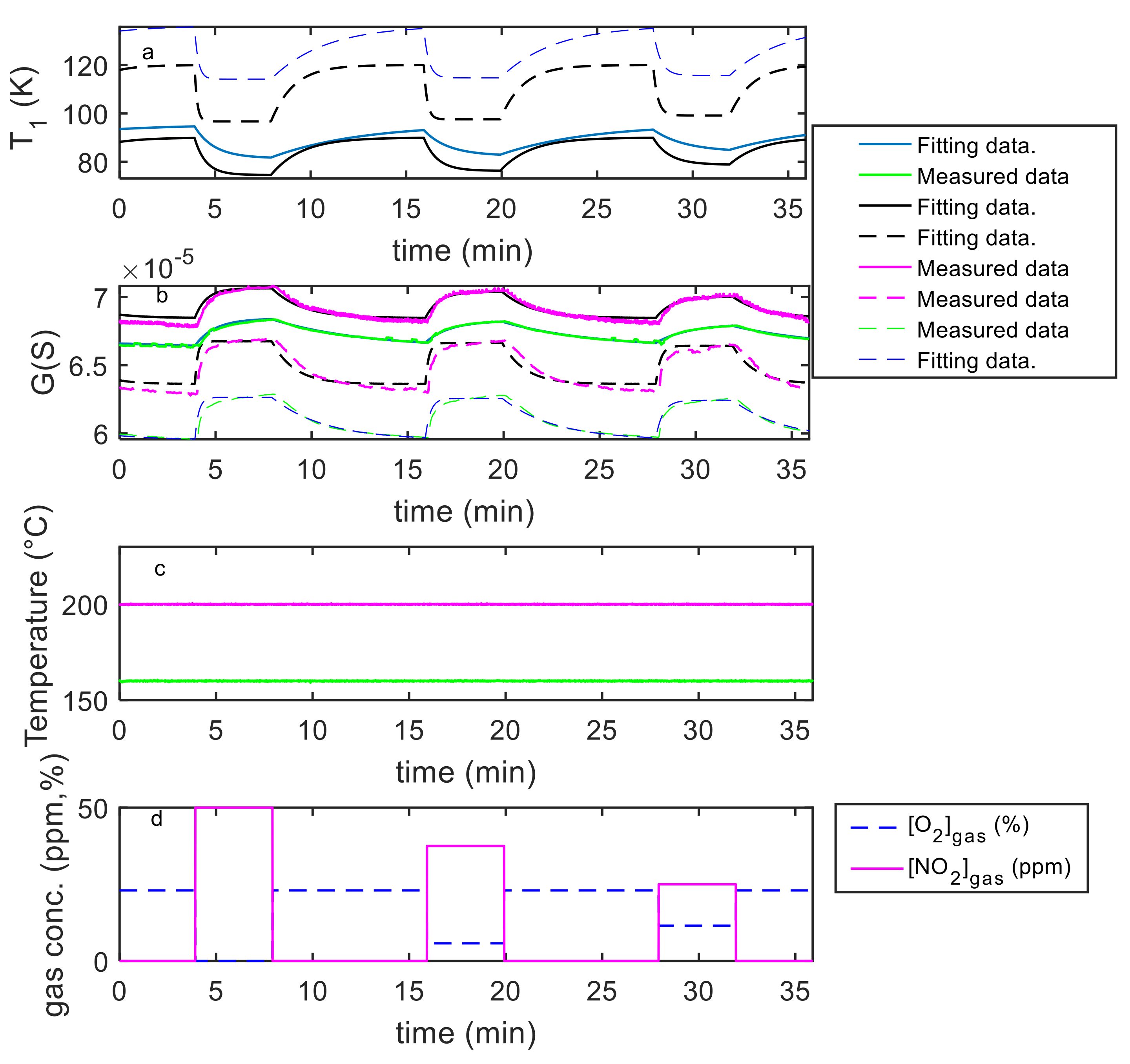
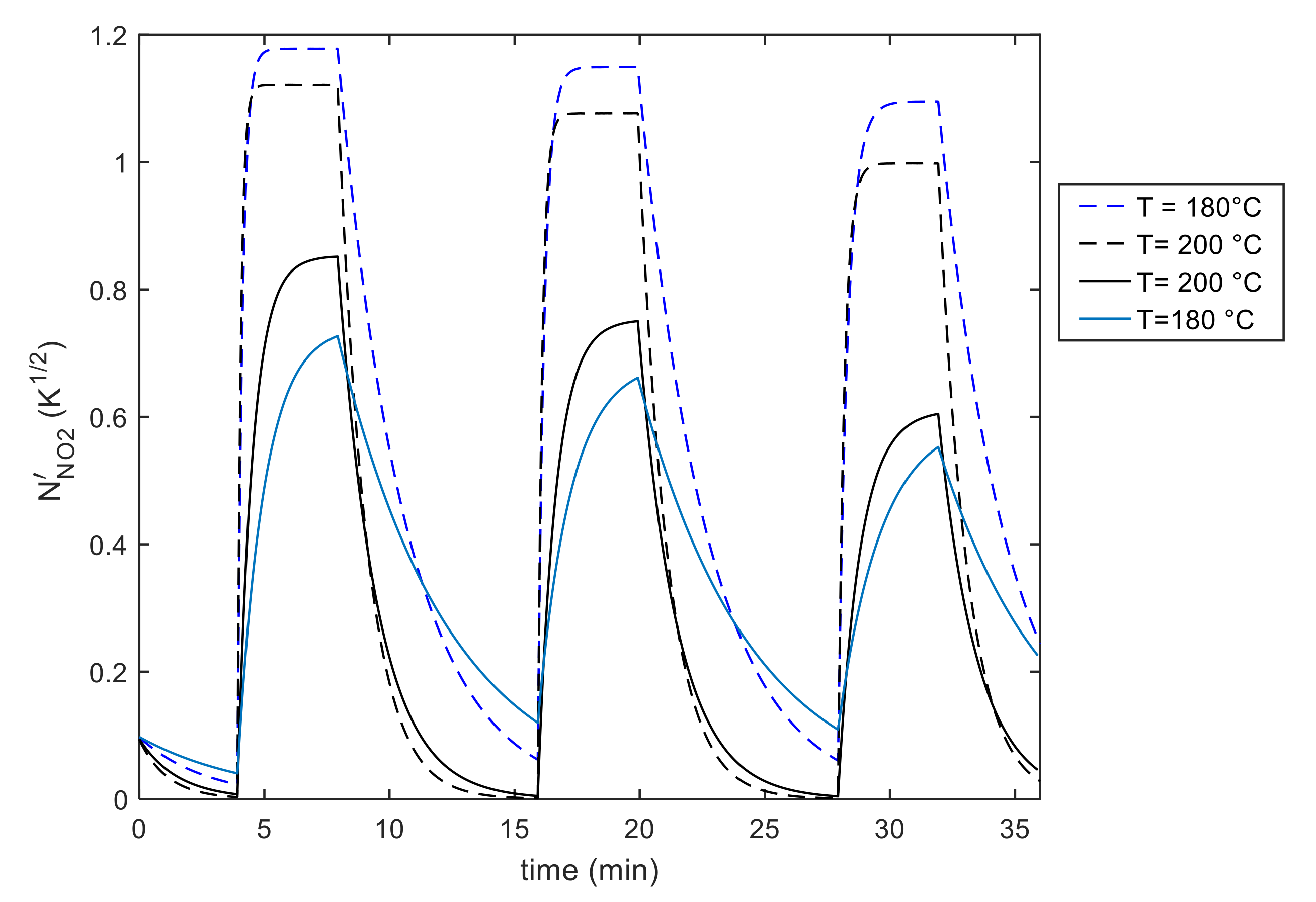
5. Model Parameter Estimation: Experimental Results and Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aasmundtveit, K.E.; Roy, A.; Ta, B.Q. Direct Integration of Carbon Nanotubes in CMOS, Towards an Industrially Feasible Process: A Review. IEEE Trans. Nanotechnol. 2020, 19, 113–122. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.L.; He, M.E.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, K.; Monika, N.; Deepak, K.; Neeraj, D.; Tankeshwar, K.; Ki-Hyun, K. Carbon nanotubes: A potential material for energy conversion and storage. Prog. Energy Combust. Sci. 2018, 64, 219–253. [Google Scholar]
- Sehrawat, P.; Julien, C.; Islam, S.S. Carbon nanotubes in Li-ion batteries: A review. Mater. Sci. Eng. B 2016, 213, 12–40. [Google Scholar] [CrossRef]
- Eshkalak, S.K.; Chinnappan, A.; Jayathilaka, W.A.D.M.; Khatibzadeh, M.; Kowsari, E.; Ramakrishna, S. A review on inkjet printing of CNT composites for smart applications. Appl. Mater. Today 2017, 9, 372–386. [Google Scholar] [CrossRef]
- Saito, R.; Fujita, M.; Dresselhaus, G.; Dresselhaus, M.S. Electronic structure of chiral graphene tubules. Appl. Phys. Lett. 1992, 60, 2204–2206. [Google Scholar] [CrossRef]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2013, 2013, 676815. [Google Scholar] [CrossRef]
- Rana, M.M.; Ibrahim, D.S.; Asyraf, M.M.; Jarin, S.; Tomal, A. A review on recent advances of CNTs as gas sensors. Sens. Rev. 2017, 37, 127–136. [Google Scholar] [CrossRef]
- Zhang, W.D.; Zhang, W.H. Carbon Nanotubes as Active Components for Gas Sensors. J. Sens. 2009, 2009, 160698. [Google Scholar] [CrossRef] [Green Version]
- Camilli, L.; Passacantando, M. Advances on Sensors Based on Carbon Nanotubes. Chemosensors 2018, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Shi, Y.; Hou, Z.; Wei, L. Carbon Nanotube-Based Chemiresistive Sensors. Sensors 2017, 17, 882. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, X.; Wu, Y.; Chen, Z.; He, C. Lead phthalocyanine modified carbon nanotubes with enhanced NH3 sensing performance. Sens. Actuators B Chem. 2012, 171, 398–404. [Google Scholar] [CrossRef]
- Hsu, H.; Jehng, J.; Sung, Y.; Wang, L.; Yang, S. The synthesis, characterization of oxidized multi-walled carbon nanotubes, and application to surface acoustic wave quartz crystal gas sensor. Mater. Chem. Phys. 2008, 109, 148–155. [Google Scholar] [CrossRef]
- Gautam, S.; Kumar, D.; Alegaonkar, P.S.; Jha, P.; Jain, N.; Rawat, J.S. Enhanced response and improved selectivity for toxic gases with functionalized CNT thin film resistors. Integr. Ferroelectr. 2018, 186, 65–70. [Google Scholar] [CrossRef]
- Pisal, S.; Bhat, T.; Deshmukh, H.; Patil, P. Functionalized Multi-Walled Carbon Nanotubes for Nitrogen Sensor. IOSR J. Appl. Chem. 2014, 7, 49–52. [Google Scholar] [CrossRef]
- Naje, A.N.; Ibraheem, R.R.; Ibrahim, F.T. Parametric Analysis of NO2 Gas Sensor Based on Carbon Nanotubes. Photonic Sens. 2016, 6, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Niu, L.; Luo, Y.; Li, Z. A highly selective chemical gas sensor based on functionalization of multi-walled carbon nanotubes with poly(ethylene glycol). Sens. Actuators B Chem. 2007, 126, 361–367. [Google Scholar] [CrossRef]
- Han, T.; Nag, A.; Mukhopadhyay, S.C.; Xu, Y. Carbon nanotubes and its gas-sensing applications: A review. Sens. Actuators A Phys. 2019, 291, 107–143. [Google Scholar] [CrossRef]
- Inoue, S.; Kokabu, T.; Matsumura, Y. Effects of physical and chemical adsorption on the electric conductance of carbon nanotube films. AIP Adv. 2018, 8, 15222. [Google Scholar] [CrossRef] [Green Version]
- Inoue, S.; Tomita, Y.; Kokabu, T.; Matsumura, Y. Principles of detection mechanism for adsorbed gases using carbon nanotube nanomat. Chem. Phys. Lett. 2018, 709, 77–81. [Google Scholar] [CrossRef]
- Kanchanatip, E.; Tulaphol, S.; Den, W.; Grisdanurak, N.; Miao, H.Y.; Yan, M. Sensing and adsorption study of gaseous phase chlorophenols on functionalized carbon nanotube membrane. Environ. Prog. Sustain. Energy 2019, 38, S315–S322. [Google Scholar] [CrossRef]
- Ijeomah, G.; Samsuri, F.; Zawawi, M.A.M. Analytical modelling of sensing performance of carbon nanotubes for gas sensing. Int. J. Nano Biomater. 2017, 7, 14–28. [Google Scholar] [CrossRef]
- Akbari, E.; Buntat, Z.; Ahmad, M.H.; Enzevaee, A.; Yousof, R.; Iqbal, S.M.Z.; Karimi, H. Analytical calculation of sensing parameters on carbon nanotube based gas sensors. Sensors 2014, 14, 5502–5515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagherzadeh-Nobari, S.; Istadeh, K.H.; Kalantarinejad, R. Computational modeling of transport properties of decorated SWCNT: Application in H 2 S gas sensor. J. Nanoparticle Res. 2020, 22, 1–17. [Google Scholar] [CrossRef]
- Hosseingholipourasl, A.; Hafizah Syed Ariffin, S.; Ahmadi, M.T.; Rahimian Koloor, S.S.; Petrů, M.; Hamzah, A. An Analytical Conductance Model for Gas Detection Based on a Zigzag Carbon Nanotube Sensor. Sensors 2020, 20, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, N.M.; Chinh, N.D.; Nguyen, T.D.; Kim, E.T.; Choi, G.; Kim, C.; Kim, D. Carbon nanotube-metal oxide nanocomposite gas sensing mechanism assessed via NO2 adsorption on n-WO3/p-MWCNT nanocomposites. Ceram. Int. 2020, 46, 29233–29243. [Google Scholar] [CrossRef]
- Fort, A.; Panzardi, E.; Al-Hamry, A.; Vignoli, V.; Mugnaini, M.; Addabbo, T.; Kanoun, O. Highly Sensitive Detection of NO2 by Au and TiO2 Nanoparticles Decorated SWCNTs Sensors. Sensors 2019, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Panzardi, E.; Lo Grasso, A.; Vignoli, V.; Mugnaini, M.; Lupetti, P.; Fort, A. NO2 Sensing with SWCNT Decorated by Nanoparticles in Temperature Pulsed Mode: Modeling and Characterization. Sensors 2020, 20, 4729. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, A.; Amadi, E.V.; Chen, Y.; Papadopoulos, C. Carbon Nanotube Assembly and Integration for Applications Nanoscale. Res. Lett. 2019, 14, 220. [Google Scholar]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube MolecularWires as Chemical Sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Kaiser, A.B.; Skakalova, V.; Roth, S. Modelling conduction in carbon nanotube networks with different thickness, chemical treatment, and irradiation. Phys. E Low-Dimens. Syst. Nanostruct. 2008, 40, 2311–2318. [Google Scholar]
- Nandi, U.; Jana, D.; Talukdar, D. Scaling description of non-ohmic direct current conduction in disordered systems. Prog. Mater. Sci. 2015, 71, 1–92. [Google Scholar] [CrossRef]
- Skákalová, V.; Kaiser, A.B.; Woo, Y.-S.; Roth, S. Electronic transport in carbon nanotubes: From individual nanotubes to thin and thick networks. Phys. Rev. B 2006, 74, 085403. [Google Scholar] [CrossRef]
- Cappelli, I.; Fort, A.; Lo Grasso, A.; Panzardi, E.; Mugnaini, M.; Vignoli, V. RH Sensing by Means of TiO2 Nanoparticles: A Comparison among Different Sensing Techniques Based on Modeling and Chemical/Physical Interpretation. Chemosensors 2020, 8, 89. [Google Scholar] [CrossRef]
- Fort, A.; Panzardi, E.; Vignoli, V.; Hjiri, M.; Aida, M.S.; Mugnaini, M.; Addabbo, T. Co3 O4/Al-ZnO nano-composites: Gas sensing properties. Sensors 2019, 19, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addabbo, T.; Bertocci, F.; Fort, A.; Mugnaini, M.; Vignoli, V.; Shahin, L.; Rocchi, S. Versatile measurement system for the characterization of gas sensing materials. In Proceedings of the 2013 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Institute of Electrical and Electronics Engineers (IEEE), Minneapolis, MN, USA, 6–9 May 2013; pp. 976–980. [Google Scholar]
- Nazhipkyzy, M.; Nurgain, A.; Zhaparova, A.A.; Seitkazinova, A.R.; Prikhodko, N.G.; Nemkayeva, R.R. Raman characteristics of multiwalled carbon nanotubes based on diatomite. Eurasian Chemico-Technol. J. 2018, 20, 319–323. [Google Scholar] [CrossRef]
- Yudianti, R.; Onggo, H.; Saito, Y.; Iwata, T.; Azuma, J.I. Analysis of functional group sited on multi-wall carbon nanotube surface. Open Mater. Sci. J. 2011, 5, 242–247. [Google Scholar] [CrossRef]





| MWCNT–COOH | MWCNT–COOH + Au | |
|---|---|---|
| (s−1) (@ 25 ppm NO2) | 9.4 | 18 |
| (s−1) | 6.1 × 106 | 7.06 |
| (K) | 3188 | 2752 |
| (K) | 9182 | 2800 |
| [S]′ (K1/2) | 0.97 | 1.68 |
| T01 (K) | 95 | 114 |
| n (K1/4) | 0.3 | 0.04 |
| RB (W) | 12,145 | 11,944 |
| MWCNT–COOH | MWCNT–COOH + Au | |||||
|---|---|---|---|---|---|---|
| T = 120 °C | T = 150 °C | T = 200 °C | T = 120 °C | T = 150 °C | T = 200 °C | |
| 1/vf (@ 25 ppm NO2) | 361 s/K1/2 | 200 s/K1/2 | 91 s/K1/2 | 9 s/K1/2 | 1.8 s/K1/2 | 3.2 s/K1/2 |
| (@ 25 ppm NO2) RESPONSE | 5.0 min | 2.3 min | 0.51 min | 1.2 min | 0.6 min | 0.35 min |
| (@ 0 ppm NO2) RECOVERY | 38 min | 7.3 min | 1.5 min | 8.5 min | 4.0 min | 2 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fort, A.; Mugnaini, M.; Panzardi, E.; Lo Grasso, A.; Al Hamry, A.; Adiraju, A.; Vignoli, V.; Kanoun, O. Modeling the Conductivity Response to NO2 Gas of Films Based on MWCNT Networks. Sensors 2021, 21, 4723. https://doi.org/10.3390/s21144723
Fort A, Mugnaini M, Panzardi E, Lo Grasso A, Al Hamry A, Adiraju A, Vignoli V, Kanoun O. Modeling the Conductivity Response to NO2 Gas of Films Based on MWCNT Networks. Sensors. 2021; 21(14):4723. https://doi.org/10.3390/s21144723
Chicago/Turabian StyleFort, Ada, Marco Mugnaini, Enza Panzardi, Anna Lo Grasso, Ammar Al Hamry, Anurag Adiraju, Valerio Vignoli, and Olfa Kanoun. 2021. "Modeling the Conductivity Response to NO2 Gas of Films Based on MWCNT Networks" Sensors 21, no. 14: 4723. https://doi.org/10.3390/s21144723








