Biopotential Signal Monitoring Systems in Rehabilitation: A Review
Abstract
:1. Introduction
2. Background
2.1. EMG and Rehabilitation
2.1.1. EMG in Neurorehabilitation
2.1.2. EMG in Stroke Rehabilitation
2.1.3. EMG in Sports Rehabilitation
2.2. EMG Signal Acquisition: General Considerations
2.2.1. EMG Signal Features
2.2.2. EMG Instrumentation Characteristics
- Accuracy: this characteristic is related to the implementation of the differential amplifier, ADC and several other components connected to inherent noise; the aim is to optimize each used component to minimize noise, ensuring accuracy;
- Sensitivity: this features on the ADC resolution and consequently the overall resolution of the system; it allows the physicians to understand the limits of their reading;
- CMRR: this is the Common-Mode Rejection Ratio, and it expresses the ability of the differential amplifier to reject common-mode signals; it plays a crucial role in avoiding 50–60 Hz power line interference;
- Input impedance: the optimization of this value is relevant in differential amplifier selections and implementations related to different user skin types and electrode interfaces;
- Input range: this specification regards hardware implementation and ADC, specifying the range of the biosignal that can be picked up without saturating the amplifier. A larger input range is preferred to acquire the entire signal, but this requires an expansion of signal resolution;
- SNR: this is the Signal-to-Noise Ratio, and it is the ratio between the signal’s amplitude and the background noise.
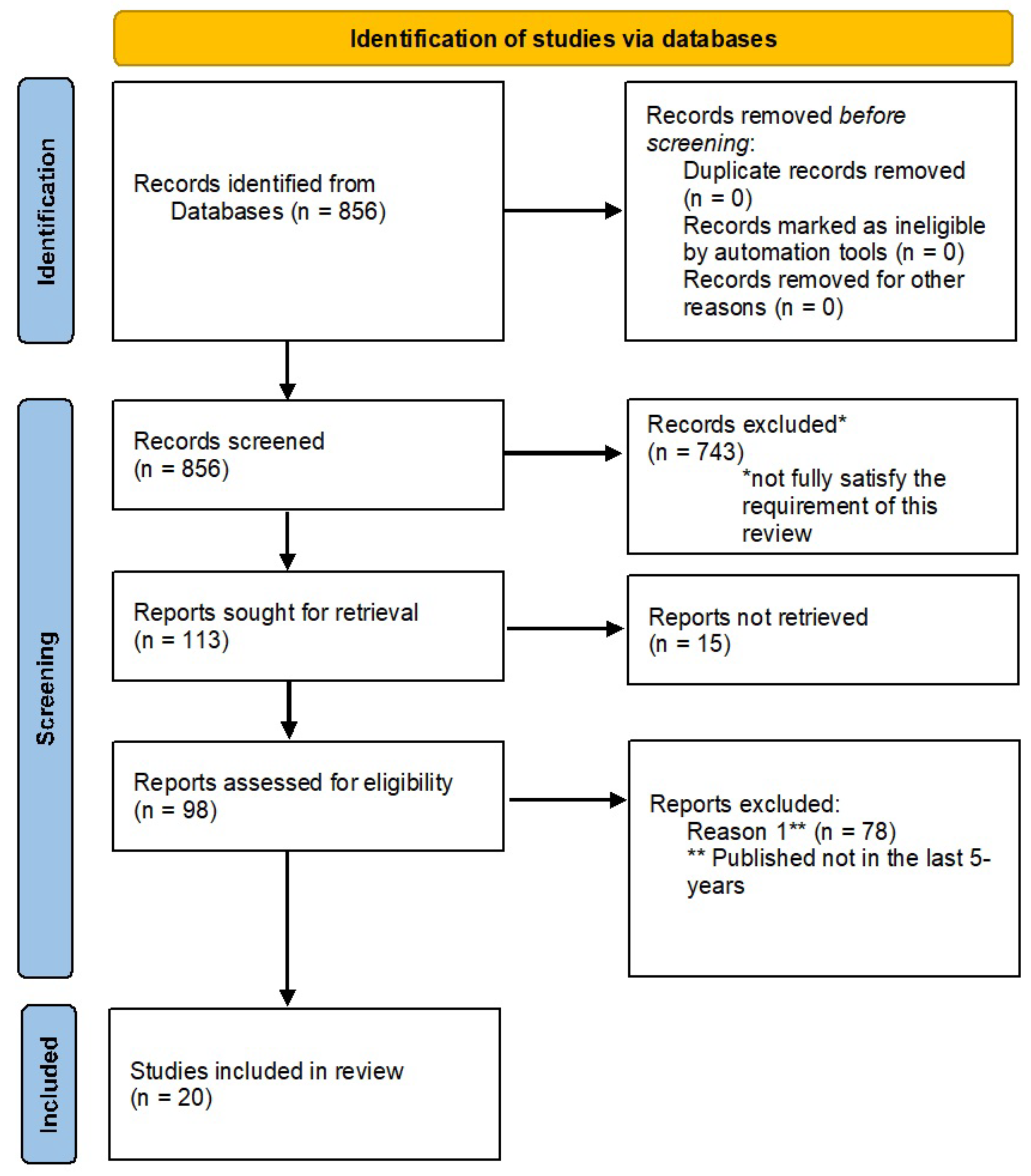
3. Research Methodology
- RQ1: what are the most recent contributions in literature?
- RQ2: what are the commonly used medical devices?
- RQ3: how do these contributions and medical devices support physiological monitoring in rehabilitation?
- RQ4: what are the future directions and opportunities for EMG signal acquisition and analysis in a rehabiliation context?
4. Wearable Devices for Rehabilitation
5. Commercial Wearable Devices
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EEG | Electroencephalogram |
| ECG | Electrocardiogram |
| EMG | Electromyogram |
| sEMG | Surface Electromyography |
| IC | Integrated Circuit |
| NMES | Neuromuscular Electrical Stimulation |
| BCI | Brain–Computer Interface |
| ADC | Analog-to-Digital Converter |
| CMRR | Common-Mode Rejection Ratio |
| SNR | Signal-to-Noise Ratio |
| AFE | Analog Front-End |
| PGA | Programmable Gain Amplifier |
| LNA | Low-Noise Amplifier |
| BLE | Bluetooth Low Energy |
| BIA | Bioelectrical Impedence Analyzer |
| CBM | Capacitive Biopotential Measurements |
| SoC | System-on-Chip |
| ASIC | Application Specified Integrated Circuit |
References
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable sensors for monitoring the physiological and biochemical profile of the athlete. NPJ Digit. Med. 2019, 2, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, A.; Calabrese, B.; Ielpo, N.; Demeco, A.; Ammendolia, A.; Corchiola, D. Cloud-based biomedical system for remote monitoring of ALS patients. In Proceedings of the 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Seoul, Korea, 16–19 December 2020; pp. 1469–1476. [Google Scholar]
- Qureshi, F.; Krishnan, S. Wearable hardware design for the internet of medical things (IoMT). Sensors 2018, 18, 3812. [Google Scholar] [CrossRef] [Green Version]
- Postolache, O.A.; Mukhopadhyay, S.C.; Jayasundera, K.P.; Swain, A.K. Sensors for Everyday Life: Healthcare Settings (Smart Sensors, Measurement and Instrumentation, 22); Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Palumbo, A.; Vizza, P.; Veltri, P.; Gambardella, A.; Pucci, F.; Sturniolo, M. Design of an electronic device for brain computer interface applications. In Proceedings of the IEEE International Workshop on Medical Measurements and Applications (MEMEA), Cetraro, Italy, 29–30 May 2009; pp. 99–103. [Google Scholar]
- Vizza, P.; Tradigo, G. On the analysis of biomedical signals for disease classification. ACM SIGBioinform. Rec. 2019, 8, 7–10. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Vizza, P.; Tradigo, G.; Curcio, A.; Indolfi, C.; Veltri, P. Intracavitary signal analysis for atrial fibrillation prediction. In Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine Workshops, Philadelphia, PA, USA, 4–7 October 2012; pp. 814–815. [Google Scholar]
- Cong, P. Circuit Design Considerations for Implantable Devices; River Publishers: Gistrup, Denmark, 2018; p. 9788793519862. [Google Scholar]
- Panja, A.; Fernandes, R.; Jondhale, S.; D’souza, P.; Uma, L.; Thale, S. A novel configurable signal acquisition system for multiple bio-signal measurements: Assistive technology for home rehabilitation. In Proceedings of the IEEE International Conference on Technological Advancements in Power and Energy (TAP Energy), Kollam, India, 21–23 December 2017; pp. 1–6. [Google Scholar]
- Li, R.T.; Kling, S.R.; Salata, M.J.; Cupp, S.A.; Sheehan, J.; Voos, J.E. Wearable performance devices in sports medicine. Sport. Health 2016, 8, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Steele, C. Applications of EMG in Clinical and Sports Medicine; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Nascimento, L.M.S.D.; Bonfati, L.V.; Freitas, M.L.B.; Mendes Junior, J.J.A.; Siqueira, H.V.; Stevan, S.L., Jr. Sensors and Systems for Physical Rehabilitation and Health Monitoring-A Review. Sensors 2020, 20, 4063. [Google Scholar] [CrossRef]
- Negrini, S.; Kiekens, C.; Bernetti, A.; Capecci, M.; Ceravolo, M.G.; Lavezzi, S.; Zampolini, M.; Boldrini, P. Telemedicine from research to practice during the pandemic. “Instant paper from the field” on rehabilitation answers to the COVID-19 emergency. Eur. J. Phys. Rehabil. Med. 2020, 56, 327–330. [Google Scholar] [CrossRef]
- Varela-Aldás, J.; Buele, J.; Ramos Lorente, P.; García-Magariño, I.; Palacios-Navarro, G. A Virtual Reality-Based Cognitive TeleRehabilitation System for Use in the COVID-19 Pandemic. Sustainability 2021, 13, 2183. [Google Scholar] [CrossRef]
- Smith, E.E.; Mountain, A.; Hill, M.D.; Wein, T.H.; Blacquiere, D.; Casaubon, L.K.; Linkewich, E.; Foley, N.; Gubitz, G.; Simard, A.; et al. Canadian stroke best practice guidance during the COVID-19 pandemic. Can. J. Neurol. Sci. 2020, 47, 474–478. [Google Scholar] [CrossRef]
- Salawu, A.; Green, A.; Crooks, M.G.; Brixey, N.; Ross, D.H.; Sivan, M. A proposal for multidisciplinary tele-rehabilitation in the assessment and rehabilitation of COVID-19 survivors. Int. J. Environ. Res. Public Health 2020, 17, 4890. [Google Scholar] [CrossRef]
- Werneke, M.W.; Deutscher, D.; Grigsby, D.; Tucker, C.A.; Mioduski, J.E.; Hayes, D. Telerehabilitation during the COVID-19 Pandemic in Outpatient Rehabilitation Settings: A Descriptive Study. Phys. Ther. 2021, 101, pzab110. [Google Scholar] [CrossRef]
- Tanaka, M.J.; Oh, L.S.; Martin, S.D.; Berkson, E.M. Telemedicine in the era of COVID-19: The virtual orthopaedic examination. J. Bone Jt. Surgery. Am. Vol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Andrenelli, E.; Negrini, F.; de Sire, A.; Arienti, C.; Patrini, M.; Negrini, S.; Ceravolo, M.G. International Multiprofessional Steering Committee of Cochrane Rehabilitation REH-COVER action. Systematic rapid living review on rehabilitation needs due to COVID-19: Update to May 31, 2020. Eur. J. Phys. Rehabil. Med. 2020, 56, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Bickton, F.M.; Chisati, E.; Rylance, J.; Morton, B. An Improvised Pulmonary Telerehabilitation Program for Postacute COVID-19 Patients Would Be Feasible and Acceptable in a Low-Resource Setting. Am. J. Phys. Med. Rehabil. 2021, 100, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Ansari, M.S. Analog front-end design for biomedical signal acquisition systems. CSI Trans. ICT 2019, 7, 199–204. [Google Scholar] [CrossRef]
- Rajeswari, J.; Jagannath, M. Advances in biomedical signal and image processing—A systematic review. Inform. Med. Unlocked 2017, 8, 13–19. [Google Scholar] [CrossRef]
- Gohel, V.; Mehendale, N. Review on electromyography signal acquisition and processing. Biophys. Rev. 2020, 12, 1361–1367. [Google Scholar] [CrossRef]
- Houssein, E.H.; Kilany, M.; Hassanien, A.E. ECG signals classification: A review. Int. J. Intell. Eng. Inform. 2017, 5, 376–396. [Google Scholar] [CrossRef]
- Faust, O.; Hagiwara, Y.; Hong, T.J.; Lih, O.S.; Acharya, U.R. Deep learning for healthcare applications based on physiological signals: A review. Comput. Methods Programs Biomed. 2018, 161, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rahul, J.; Sora, M.; Sharma, L.D. An overview on biomedical signal analysis. Int. J. Recent Technol. Eng. 2019, 7, 206–209. [Google Scholar]
- Ielpo, N.; Calabrese, B.; Cannataro, M.; Palumbo, A.; Ciliberti, S.; Grillo, C.; Iocco, M. EMG-Miner: Automatic Acquisition and Processing of Electromyographic Signals: First Experimentation in a Clinical Context for Gait Disorders Evaluation. In Proceedings of the 2014 IEEE 27th International Symposium on Computer-Based Medical Systems, New York, NY, USA, 27–29 May 2014; pp. 441–446. [Google Scholar]
- Uktveris, T.; Jusas, V. Development of a modular board for EEG signal acquisition. Sensors 2018, 18, 2140. [Google Scholar] [CrossRef] [Green Version]
- Aljalal, M.; Ibrahim, S.; Djemal, R.; Ko, W. Comprehensive review on brain-controlled mobile robots and robotic arms based on electroencephalography signals. Intell. Serv. Robot. 2020, 13, 539–563. [Google Scholar] [CrossRef]
- Merletti, R.; Botter, A.; Cescon, C.; Minetto, M.A.; Vieira, T.M. Advances in surface EMG: Recent progress in clinical research applications. Crit. Rev. Biomed. Eng. 2010, 38, 347–379. [Google Scholar] [CrossRef]
- Drost, G.; Stegeman, D.F.; van Engelen, B.G.M.; Zwarts, M.J. Clinical applications of high-density surface EMG: A systematic review. J. Electromyogr. Kinesiol. 2006, 16, 586–602. [Google Scholar] [CrossRef]
- Campanini, I.; Disselhorst-Klug, C.; Rymer, W.Z.; Merletti, R. Surface EMG in Clinical Assessment and Neurorehabilitation: Barriers limiting its use. Front. Neurol. 2020, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Demeco, A.; Marotta, N.; Moggio, L.; Pino, I.; Marinaro, C.; Barletta, M.; Petraroli, A.; Palumbo, A.; Ammendolia, A. Quantitative analysis of movements in facial nerve palsy with surface electromyography and kinematic analysis. J. Electromyogr. Kinesiol. 2021, 56, 102485. [Google Scholar] [CrossRef]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the State-of-the-Art and Areas of Application. JMIR Rehabil. Assist. Technol. 2017, 4, e7. [Google Scholar] [CrossRef]
- Mani, S.; Sharma, S.; Omar, B.; Paungmali, A.; Joseph, L. Validity and reliability of Internet-based physiotherapy assessment for musculoskeletal disorders: A systematic review. J. Telemed. Telecare 2017, 23, 379–391. [Google Scholar] [CrossRef]
- Busch, C.; Baumbach, C.; Willemsen, D.; Nee, O.; Gorath, T.; Hein, A.; Scheffold, T. Supervised training with wireless monitoring of ECG, blood pressure and oxygen-saturation in cardiac patients. J. Telemed. Telecare 2009, 15, 112–114. [Google Scholar] [CrossRef]
- Gal, N.; Andrei, D.; Nemeş, D.I.; Nădăşan, E.; Stoicu-Tivadar, V. A Kinect based intelligent e-rehabilitation system in physical therapy. Stud. Health Technol. Inform. 2015, 210, 489–493. [Google Scholar]
- Bao, S.; Yin, S.; Chen, H.; Chen, W. A wearable multimode system with soft sensors for lower limb activity evaluation and rehabilitation. In Proceedings of the IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Houston, TX, USA, 14–17 May 2018; pp. 1–6. [Google Scholar]
- Zhao, Y.; Wang, J.; Zhang, Y.; Liu, H.; Chen, Z.A.; Lu, Y.; Dai, Y.; Xu, L.; Gao, S. Flexible and Wearable EMG and PSD Sensors Enabled Locomotion Mode Recognition for IoHT Based In-home Rehabilitation. IEEE Sens. J. 2021, 1. [Google Scholar] [CrossRef]
- Gargiulo, G.; McEwan, A. Applied Biomedical Engineering; InTechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Jiang, N.; Falla, D.; d’Avella, A.; Graimann, B.; Farina, D. Myoelectric control in neurorehabilitation. Crit. Rev. Biomed. Eng. 2010, 38, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.; Bueno, D.R.; Gallego, J.A.; Jaramillo, P.; Kilicarslan, A. Surface emg in neurorehabilitation and ergonomics: State of the art and future perspectives. Emerg. Ther. Neurorehabilit. 2014, 267–284. [Google Scholar] [CrossRef]
- Manca, A.; Cereatti, A.; Bar-On, L.; Botter, A.; Della Croce, U.; Knaflitz, M.; Maffiuletti, N.A.; Mazzoli, D.; Merlo, A.; Roatta, S.; et al. A survey on the use and barriers of surface electromyography in neurorehabilitation. Front. Neurol. 2020, 11, 1137. [Google Scholar] [CrossRef] [PubMed]
- Wohrle, H.; Tabie, M.; Kim, S.K.; Kirchner, F.; Kirchner, E.A. A hybrid FPGA-based system for EEG-and EMG-based online movement prediction. Sensors 2017, 17, 1552. [Google Scholar] [CrossRef] [Green Version]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 277, 1693–1702. [Google Scholar] [CrossRef]
- Lin, S.H.; Dionne, T.P. Interventions to improve movement and functional outcomes in adult stroke rehabilitation: Review and evidence summary. J. Particip. Med. 2018, 10, e8929. [Google Scholar] [CrossRef]
- Aceves-Fernandez, M.A. Artificial Intelligence: Applications in Medicine and Biology; Intech Open: London, UK, 2019. [Google Scholar]
- Lyu, M.; Chen, W.H.; Ding, X.; Wang, J.; Pei, Z.; Zhang, B. Development of an EMG-controlled knee exoskeleton to assist home rehabilitation in a game context. Front. Neurorobotics 2019, 13, 67. [Google Scholar] [CrossRef] [Green Version]
- Androwis, G.J.; Pilkar, R.; Ramanujam, A.; Nolan, K.J. Electromyography assessment during gait in a robotic exoskeleton for acute stroke. Front. Neurol. 2018, 9, 630. [Google Scholar] [CrossRef] [Green Version]
- Santisteban, L.; Térémetz, M.; Bleton, J.P.; Baron, J.C.; Maier, M.A.; Lindberg, P.G. Upper limb outcome measures used in stroke rehabilitation studies: A systematic literature review. PLoS ONE 2016, 11, e0154792. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Nam, C.; Rong, W.; Li, W.; Guo, Z.; Huang, Y.; Hu, X.; Zheng, Y.; Poon, W. Robotic and neuromuscular electrical stimulation (NMES) hybrid system. In Intelligent Biomechatronics in Neurorehabilitation; Academic Press: Cambridge, MA, USA, 2020; pp. 147–166. [Google Scholar]
- Monte-Silva, K.; Piscitelli, D.; Norouzi-Gheidari, N.; Batalla, M.A.P.; Archambault, P.; Levin, M.F. Electromyogram-related neuromuscular electrical stimulation for restoring wrist and hand movement in poststroke hemiplegia: A systematic review and meta-analysis. Neurorehabilit. Neural Repair 2019, 33, 96–111. [Google Scholar] [CrossRef]
- Hameed, H.K.; Hassan, W.Z.W.; Shafie, S.; Ahmad, S.A.; Jaafar, H. A review on surface electromyography-controlled hand robotic devices used for rehabilitation and assistance in activities of daily living. J. Prosthetics Orthot. 2020, 32, 3–13. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Garcia-Cossio, E.; Birbaumer, N.; Burdet, E.; Ramos-Murguialday, A. Is EMG a viable alternative to BCI for detecting movement intention in severe stroke? IEEE Trans. Biomed. Eng. 2018, 65, 2790–2797. [Google Scholar] [CrossRef]
- Shenoy, S. EMG in sports rehabilitation. Br. J. Sport. Med. 2010, 44, i10. [Google Scholar] [CrossRef] [Green Version]
- Felici, F.; Vecchio, A.D. Surface Electromyography: What limits its use in exercise and sport physiology? Front. Neurol. 2020, 11, 1508. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Demeco, A.; Marotta, N.; Moggio, L.; Palumbo, A.; Iona, T.; Ammendolia, A. Anterior Cruciate Ligament Injury Prevention Exercises: Could a Neuromuscular Warm-Up Improve Muscle Pre-Activation before a Soccer Game? A Proof-of-Principle Study on Professional Football Players. Appl. Sci. 2021, 11, 4958. [Google Scholar] [CrossRef]
- Turker, H.; Sze, H. Surface electromyography in sports and exercise. In Electrodiagnosis in New Frontiers of Clinical Research; IntechOpen: Rijeka, Croatia, 2013; pp. 175–194. [Google Scholar]
- Massó, N.; Rey, F.; Romero, D.; Gual, G.; Costa, L.; Germán, A. Surface electromyography applications in the sport. Apunt. Sport. Med. 2010, 45, 121–130. [Google Scholar]
- Hussain, J.; Sundaraj, K.; Low, Y.F.; Kiang, L.C.; Sundaraj, S.; Ali, M.A. A systematic review on fatigue analysis in triceps brachii using surface electromyography. Biomed. Signal Process. Control 2018, 40, 396–414. [Google Scholar] [CrossRef]
- Edwards, P.K.; Ebert, J.R.; Littlewood, C.; Ackland, T.; Wang, A. A systematic review of electromyography studies in normal shoulders to inform postoperative rehabilitation following rotator cuff repair. J. Orthop. Sport. Phys. Ther. 2017, 47, 931–944. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Adams, D.P.; González-Bernal, J.J.; Fernández Araque, A.; Cano García, A.; Fernández-Lázaro, C.I. Electromyography: A Simple and Accessible Tool to Assess Physical Performance and Health during Hypoxia Training. Syst. Rev. Sustain. 2020, 12, 9137. [Google Scholar] [CrossRef]
- Ray, T.; Choi, J.; Reeder, J.; Lee, S.P.; Aranyosi, A.J.; Ghaffari, R.; Rogers, J.A. Soft, skin-interfaced wearable systems for sports science and analytics. Curr. Opin. Biomed. Eng. 2019, 9, 47–56. [Google Scholar] [CrossRef]
- Jiang, Y. Combination of wearable sensors and internet of things and its application in sports rehabilitation. Comput. Commun. 2020, 150, 167–176. [Google Scholar] [CrossRef]
- Lynn, S.K.; Watkins, C.; Wong, M.A.; Balfany, K.; Feeney, D.F. Validity and reliability of surface electromyography measurements from a wearable athlete performance system. J. Sport. Sci. Med. 2018, 17, 205. [Google Scholar]
- Jull, G.; Moore, A.; Falla, D.; Lewis, J.; McCarthy, C.; Sterling, M. Grieve’s Modern Musculoskeletal Physiotherapy E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Tankisi, H.; Burke, D.; Cui, L.; de Carvalho, M.; Kuwabara, S.; Nandedkar, S.D.; Rutkove, S.; Stalberg, E.; van Putten, M.J.A.M.; Fuglsang-Frederiksen, A. Standards of instrumentation of EMG. Clin. Neurophysiol. 2020, 131, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Zouridakis, G. Biomedical Technology and Devices Handbook; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Reaz, M.B.I.; Hussain, M.S.; Mohd-Yasin, F. Techniques of EMG signal analysis: Detection, processing, classification and applications. Biol. Proced. Online 2006, 8, 11–35. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.Y.K. Biomedical Device Technology: Principles and Design; Charles C. Thomas Publisher, Limited: Springfield, IL, USA, 2016. [Google Scholar]
- Merletti, R.; Farina, D. Surface Electromyography: Physiology, Engineering, and Application (IEEE Press Series on Biomedical Engineering), 1st ed.; Wiley-IEEE Press: Hoboken, NJ, USA, 2016. [Google Scholar]
- McManus, L.; De Vito, G.; Lowery, M.M. Analysis and biophysics of surface EMG for physiotherapists and kinesiologists: Toward a common language with rehabilitation engineers. Front. Neurol. 2020, 11, 576729. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, J.; Yang, X. Virtual rehabilitation training system based on surface emg feature extraction and analysis. J. Med. Syst. 2019, 43, 48. [Google Scholar] [CrossRef]
- Song, H.; Park, Y.; Kim, H.; Ko, H. Fully integrated biopotential acquisition analog front-end IC. Sensors 2015, 15, 25139–25156. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Maceira-Elvira, P.; Popa, T.; Schmid, A.C.; Hummel, F.C. Wearable technology in stroke rehabilitation: Towards improved diagnosis and treatment of upper-limb motor impairment. J. Neuroeng. Rehabil. 2019, 16, 1–18. [Google Scholar] [CrossRef]
- Porciuncula, F.; Roto, A.V.; Kumar, D.; Davis, I.; Roy, S.; Walsh, C.J.; Awad, L.N. Wearable movement sensors for rehabilitation: A focused review of technological and clinical advances. J. Inj. Funct. Rehabil. 2018, 10, S220–S232. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, M.M.; Alon, G.; Pai, V.M.; Conroy, R.S. Wearable technologies for active living and rehabilitation: Current research challenges and future opportunities. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668319839607. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Un, K.F.; Mak, P.I.; Chen, Y.; Muñoz-Ferreras, J.M.; Yang, Z.; Gómez-García, R. Overview of recent development on wireless sensing circuits and systems for healthcare and biomedical applications. IEEE J. Emerg. Sel. Top. Circuits Syst. 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Baig, M.M.; GholamHosseini, H.; Moqeem, A.A.; Mirza, F.; Lindén, M. A systematic review of wearable patient monitoring systems—Current challenges and opportunities for clinical adoption. J. Med. Syst. 2017, 41, 1–9. [Google Scholar] [CrossRef]
- Kumari, P.; Mathew, L.; Syal, P. Increasing trend of wearables and multimodal interface for human activity monitoring: A review. Biosens. Bioelectron. 2017, 90, 298–307. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Gong, Z.; Lei, Y.; OuYang, X.; Chan, C.C.; Ruan, S. Wearable Physiological Monitoring System Based on Electrocardiography and Electromyography for Upper Limb Rehabilitation Training. Sensors 2020, 20, 4861. [Google Scholar] [CrossRef]
- Liu, S.H.; Wang, J.J.; Tan, T.H. A Portable and Wireless Multi-Channel Acquisition System for Physiological Signal Measurements. Sensors 2019, 19, 5314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Cho, J.; Na, K.; Yoon, E. Modular 128-Channel Δ-ΔΣ Analog Front-End Architecture Using Spectrum Equalization Scheme for 1024-Channel 3-D Neural Recording Microsystems. IEEE J.-Solid-State Circuits 2017, 53, 501–514. [Google Scholar] [CrossRef]
- Raheem, M.A.; Manjunathachari, K. A Two Channel Analog Front end Design AFE Design with Continuous Time Σ-Δ Modulator for ECG Signal. Int. J. Electr. Comput. Eng. 2018, 8, 5041. [Google Scholar] [CrossRef] [Green Version]
- Kast, C.; Krenn, M.; Aramphianlert, W.; Hofer, C.; Aszmann, O.C.; Mayr, W. Modular Multi-channel Real-time Bio-signal Acquisition System. In Proceedings of the International Conference on Advancements of Medicine and Health Care through Technology, Cluj-Napoca, Romania, 12–15 October 2016; Springer: Cham, Switzerland, 2017; pp. 95–98. [Google Scholar]
- Tran, L.; Cha, H.K. An ultra-low-power neural signal acquisition analog front-end IC. Microelectron. J. 2021, 107, 104950. [Google Scholar] [CrossRef]
- Piccinini, D.J.; Andino, N.B.; Ponce, S.D.; Roberti, M.A. Wearable system for acquisition and monitoring of biological signals. J. Phys. Conf. Ser. 2016, 705, 012009. [Google Scholar] [CrossRef]
- Sarker, V.K.; Jiang, M.; Gia, T.N.; Anzanpour, A.; Rahmani, A.M.; Liljeberg, P. Portable multipurpose bio-signal acquisition and wireless streaming device for wearables. In Proceedings of the 2017 IEEE Sensors Applications Symposium (SAS), Glassboro, NJ, USA, 13–15 March 2017; pp. 1–6. [Google Scholar]
- Mazzetta, I.; Gentile, P.; Pessione, M.; Suppa, A.; Zampogna, A.; Bianchini, E.; Irrera, F. Stand-alone wearable system for ubiquitous real-time monitoring of muscle activation potentials. Sensors 2018, 18, 1748. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, M.S.; Wang, H.; Alam, E.E.; Fang, H. A real time and non-contact multiparameter wearable device for health monitoring. In Proceedings of the 2016 IEEE Global Communications Conference (GLOBECOM), Washington, DC, USA, 4–8 December 2016; pp. 1–6. [Google Scholar]
- Kim, I.; Bhagat, Y.A.; Homer, J.; Lobo, R. Multimodal analog front end for wearable bio-sensors. IEEE Sens. J. 2016, 16, 8784–8791. [Google Scholar] [CrossRef]
- Nakamura, H.; Sakajiri, Y.; Ishigami, H.; Ueno, A. A Novel Analog Front End with Voltage-Dependent Input Impedance and Bandpass Amplification for Capacitive Biopotential Measurements. Sensors 2020, 20, 2476. [Google Scholar] [CrossRef]
- Biagetti, G.; Crippa, P.; Falaschetti, L.; Orcioni, S.; Turchetti, C. Human activity monitoring system based on wearable sEMG and accelerometer wireless sensor nodes. Biomed. Eng. Online 2018, 17, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Biagetti, G.; Crippa, P.; Falaschetti, L.; Turchetti, C. A multi-channel electromyography, electrocardiography and inertial wireless sensor module using Bluetooth low-energy. Electronics 2020, 9, 934. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y. NCMB-button: A wearable non-contact system for long-term multiple biopotential monitoring. In Proceedings of the 2017 IEEE/ACM International Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE), Philadelphia, PA, USA, 17–19 July 2017; pp. 348–355. [Google Scholar]
- Yin, S.; Li, G.; Luo, Y.; Yang, S.; Tain, H.; Lin, L. A Single-Channel Amplifier for Simultaneously Monitoring Impedance Respiration Signal and ECG Signal. Circuits Syst. Signal Process. 2021, 40, 559–571. [Google Scholar] [CrossRef]
- Senapati, B.; Kumar, M.G.L.; Ray, K.B. High resolution reconfigurable bio-potential processor for portable biomedical application. In Devices for Integrated Circuit (DevIC); IEEE: New York, NY, USA, 2017; pp. 517–521. [Google Scholar]
- Lee, S.C.; Lin, Y.S.; Chen, Y.J.; Chiueh, H. A wireless multi-channel physiological signal acquisition system-on-chip for wearable devices. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar] [CrossRef]
- Augustyniak, P. Remotely programmable architecture of a multi-purpose physiological recorder. Microprocess. Microsyst. 2016, 46, 55–66. [Google Scholar] [CrossRef]
- Bhamra, H.; Lynch, J.; Ward, M.; Irazoqui, P. A noise-power-area optimized biosensing front end for wireless body sensor nodes and medical implantable devices. IEEE Trans. Very Large Scale Integr. (Vlsi) Syst. 2017, 25, 2917–2928. [Google Scholar] [CrossRef]
- Biometrics Ltd. Available online: Https://www.biometricsltd.com/index.htm (accessed on 24 April 2021).
- Surface EMG Sensor. Available online: Https://www.biometricsltd.com/surface-emg-sensor.htm (accessed on 15 April 2021).
- Individual Sensors. Available online: Http://www.shimmersensing.com/products/individual-sensors/ (accessed on 4 March 2021).
- Shimmer3 EMG Unit. Available online: Http://www.shimmersensing.com/products/shimmer3-emg-sensor#related-tab (accessed on 4 March 2021).
- Shimmer3 ECG Unit. Available online: Http://www.shimmersensing.com/products/shimmer3-ecg-sensor (accessed on 15 April 2021).
- BioSemi Products. Available online: Https://www.biosemi.com/products.htm (accessed on 18 February 2021).
- Plux. Available online: Https://plux.info/ (accessed on 3 March 2021).
- BITalino. Available online: Https://plux.info/14-bitalino (accessed on 3 March 2021).
- Biosignalsplux. Available online: Https://plux.info/12-biosignalsplux (accessed on 3 March 2021).
- Delsys. Available online: Https://delsys.com/ (accessed on 3 March 2021).


| Database | URL | Date Access |
|---|---|---|
| PubMed | https://pubmed.ncbi.nlm.nih.gov/ | 30 June 2021 |
| MDPI | https://www.mdpi.com/ | 30 June 2021 |
| Springer | https://link.springer.com/ | 30 June 2021 |
| ACM Digital Library | https://dl.acm.org/ | 30 June 2021 |
| Science Direct | https://www.sciencedirect.com/ | 30 June 2021 |
| Authors | Signals | Channels | Platform Characteristics | Features |
|---|---|---|---|---|
| Tran et al., 2021 | Bio-potentials | 4 channels | Four-channel neural recording analog front-end composed by a low-noise amplifier (LNA), a programmable gain amplifier (PGA) and buffers; 4-to-1 multiplexer (MUX) and analog-to-digital converter (ADC) | Programmable gain from 45 dB to 63 dB, input-referred noise of 3.16 μVRMS within the 10 kHz bandwidth, noise efficiency factor of 2.04, power efficiency factor of 4.16, power consumption of 2.82 μW per channel powered from 1 V supply voltage |
| Yin et al., 2021 | Bio-potentials, impedance respiration | Single 1 channel | Oversampling and fast digital lock-in technology, ADS1294R, STM32F103RET6 for signal processing | Improve the common-mode rejection ratio (CMRR) and the signal-to-noise ratio (SNR) of the signal |
| Zhao et al., 2020 | ECG/EMG | N.A. | Low-energy Bluetooth module | Wearable monitoring device, software platform for data analysis |
| Biagetti et al., 2020 | Bio-potentials | 3 channels | Six electrodes, 24 bits of resolution and a sampling rate up to 3.2 kHz for each channel, Bluetooth Low Energy wireless link | Wireless sensor, real-time acquisition, maximization of the available bandwidth, reliability of the transmission |
| Nakamura et al., 2020 | ECG/EMG | N.A. | Analog front-end (AFE) | Capacitive measurements |
| Liu et al., 2019 | Bio-potentials | 8 channels | Powerful microcontroller unit, lithium battery, Bluetooth 3.0 data transmission and built-in 2 GB flash memory | Portable device with a graphic user interface (GUI) and an application program for displaying the signals on a computer or a smart device |
| Park et al., 2018 | Bio-potentials | 128 channels | Energy-efficient integrated circuit architecture of a -modulated AFE with multi-shank neural probes connected to individual AFEs | The - AFE is characterized by a consume of each single-channel AFE of 3.05 μW from 0.5 and 1.0 V supplies in an area of 0.05 mm2 with 63.8 dB signal-to-noise-and-distortion ratio and 3.02 noise efficiency factor |
| Raheem et al., 2018 | Bio-potentials | 2 channels | Programmable gain amplifier (PGA) and 10-bit (SDM-ADC) | High impedance, power consumption of 11 mW, programmable gains from 52.6 dB to 72 dB and input referred noise of 3.5 µV in the amplifier bandwidth |
| Mazzetta et al., 2018 | EMG | Differential 1 channel | 32 bit ARM® Cortex®-M4, microSD, Bluetooth 4.0, 592 mWh battery, micro-USB connector, 30 × 30 × 15 mm dimensions, weight of 10 g | Power consumption, compactness and energy autonomy, wireless and comfortably wearable |
| Biagetti et al., 2018 | sEMG | N.A. | Ultralight wireless sensing nodes, base station for data transmission through a 2.4 GHz radio link, communication protocol designed on top of the IEEE 802.15.4 physical layer | Low-cost wearable wireless system, user interface software for viewing, recording and analyzing data |
| Kast et al., 2017 | Bio-potentials | Bipolar 64 channels | Up to eight front-end acquisition modules with synchronization module, a separated universal serial bus data-link to the computer and an ADS1299 | Raw data are analyzed and stored on a personal computer or a single-board computer |
| Sarker et al., 2017 | ECG/EMG | 8 channels | 24 bit resolution/channel and 500 samples/s, IoT-based system | Compact and wearable portable bio-signal acquisition device, real-time data wireless transmission, low energy consumption |
| Li et al., 2017 | ECG/EMG | N.A. | 150 mAh rechargeable Li-ion battery, packaged into a 39 × 32 × 17 mm 3D printed small box, total weight of 24.0 g, power management circuit, dual power supply for operational amplifiers | Wearable wireless non-contact system, ultra-high input impedance, feasibility of long-term biopotential monitoring |
| Senepati et al., 2017 | ECG/EMG | N.A. | Band pass and band stop FIR filters, Successive Approximation Register (SAR) DAC, Spartan-3E FPGA and 0.18 μm CMOS TSMC technology | Area of 33,005 μm2 area, power consumption of 0.382 mW, suppressing of baselines wander and power line interference noise (50/60 Hz) |
| Bhamra et al., 2017 | ECG/EMG | N.A. | ASIC technology in a 0.18 μm CMOS process, high-pass and low-pass cutoff frequencies being 0.5–300 Hz and 150 Hz–10 kHz, antialiasing filter, successive approximation register (SAR) analog-to-digital converter (ADC), power management | Wireless, programmable gain from 38 to 72 dB, AFE and ADC dissipation of 5.74 μW and 306 nW, measured input-referred noise of 2.98 μVrms, noise efficiency factor of 2.6, power efficiency factor of 9.46, area of the AFE of 0.0228 mm2 |
| Kim et al., 2016 | Bio-potentials, PPG, BIA | N.A. | CMOS technology, low-power and multimodal analog front-end (AFE) | Wearable health monitoring, low dimension and power consumption |
| Mahmud et al., 2016 | ECG | N.A. | Fully integrated analog front-end (AFE), temperature sensor, accelerometer, Bluetooth Low Energy (BLE) module | Multiparameter real time monitoring, small dimensions, Android application, alerts |
| Piccinini et al., 2016 | ECG/EMG | N.A. | ADS1294 Medical Analog Front End, CC3200 microcontroller, two Li-ion charged batteries | Portable solution, size physical reduction, robustness in wireless transmission, reliability in data acquisition and processing |
| Lee et al., 2016 | ECG/EMG | N.A. | Mixed-signal processor system-on-chip (SoC), Bluetooth Low Energy (BLE) chip, 200 mAh battery | Wireless transmission, power efficiency, 12 h of continuous recording |
| Augustyniak et al., 2016 | Bio-potentials | Single-ended 5 channels | Programmable AFE ADAS1000, 24-bit resolution analog-to-digital converter with programmable data rate up to 128 kHz | Wired and wireless body sensor networks, configurable gain for channel |
| Features | Biometric | Shimmer | Biosemi | BTS Bioengineering | Biosignal Plux | BITalino | Delsys |
|---|---|---|---|---|---|---|---|
| Type of sensor | Wireless EMG Sensor | Shimmer3 EMG Unit | ActiveTwo | FreeEMG 1000 H2O | Electro-myography Sensor | Electro-myography Sensor | Trigno Avanti Sensor |
| Size (mm × mm × mm) | 42 × 24 × 14 | 65 × 32 × 12 | 120 × 150 × 190 | Probes: 41.5 × 24.8 × 14 | 28 × 70 × 12 | 12 × 27 | 27 × 37 × 13 |
| Weight | 17 g | 31 g | 1.1 kg | 13 g—battery included | 25 g | N.A. | 14 g |
| # channels | 1 | 2 | 8 up to 256 | 1 | 1 | 1 | 1 differential input |
| Input impedance | >100 Mohms | N.A. | >100 M @ 50 Hz | >100 GOhm | 10/7.5 GOhm/pF | ||
| Input range | +/−6 mV | Approx. 800 mV @ gain = 6 | +262 mV to −262 mV | N.A. | Up to 10 mV | ±1.64 mV @ VCC = 3.3 V | 11 mV/22 mV rti |
| Gain | +/−60 mV to +/−6000 mV | 1,2,3,4,6,8,12 (software configurable) | N.A. | N.A. | 1000 | 1009 | 11 mV/22 mV rti |
| CMRR | >96 dB (typically 110 dB) @ 60 Hz | N.A. | >90 dB @ 50 Hz | N.A. | 100 dB | 86 dB | <−80 dB |
| Consumption | N.A. | N.A. | 4 Watt @ 280 channels | N.A. | 1 mA | 0.17 mA | N.A. |
| Bandwith | 0–250, 470, 950, 5000 Hz | 8.4 kHz | Up to DC—3200 Hz @ –3 dB | N.A. | 25–500 Hz | 25–482 Hz | 10–850 Hz 20–450 Hz |
| Data transmission | Wireless | Bluetooth Radio – RN-42 | Fiber optic | Wireless IEEE 802.15.4 | Bluetooth Low Energy | N.A. | 2.400-2.483 GHz ISM Band, Proprietary RF Protocol - BLE V4.2 |
| Resolution | N.A. | 24 bit | 24 bit | 16 bit | 12 bit | N.A. | 16 bit |
| Sample rate | N.A. | 125, 250, 500, 1000, 2000, 4000, 8000 SPS | 2048 Hz–4096 Hz–8192 Hz–16,384 Hz | N.A. | N.A. | N.A. | 4370 sa/sec |
| Battery type and life | Rechargeable Li-ion Polymer, Up to 8 h | 450 mAh rechargeable Li-ion battery | Battery power with >10 h @ 144 channels, >72 h @ 16 channels | Battery Li-Po, Up to 6 h | N.A. | Battery Li-Po 700 mAh | Rechargeable Li-Po Battery Up to 8 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, A.; Vizza, P.; Calabrese, B.; Ielpo, N. Biopotential Signal Monitoring Systems in Rehabilitation: A Review. Sensors 2021, 21, 7172. https://doi.org/10.3390/s21217172
Palumbo A, Vizza P, Calabrese B, Ielpo N. Biopotential Signal Monitoring Systems in Rehabilitation: A Review. Sensors. 2021; 21(21):7172. https://doi.org/10.3390/s21217172
Chicago/Turabian StylePalumbo, Arrigo, Patrizia Vizza, Barbara Calabrese, and Nicola Ielpo. 2021. "Biopotential Signal Monitoring Systems in Rehabilitation: A Review" Sensors 21, no. 21: 7172. https://doi.org/10.3390/s21217172






