Colorimetric Detection of Chromium(VI) Ions in Water Using Unfolded-Fullerene Carbon Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of N-CDs
2.3. Instrumentation for Characterization and Sensitivity Measurements
2.4. Procedure and Optimization for Sensitivity Measurements
3. Results and Discussion
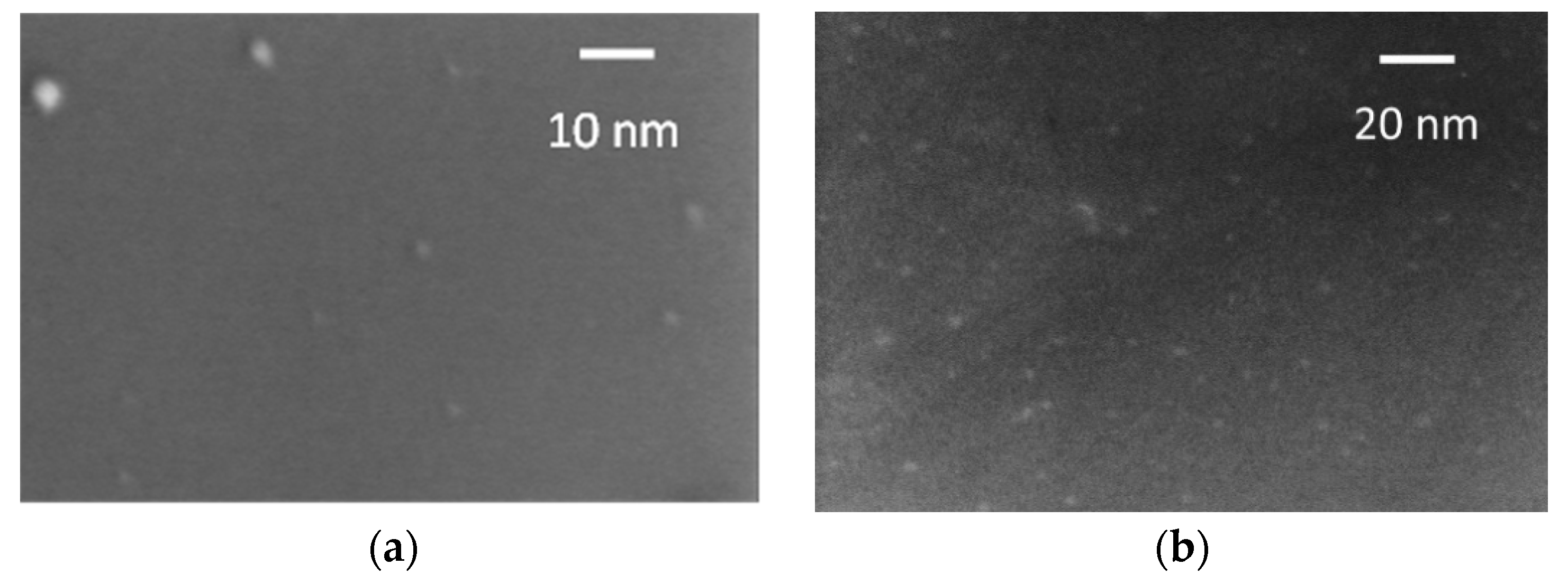
3.1. Morphological, Structural and Optical Characterization of N-CDs
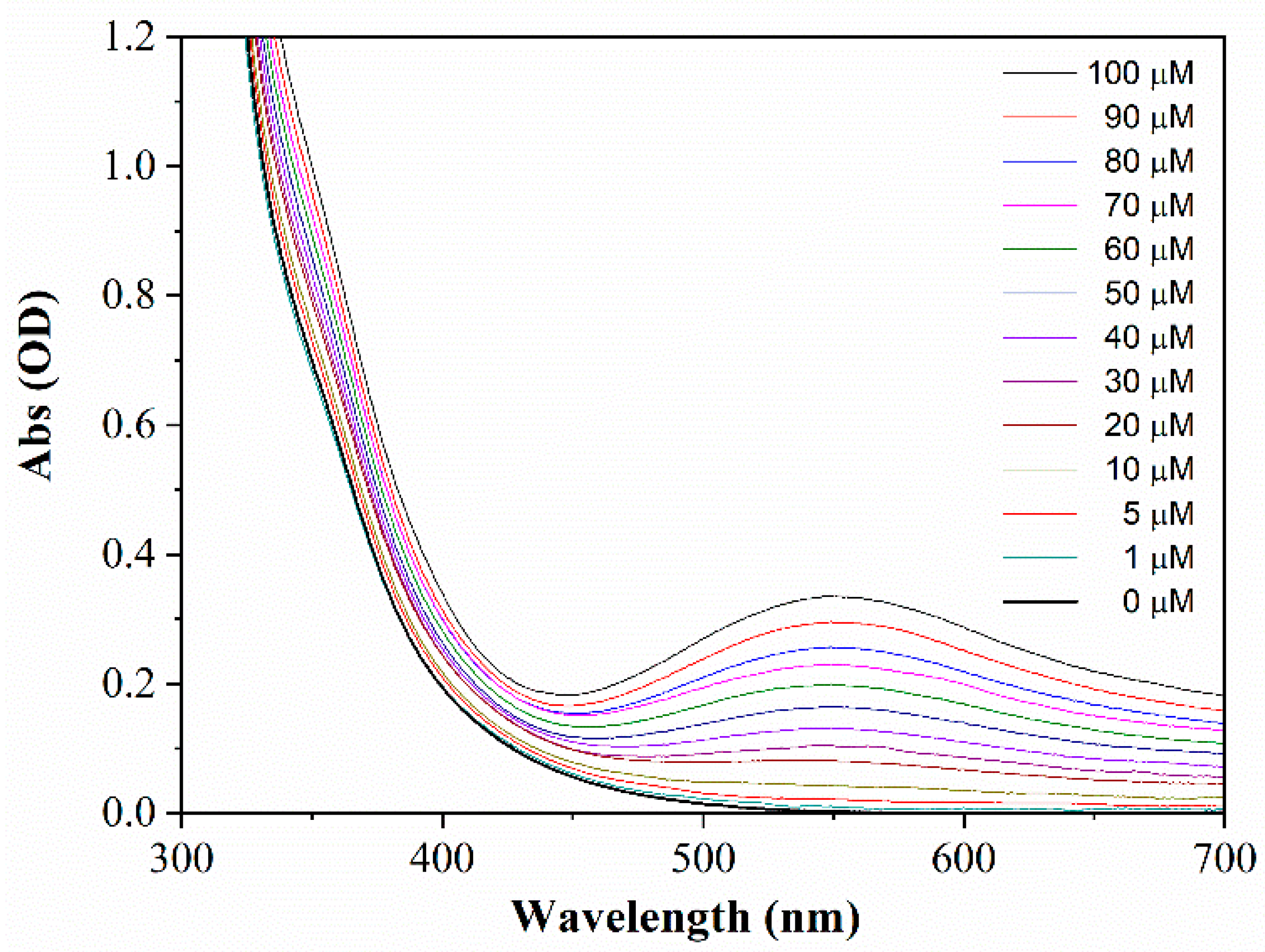
3.2. Colorimetric Sensing of Cr(VI) Ions
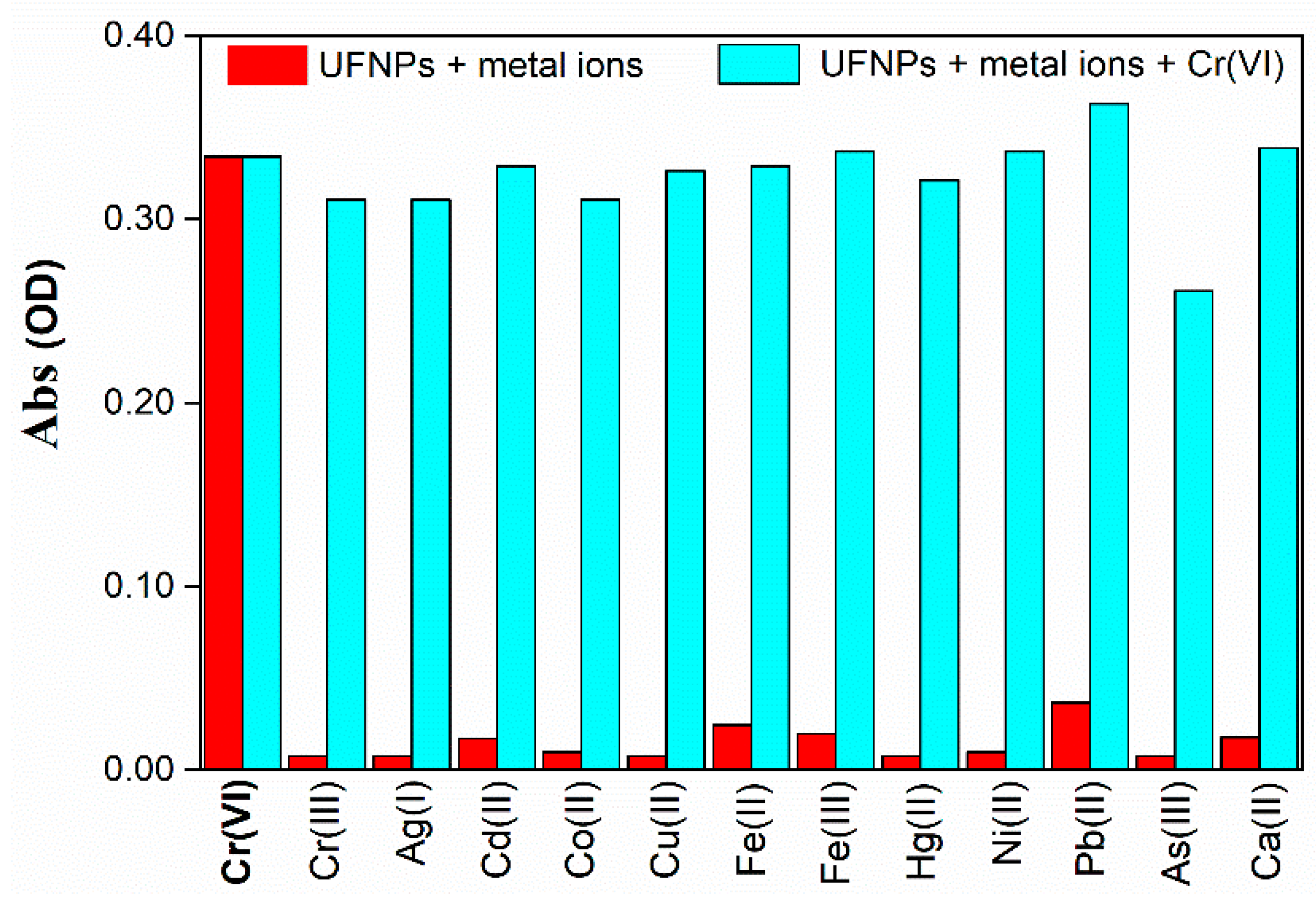
3.2.1. Selectivity for Cr(VI) Ions
3.2.2. Interfering Effects from Other HMs
3.2.3. Practical Application to Real Samples
3.2.4. Comparison with Other Sensing Materials and Techniques
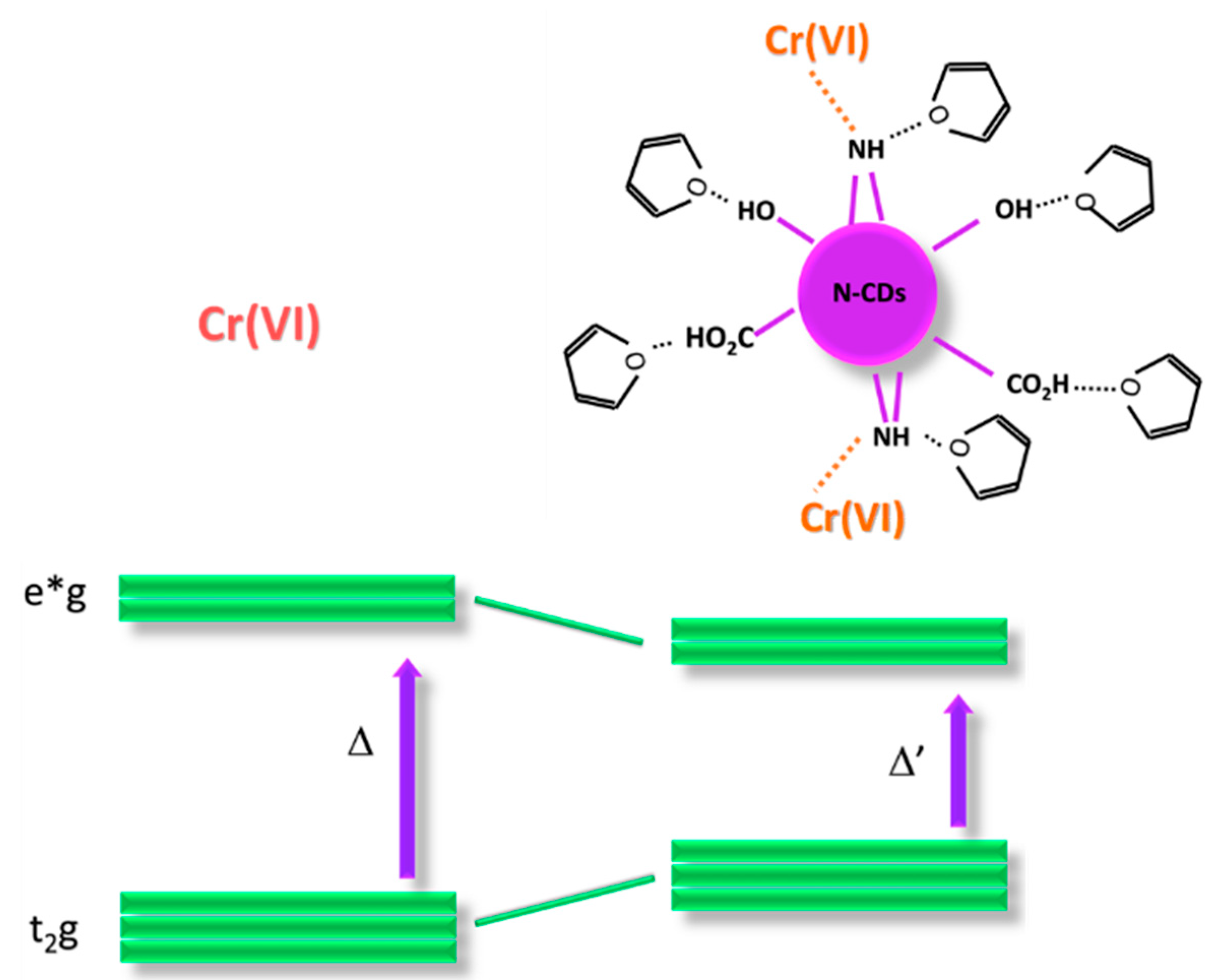
3.2.5. Possible Mechanism for the Interaction of Cr(VI) with N-CDs-W-THF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandez-Luqueno, F.; López-Valdez, F.; Gamero-Melo, P.; Luna-Suárez, S.; Aguilera-González, E.N.; Martínez, A.I.; García-Guillermo, M.D.S.; Hernández-Martínez, G.; Herrera-Mendoza, R.; Pérez-Velázquez, I.R. Heavy metal pollution in drinking water-a global risk for human health: A review. Afr. J. Environ. Sci. Technol. 2013, 7, 567–584. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology. Experientia Supplementum; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar]
- Gordon, B.; Callan, P.; Vickers, C. WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Ressalan, S.; Chauhan, R.S.; Goswami, A.K.; Purohit, D.N. Review of Spectrophotometric Methods for Determination of Chromium. Rev. Anal. Chem 1997, 16, 69–171. [Google Scholar] [CrossRef]
- Parks, J.L.; McNeill, L.; Frey, M.; Eaton, A.D.; Haghani, A.; Ramirez, L.; Edwards, M. Determination of total chromium in environmental water samples. Water Res. 2004, 38, 2827–2838. [Google Scholar] [CrossRef]
- Ackerman, A.H.; Creed, P.A.; Parks, A.N.; Fricke, M.W.; Schwegel, C.A.; Creed, J.T.; Heitkemper, D.T.; Vela, N.P. Comparison of a chemical and enzymatic extraction of arsenic from rice and an assessment of the arsenic absorption from contaminated water by cooked rice. Environ. Sci. Technol. 2005, 39, 5241–5246. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, W.; Jian, D.; Li, J.; Ding, H.; Yi, D.; Dingrong, Y.; Liu, F.; Wang, S. Quantitative remote and on-site Hg2+ detection using the handheld smartphone based optical fiber fluorescence sensor (SOFFS). Sens. Actuators B Chem. 2019, 301, 127168. [Google Scholar] [CrossRef]
- Li, H.F.; Lin, J.M. Applications of microfluidic systems in environmental analysis. Anal. Bioanal. Chem. 2008, 393, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Lace, A.; Ryan, D.; Bowkett, M.; Cleary, J. Chromium monitoring in water by colorimetry using optimised 1, 5-diphenylcarbazide method. Int. J. Environ. Res. Public Health 2019, 16, 1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. TrAC Trend Anal. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Li, F.M.; Liu, J.M.; Wang, X.X.; Lin, L.P.; Cai, W.L.; Lin, X.; Zeng, Y.N.; Li, Z.M.; Lin, S.Q. Non-aggregation based label free colorimetric sensor for the detection of Cr(VI) based on selective etching of gold nanorods. Sens. Actuators B Chem. 2011, 155, 817–822. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X. Colorimetric speciation of Cr(III) and Cr(VI) with a gold nanoparticle probe. Anal. Methods 2013, 5, 1442–1448. [Google Scholar] [CrossRef]
- He, S.; Lin, X.; Liang, H.; Xiao, F.; Li, F.; Liu, C.; Fan, P.; Yang, S.; Liu, Y. Colorimetric detection of Cr(VI) using silver nanoparticles functionalized with PVP. Anal. Methods 2019, 11, 5819–5825. [Google Scholar] [CrossRef]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of carbon dots in environmental pollution control: A review. Chem. Eng. J. 2020, 406, 126848. [Google Scholar] [CrossRef]
- Xu, D.; Lin, Q.; Chang, H.T. Recent advances and sensing applications of carbon dots. Small Methods 2020, 4, 1900387. [Google Scholar] [CrossRef]
- Nghia, N.N.; Lee, Y.I. Colorimetric detection of chromium(VI) using graphene oxide nanoparticles acting as a peroxidase mimetic catalyst and 8-hydroxyquinoline as an inhibitor. Microchim. Acta 2019, 186, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ciotta, E.; Paoloni, S.; Richetta, M.; Prosposito, P.; Tagliatesta, P.; Lorecchio, C.; Venditti, I.; Fratoddi, I.; Casciardi, S.; Pizzoferato, R. Sensitivity to Heavy-Metal Ions of Unfolded Fullerene Quantum Dots. Sensors 2017, 17, 2614. [Google Scholar] [CrossRef] [Green Version]
- Ciotta, E.; Prosposito, P.; Tagliatesta, P.; Lorecchio, C.; Stella, L.; Kaciulis, S.; Soltani, P.; Placidi, E.; Pizzoferrato, R. Discriminating between different heavy metal ions with fullerene-derived nanoparticles. Sensors 2018, 18, 1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.X.; Liu, C.F.; Wu, Z.L.; Zeng, Q.L.; Yang, X.X.; Wu, W.B.; Lia, W.F.; Huang, C.Z. A surfactant-assisted redox hydrothermal route to prepare highly photoluminescent carbon quantum dots with aggregation-induced emission enhancement properties. Chem. Commun. 2013, 49, 8015–8017. [Google Scholar] [CrossRef]
- Limosani, F.; Bauer, E.M.; Cecchetti, D.; Biagioni, S.; Orlando, V.; Pizzoferrato, R.; Prosposito, P.; Carbone, M. Top-Down N-Doped Carbon Quantum Dots for Multiple Purposes: Heavy Metal Detection and Intracellular Fluorescence. Nanomaterials 2021, 11, 2249. [Google Scholar] [CrossRef]
- Scarpellini, D.; Paoloni, S.; Medaglia, P.G.; Pizzoferrato, R.; Orsini, A.; Falconi, C. Structural and optical properties of dense vertically aligned ZnO nanorods grown onto silver and gold thin films by galvanic effect with iron contamination. Mater. Res. Bull. 2015, 65, 231–237. [Google Scholar] [CrossRef]
- Zygouri, P.; Spyrou, K.; Mitsari, E.; Barrio, M.; Macovez, R.; Patila, M.; Stamatis, H.; Verginadis, I.I.; Velapoulou, A.P.; Evangelou, A.M.; et al. A facile approach to hydrophilic oxidized fullerenes and their derivatives as cytotoxic agents and supports for nanobiocatalytic systems. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Li, J.; Xie, Z.; Sun, Z. Tailoring color emissions from N-doped graphene quantum dots for bioimaging applications. Light Sci. Appl. 2015, 4, e364. [Google Scholar]
- Qu, D.; Zheng, M.; Du, P.; Zhou, Y.; Ligong, Z.; Li, D.; Tan, H.; Zhao, Z.; Xie, Z.; Sun, Z. Highly luminescent S,N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 2013, 5, 12272. [Google Scholar] [CrossRef] [PubMed]
- Afreen, S.; Kokubo, K.; Muthoosamy, K.; Manickam, S. Hydration or hydroxylation: Direct synthesis of fullerenol from pristine fullerene [C60] via acoustic cavitation in the presence of hydrogen peroxide. RSC Adv. 2017, 7, 31930. [Google Scholar] [CrossRef] [Green Version]
- Acik, M.; Lee, G.; Mattevi, C.; Chhowalla, M.; Cho, K.; Chabal, Y.J. Unusual infrared-absorption mechanism in thermally reduced graphene oxide. Nat. Mater. 2010, 9, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.S.; Sahatiya, P.; Badhulika, S. One step, high yield synthesis of amphiphilic carbon quantum dots derived from chia seeds: A solvatochromic study. New J. Chem. 2017, 41, 13130–13139. [Google Scholar] [CrossRef]
- Lin, H.; Ding, L.; Zhang, B.; Huang, J. Detection of nitrite based on fluorescent carbon dots by the hydrothermal method with folic acid. R. Soc. Open Sci. 2018, 5, 172149. [Google Scholar] [CrossRef] [Green Version]
- Ghayyem, S.; Swaidan, A.; Barras, A.; Dolci, M.; Faridbod, F.; Szunerits, S.; Boukherroub, R. Colorimetric detection of chromium (VI) ion using poly (N-phenylglycine) nanoparticles acting as a peroxidase mimetic catalyst. Talanta 2021, 226, 122082. [Google Scholar] [CrossRef]
- Gao, Y.; Jiao, Y.; Lu, W.; Liu, Y.; Han, H.; Gong, X.; Xian, M.; Shuang, S.; Dong, C. Carbon dots with red emission as a fluorescent and colorimetric dual-readout probe for the detection of chromium(VI) and cysteine and its logic gate operation. J. Mater. Chem. B 2018, 6, 6099–6107. [Google Scholar] [CrossRef]
- Borthakur, P.; Boruah, P.K.; Das, M.R.; Szunerits, S.; Boukherroub, R. Cu(0) nanoparticle-decorated functionalized reduced graphene oxide sheets as artificial peroxidase enzymes: Application for colorimetric detection of Cr(VI) ions. New J. Chem. 2019, 43, 1404–1414. [Google Scholar] [CrossRef]
- Guo, J.F.; Huo, D.Q.; Yang, M.; Hou, C.J.; Li, J.J.; Fa, H.B.; Luo, H.B.; Yang, P. Colorimetric detection of Cr(VI) based on the leaching of gold nanoparticles using a paper-based sensor. Talanta 2016, 161, 819–825. [Google Scholar] [CrossRef]
- Dong, C.; Wu, G.; Wang, Z.; Ren, W.; Zhang, Y.; Shen, Z.; Li, T.; Wu, A. Selective colorimetric detection of Cr(III) and Cr(VI) using gallic acid capped gold nanoparticles. Dalton Trans. 2016, 45, 8347–8354. [Google Scholar] [CrossRef]
- Mutuyimana, F.P.; Liu, J.; Nsanzamahoro, S.; Na, M.; Chen, H.; Chen, X. Yellow-emissive carbon dots as a fluorescent probe for chromium (VI). Microchim. Acta 2019, 186, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Bao, Q.; Wu, C.; Hu, M.; Chen, Y.; Wang, L.; Chen, W. Carbon dots derived from Poria cocos polysaccharide as an effective” on-off” fluorescence sensor for chromium(VI) detection. J. Pharm. Anal. 2021, in press. [Google Scholar] [CrossRef]
- Breslin, C.B.; Branagan, D.; Garry, L.M. Electrochemical detection of Cr(VI) with carbon nanotubes decorated with gold nanoparticles. J. Appl. Electrochem. 2019, 49, 195–205. [Google Scholar] [CrossRef]
- Liu, S.; Cui, J.; Huang, J.; Tian, B.; Jia, F.; Wang, Z. Facile one-pot synthesis of highly fluorescent nitrogen-doped carbon dots by mild hydrothermal method and their applications in detection of Cr(VI) ions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 65–71. [Google Scholar] [CrossRef]
- Li, P.; Li, S.F.Y. Recent advances in fluorescence probes based on carbon dots for sensing and speciation of heavy metals. Nanophotonics 2021, 10, 877–908. [Google Scholar] [CrossRef]
- Devi, P.; Rajput, P.; Thakur, A.; Kim, K.H.; Kumar, P. Recent advances in carbon quantum dot-based sensing of heavy metals in water. TrAC Trend Anal. Chem. 2019, 114, 171–195. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, S.; Liu, J.; Li, J.; Lu, X. Efficient Visual Chemosensor for Hexavalent Chromium via a Controlled Strategy for Signal Amplification in Water. Anal. Chem. 2020, 92, 3426–3433. [Google Scholar] [CrossRef]




| Sample | Addition | Diameter (nm) | Error (nm) |
|---|---|---|---|
| N-CDs-W | 3.50 | ±0.20 | |
| N-CDs-W-THF | 3.12 | ±0.30 | |
| N-CDs-W-THF | Cr (IV) 5 mM | 4.00 | ±0.09 |
| Sample | Spiked Cr(VI) (µM) | Found Cr(VI) (µM) | Recovery (%) |
|---|---|---|---|
| Lake water Lago Albano (Italy) | 50 | 53 | 106 |
| 10 | 9.8 | 98 | |
| 5 | 5.4 | 108 | |
| 2 | 2.2 | 110 | |
| 1 | 1.1 | 110 | |
| 50 | 47 | 95 | |
| Tap water | 10 | 10.7 | 107 |
| (Rome, Italy) | 5 | 5.2 | 104 |
| 2 | 2.2 | 110 | |
| 1 | 1.21 | 121 |
| Materials | Method | Linear Range (µM) | Limit of Detection (µM) | Reference |
|---|---|---|---|---|
| Poly (N-Phenylglycine) NPs | Colorimetric | 0.01–0.1 | 0.012 | [29] |
| “ ” | Colorimetric | 0.05–12.5 | 0.52 | [29] |
| Cu-decorated GO | Colorimetric | 0–0.2 | 0.067 | [31] |
| Au NPs | Colorimetric | 0.5–50 | 0.280 | [32] |
| Au nanorods | Colorimetric | 0.1–20 | 0.088 | [11] |
| PVP-Ag NPs | Colorimetric | 0.1–2.4 | 0.034 | [13] |
| Au NPs | Colorimetric | 0.15–2 | 0.1 | [33] |
| citrate-capped Au NPs | Colorimetric | 2–10 | 0.3 | [12] |
| GO NPs | Colorimetric | 0.07–0.43 | 0.0058 | [16] |
| Carbon dots | Fluorescence | 0.5–260 | 0.260 | [30] |
| Carbon dots | Fluorescence | 1–400 * | 0.13 | [34] |
| Carbon dots | Fluorescence | 1–100 | 0.25 | [35] |
| 1,5-Diphenylcarbazide | Colorimetric | 0.6–140 | 0.46 | [9] |
| Au decorated/CNTs | Electrochemistry | 0.8–230 | 0.72 | [36] |
| N-CDs-W-THF | Colorimetric | 0–100 | 0.30 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babazadeh, S.; Bisauriya, R.; Carbone, M.; Roselli, L.; Cecchetti, D.; Bauer, E.M.; Sennato, S.; Prosposito, P.; Pizzoferrato, R. Colorimetric Detection of Chromium(VI) Ions in Water Using Unfolded-Fullerene Carbon Nanoparticles. Sensors 2021, 21, 6353. https://doi.org/10.3390/s21196353
Babazadeh S, Bisauriya R, Carbone M, Roselli L, Cecchetti D, Bauer EM, Sennato S, Prosposito P, Pizzoferrato R. Colorimetric Detection of Chromium(VI) Ions in Water Using Unfolded-Fullerene Carbon Nanoparticles. Sensors. 2021; 21(19):6353. https://doi.org/10.3390/s21196353
Chicago/Turabian StyleBabazadeh, Saeedeh, Ramanand Bisauriya, Marilena Carbone, Ludovica Roselli, Daniele Cecchetti, Elvira Maria Bauer, Simona Sennato, Paolo Prosposito, and Roberto Pizzoferrato. 2021. "Colorimetric Detection of Chromium(VI) Ions in Water Using Unfolded-Fullerene Carbon Nanoparticles" Sensors 21, no. 19: 6353. https://doi.org/10.3390/s21196353






