UV Hyperspectral Imaging as Process Analytical Tool for the Characterization of Oxide Layers and Copper States on Direct Bonded Copper
Abstract
:1. Introduction
2. Materials and Methods
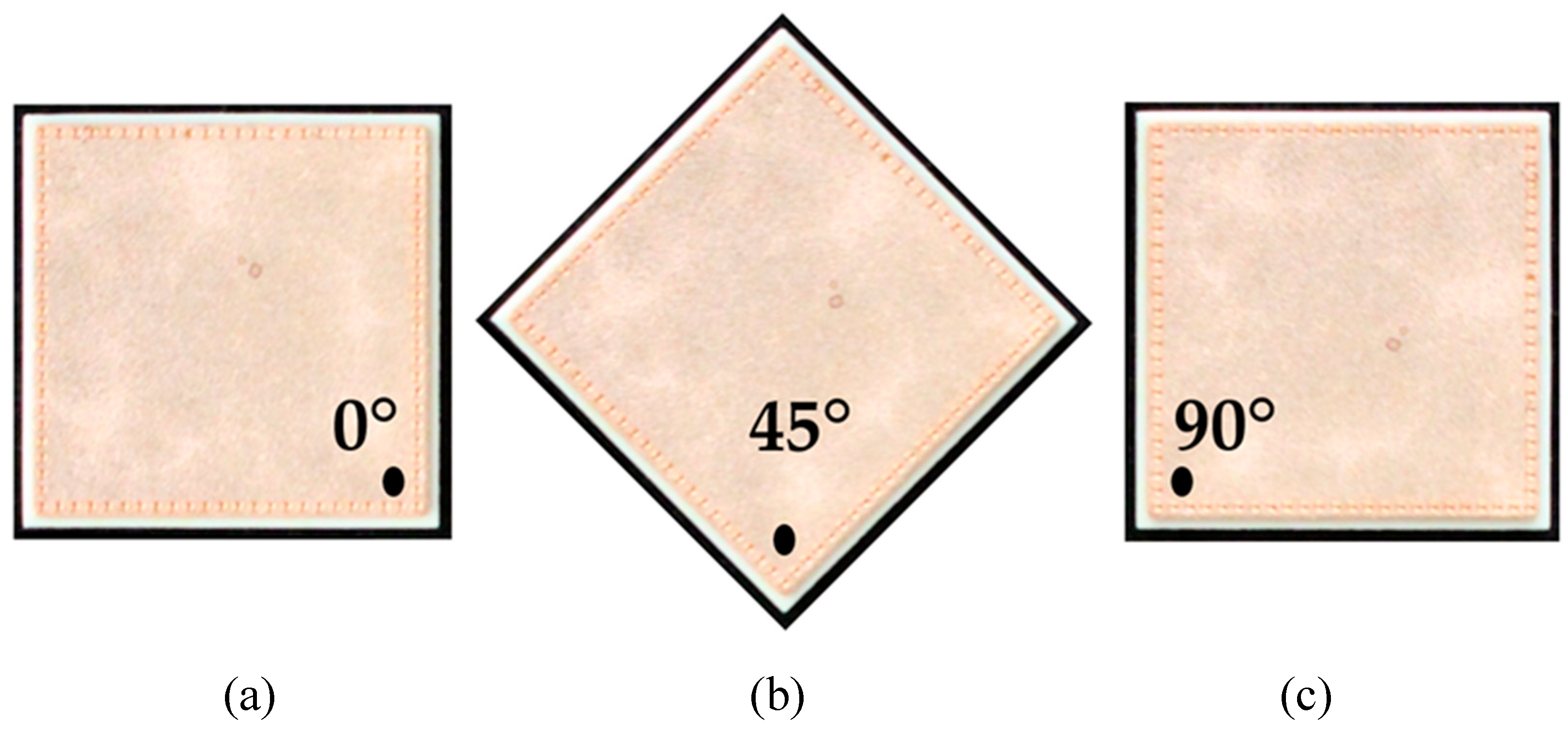
2.1. Samples
2.2. Oxide Layer Thickness Measurement
2.3. UV Spectroscopy
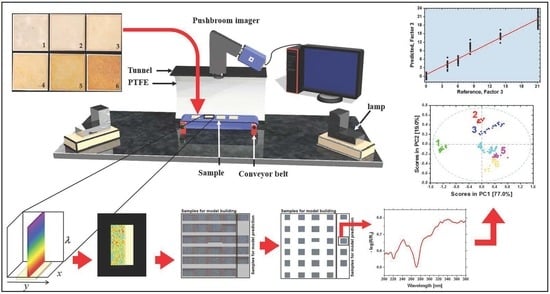
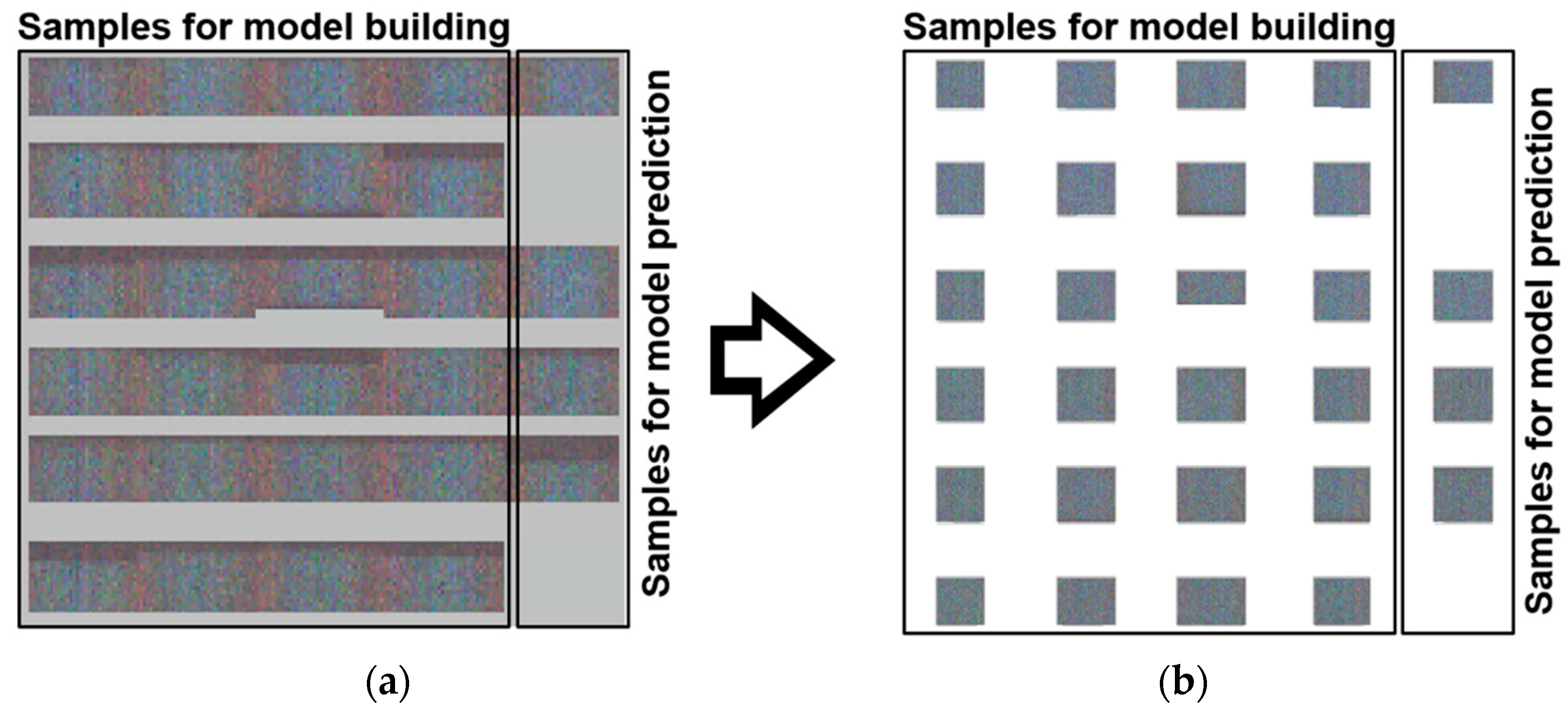
2.4. Data Collection and Preprocessing
2.5. Multivariate Data Analysis and Data Handling
3. Results and Discussion
3.1. UV Spectroscopy
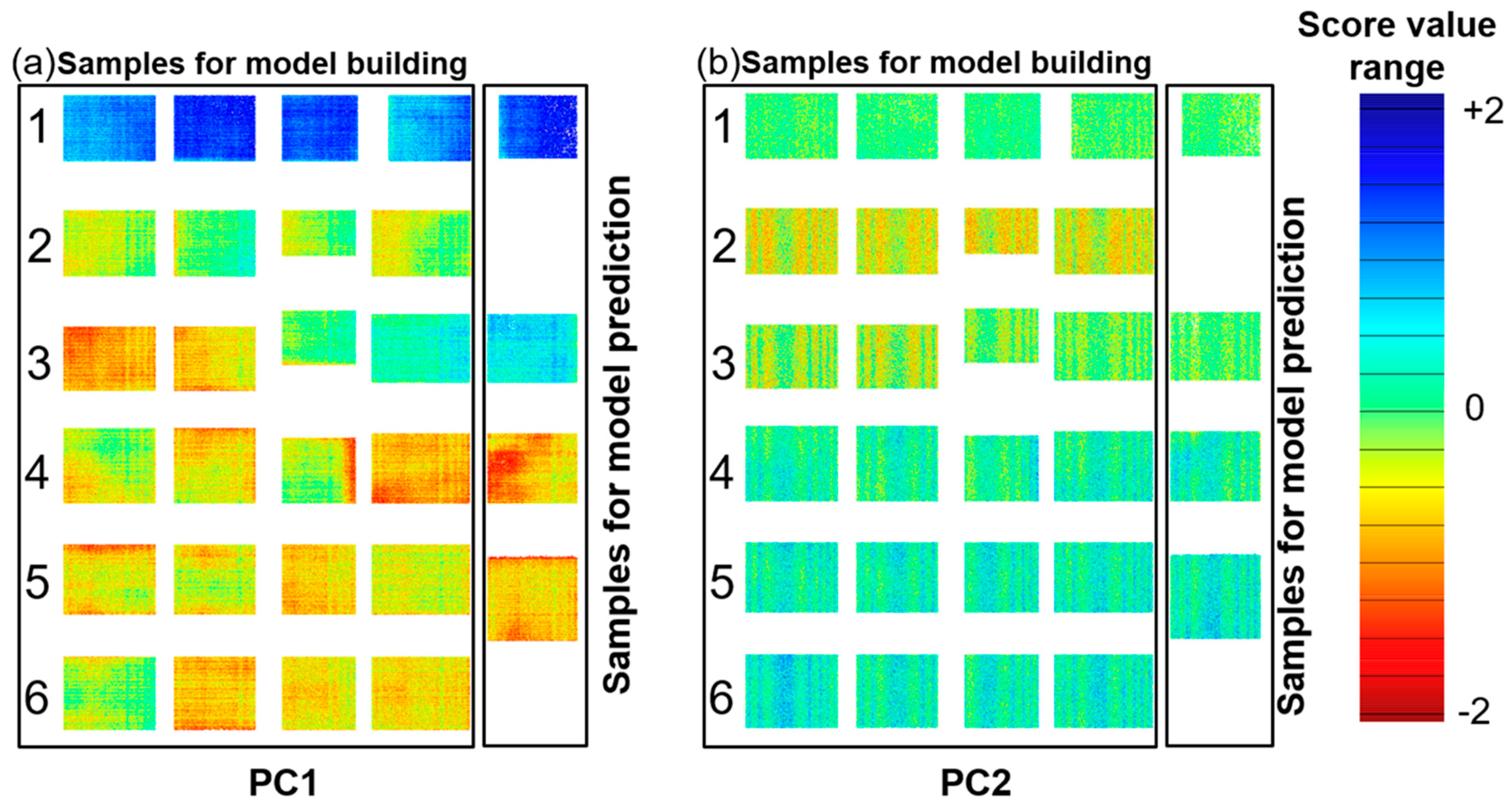
3.2. UV Hyperspectral Imaging
3.3. PLS-R
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esa, S.R.; Yahya, R.; Hassan, A.; Omar, G. Nano-scale copper oxidation on leadframe surface. Ionics 2017, 23, 319–329. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.M.; Abd El-Aziz, S.E.; Marei, S.S. A review: Application of remote sensing as a promising strategy for insect pests and diseases management. Environ. Sci. Pollut. Res. 2020, 27, 33503–33515. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Expanding the Vision of Sensor Materials; National Academies Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Al Ktash, M.; Hauler, O.; Ostertag, E.; Brecht, M. Ultraviolet-visible/near infrared spectroscopy and hyperspectral imaging to study the different types of raw cotton. J. Spectr. Imaging 2020, 9, a18. [Google Scholar] [CrossRef]
- Gowen, A.A.; O’Donnell, C.P.; Cullen, P.J.; Downey, G.; Frias, J.M. Hyperspectral imaging–an emerging process analytical tool for food quality and safety control. Trends Food Sci. Technol. 2007, 18, 590–598. [Google Scholar] [CrossRef]
- Calvini, R.; Ulrici, A.; Amigo, J. Sparse-Based Modeling of Hyperspectral Data. In Data Handling in Science and Technology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 30, pp. 613–634. [Google Scholar]
- Tschannerl, J.; Ren, J.; Jack, F.; Krause, J.; Zhao, H.; Huang, W.; Marshall, S. Potential of UV and SWIR hyperspectral imaging for determination of levels of phenolic flavour compounds in peated barley malt. Food Chem. 2019, 270, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boldrini, B.; Kessler, W.; Rebner, K.; Kessler, R.W. Hyperspectral imaging: A review of best practice, performance and pitfalls for in-line and on-line applications. J. Near Infrared Spectrosc. 2012, 20, 483–508. [Google Scholar] [CrossRef]
- Lodhi, V.; Chakravarty, D.; Mitra, P. Hyperspectral imaging system: Development aspects and recent trends. Sens. Imaging 2019, 20, 35. [Google Scholar] [CrossRef]
- Jin, S.; Hui, W.; Wang, Y.; Huang, K.; Shi, Q.; Ying, C.; Liu, D.; Ye, Q.; Zhou, W.; Tian, J. Hyperspectral imaging using the single-pixel Fourier transform technique. Sci. Rep. 2017, 7, 45209. [Google Scholar] [CrossRef] [PubMed]
- Al Ktash, M.; Stefanakis, M.; Boldrini, B.; Ostertag, E.; Brecht, M. Characterization of Pharmaceutical Tablets Using UV Hyperspectral Imaging as a Rapid In-Line Analysis Tool. Sensors 2021, 21, 4436. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, C.T.; Folkman, M.A.; Figueroa, M.A. Application of hyperspectral-imaging spectrometer systems to industrial inspection. In Proceedings of the Three-Dimensional and Unconventional Imaging for Industrial Inspection and Metrology, Philadelphia, PA, USA, 23–25 October 1995; pp. 264–272. [Google Scholar]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 010901. [Google Scholar] [CrossRef] [PubMed]
- Manolakis, D.; Shaw, G. Detection algorithms for hyperspectral imaging applications. IEEE Signal Process. Mag. 2002, 19, 29–43. [Google Scholar] [CrossRef]
- Rebner, K. Hyperspectral Imaging for Quality Analysis and Control. In Proceedings of the Applied Industrial Optics: Spectroscopy, Imaging and Metrology, Heidelberg Germany, 25–28 July 2016. [Google Scholar]
- Stiedl, J.; Boldrini, B.; Green, S.; Chassé, T.; Rebner, K. Characterisation of oxide layers on technical copper based on visible hyperspectral imaging. J. Spectr. Imaging 2019, 8, a10. [Google Scholar] [CrossRef]
- Stiedl, J.; Green, S.; Chassé, T.; Rebner, K. Characterization of oxide layers on technical copper material using ultraviolet visible (UV–Vis) spectroscopy as a rapid on-line analysis tool. Appl. Spectrosc. 2019, 73, 59–66. [Google Scholar] [PubMed]
- Stiedl, J.; Green, S.; Chassé, T.; Rebner, K. Auger electron spectroscopy and UV–Vis spectroscopy in combination with multivariate curve resolution analysis to determine the Cu2O/CuO ratios in oxide layers on technical copper surfaces. Appl. Surf. Sci. 2019, 486, 354–361. [Google Scholar] [CrossRef]
- Ojeda, C.B.; Rojas, F.S. Process analytical chemistry: Applications of ultraviolet/visible spectrometry in environmental analysis: An overview. Appl. Spectrosc. Rev. 2009, 44, 245–265. [Google Scholar] [CrossRef]
- Mazzeo, G.; Prestopino, G.; Conte, G.; Salvatori, S. Metal-diamond-metal planar structures for off-angle UV beam positioning with high lateral resolution. Sens. Actuators A Phys. 2005, 123, 199–203. [Google Scholar] [CrossRef]
- Reyes, G.; Diaz, W.; Toro, C.; Balladares, E.; Torres, S.; Parra, R.; Vásquez, A. Copper Oxide Spectral Emission Detection in Chalcopyrite and Copper Concentrate Combustion. Processes 2021, 9, 188. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef] [Green Version]
- Obeidat, S.M.; Al-Ktash, M.M.; Al-Momani, I.F. Study of fuel assessment and adulteration using EEMF and multiway PCA. Energy Fuels 2014, 28, 4889–4894. [Google Scholar] [CrossRef]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- INNO-SPEC GmbH. BlueEye UV Hyperspectral Imaging Camera (220–380 nm). Available online: https://inno-spec.de/wp-content/uploads/2021/10/210928_BlueEye.pdf (accessed on 29 October 2021).
- PCO AG. pco.edge 4.2 bi Cooled sCMOS Camera. Available online: https://www.pco.de/fileadmin/user_upload/pco-product_sheets/DS_PCOEDGE42BI_V104.pdf (accessed on 29 October 2021).
- Schlapfer, D.R.; Kaiser, J.W.; Brazile, J.; Schaepman, M.E.; Itten, K.I. Calibration concept for potential optical aberrations of the APEX pushbroom imaging spectrometer. In Proceedings of the Sensors, Systems, and Next-Generation Satellites VII, Barcelona, Spain, 8–10 September 2003; pp. 221–231. [Google Scholar]
- Quantum Desgin Europe GmbH. Lamp Spectra and Irradiance. Available online: https://qd-europe.com/fileadmin/Mediapool/products/lightsources/en/LQ_Lamp_spectra_and_irradiance_en.pdf (accessed on 27 September 2021).




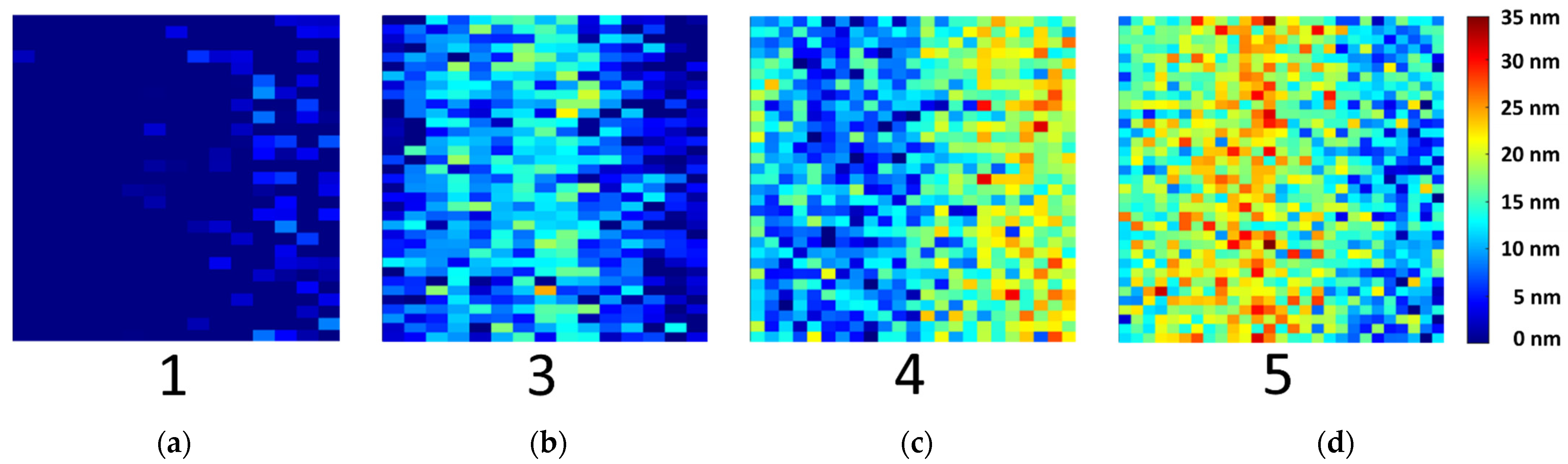
| Sample Type | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Number of measured samples | 5 * | 4 | 5 * | 5 * | 5 * | 4 |
| Temperature/°C | NA | 110.0 | 142.5 | 142.5 | 175.0 | 175.0 |
| Time/min | NA | 2 | 11 | 20 | 11 | 20 |
| Mean oxide layer thickness/nm | 0 | 4.0 | 6.0 | 8.3 | 14.0 | 21.1 |
| Standard deviation oxide layer thickness/nm | 0 | 5.9 | 3.0 | 4.5 | 7.0 | 8.2 |
| Method | Number of Factors | Parameters Calibration | Parameters Validation | ||
|---|---|---|---|---|---|
| R2c | RMSEC/nm | R2cv | RMSECV/nm | ||
| UV spectroscopy | 3 | 0.94 | 1.64 | 0.93 | 1.74 |
| UV hyperspectral imaging | 3 | 0.94 | 1.76 | 0.93 | 1.88 |
| Method | Sample Type | Reference/nm | Predicted/nm | Deviation/nm |
|---|---|---|---|---|
| UV spectroscopy | 1 | 0 | 1.59 | 0.93 |
| 3 | 6 | 6.00 | 1.02 | |
| 4 | 8.3 | 7.86 | 1.44 | |
| 5 | 14 | 15.25 | 1.53 | |
| UV hyperspectral imaging | 1 | 0 | –0.87 | 1.49 |
| 3 | 6 | 5.51 | 2.08 | |
| 4 | 8.3 | 11.74 | 1.91 | |
| 5 | 14 | 14.35 | 1.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Ktash, M.; Stefanakis, M.; Englert, T.; Drechsel, M.S.L.; Stiedl, J.; Green, S.; Jacob, T.; Boldrini, B.; Ostertag, E.; Rebner, K.; et al. UV Hyperspectral Imaging as Process Analytical Tool for the Characterization of Oxide Layers and Copper States on Direct Bonded Copper. Sensors 2021, 21, 7332. https://doi.org/10.3390/s21217332
Al Ktash M, Stefanakis M, Englert T, Drechsel MSL, Stiedl J, Green S, Jacob T, Boldrini B, Ostertag E, Rebner K, et al. UV Hyperspectral Imaging as Process Analytical Tool for the Characterization of Oxide Layers and Copper States on Direct Bonded Copper. Sensors. 2021; 21(21):7332. https://doi.org/10.3390/s21217332
Chicago/Turabian StyleAl Ktash, Mohammad, Mona Stefanakis, Tim Englert, Maryam S. L. Drechsel, Jan Stiedl, Simon Green, Timo Jacob, Barbara Boldrini, Edwin Ostertag, Karsten Rebner, and et al. 2021. "UV Hyperspectral Imaging as Process Analytical Tool for the Characterization of Oxide Layers and Copper States on Direct Bonded Copper" Sensors 21, no. 21: 7332. https://doi.org/10.3390/s21217332