Combined SPRi Sensor for Simultaneous Detection of Nitrate and Ammonium in Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Samples
2.2. Surface Plasmon Resonance Imaging (SPRi) and Biochips Preparation
2.2.1. Biochips with Monolayers of Nitrate Reductase or Glutamine Synthetase
2.2.2. Combined Biochip with Spots of Nitrate Reductase and Glutamine Synthetase
2.2.3. Surface Plasmon Resonance Imaging (SPRi)
2.3. Ion Chromatography
2.4. UV-VIS Spectrophotometry
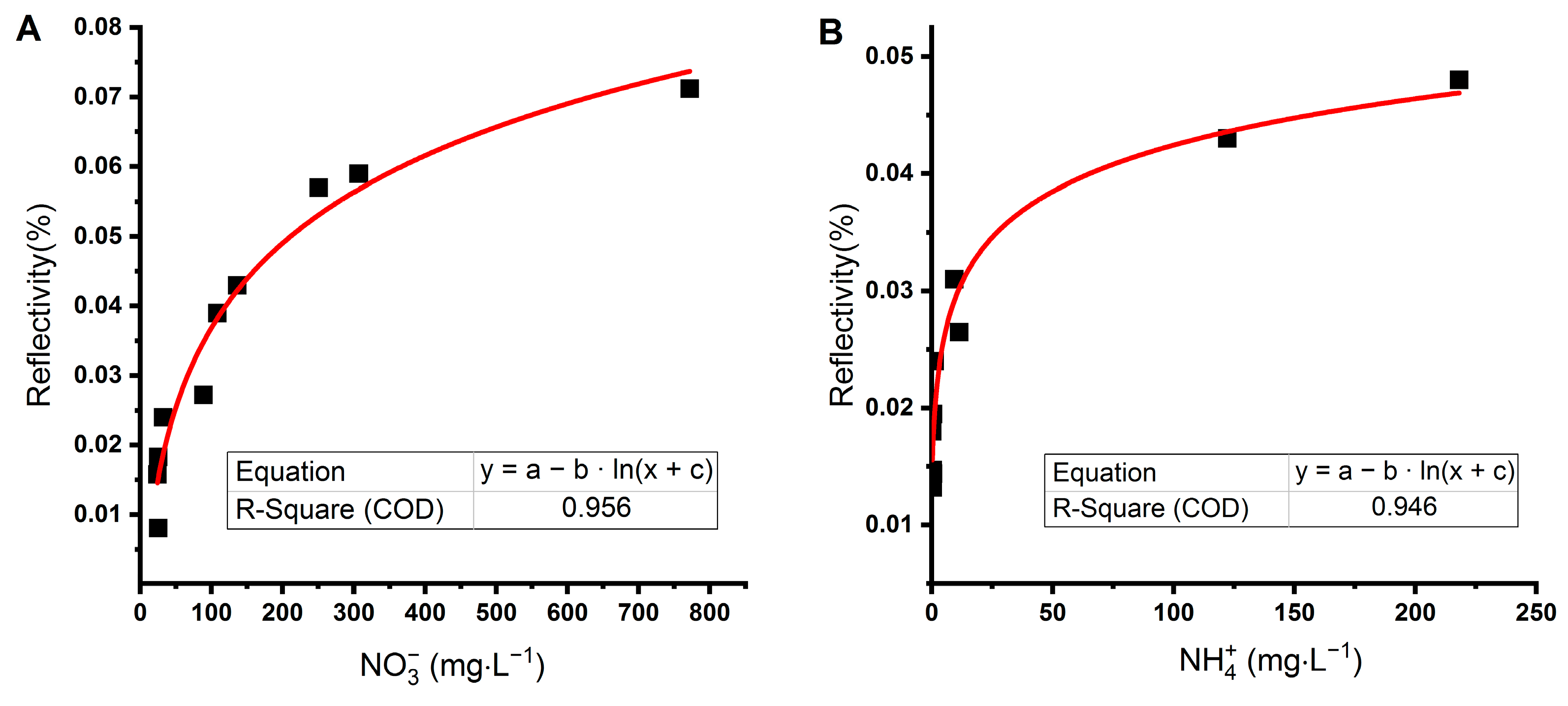
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mateo-Sagasta, J.; Marjani Zadeh, S.; Turral, H. More People, More Food, Worse Water?: A Global Review of Water Pollution from Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Evans, A.E.; Mateo-Sagasta, J.; Qadir, M.; Boelee, E.; Ippolito, A. Agricultural water pollution: Key knowledge gaps and research needs. Curr. Opin. Environ. Sustain. 2019, 36, 20–27. [Google Scholar] [CrossRef]
- Cui, M.; Zeng, L.; Qin, W.; Feng, J. Measures for reducing nitrate leaching in orchards: A review. Environ. Pollut. 2020, 263, 114553. [Google Scholar] [CrossRef] [PubMed]
- Rabalais, N.N.; Turner, R.E.; Díaz, R.J.; Justić, D. Global change and eutrophication of coastal waters. ICES J. Mar. Sci. 2009, 66, 1528–1537. [Google Scholar] [CrossRef]
- Spalding, R.F.; Exner, M.E. Occurrence of Nitrate in groundwater—A review. J. Environ. Qual. 1993, 22, 392–402. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, A.; Salamanca, A. Nitrate toxicity to aquatic animals: A review with new data for freshwater invertebrates. Chemosphere 2005, 58, 1255–1267. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; De Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; Van Breda, S.G. Drinking water Nitrate and Human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [Green Version]
- Brender, J.D.; Weyer, P.J.; Romitti, P.A.; Mohanty, B.P.; Shinde, M.U.; Vuong, A.M.; Sharkey, J.R.; Dwivedi, D.; Horel, S.A.; Kantamneni, J.; et al. Prenatal Nitrate intake from drinking water and selected birth defects in offspring of participants in the national birth defects prevention study. Environ. Health Perspect. 2013, 121, 1083–1089. [Google Scholar] [CrossRef] [Green Version]
- Blaisdell, J.; Turyk, M.E.; Almberg, K.S.; Jones, R.M.; Stayner, L. Prenatal exposure to nitrate in drinking water and the risk of congenital anomalies. Environ. Res. 2019, 176, 108553. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating First Addendum, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Milne, I.; Seager, J.; Mallett, M.; Sims, I. Effects of short-term pulsed ammonia exposure on fish. Environ. Toxicol. Chem. 2000, 19, 2929–2936. [Google Scholar] [CrossRef]
- Moorcroft, M.J. Detection and determination of nitrate and nitrite: A review. Talanta 2001, 54, 785–803. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Yu, L.-J.; Liu, Y.; Lin, L.; Lu, R.-G.; Zhu, J.-P.; He, L.; Lu, Z.-L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Adeloju, S.B. Nitrate biosensors and biological methods for nitrate determination. Talanta 2016, 153, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Borcherding, H.; Leikefeld, S.; Frey, C.; Diekmann, S.; Steinrücke, P. Enzymatic microtiter plate-based Nitrate detection in environmental and medical analysis. Anal. Biochem. 2000, 282, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple spectrophotometric method for simultaneous detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Ivancic, I. An optimal manual procedure for ammonia analysis in natural waters by the indophenol blue method. Water Res. 1984, 18, 1143–1147. [Google Scholar] [CrossRef]
- Hashihama, F.; Kanda, J.; Tauchi, A.; Kodama, T.; Saito, H.; Furuya, K. Liquid waveguide spectrophotometric measurement of nanomolar ammonium in seawater based on the indophenol reaction with O-Phenylphenol (OPP). Talanta 2015, 143, 374–380. [Google Scholar] [CrossRef]
- Li, P.; Deng, Y.; Shu, H.; Lin, K.; Chen, N.; Jiang, Y.; Chen, J.; Yuan, D.; Ma, J. High-frequency underway analysis of ammonium in coastal waters using an integrated syringe-pump-based environmental-water analyzer (iSEA). Talanta 2019, 195, 638–646. [Google Scholar] [CrossRef]
- Lin, K.; Li, P.; Wu, Q.; Feng, S.; Ma, J.; Yuan, D. Automated determination of ammonium in natural waters with reverse flow injection analysis based on the indophenol blue method with O-Phenylphenol. Microchem. J. 2018, 138, 519–525. [Google Scholar] [CrossRef]
- Ma, J.; Li, P.; Lin, K.; Chen, Z.; Chen, N.; Liao, K.; Yuan, D. Optimization of a salinity-interference-free indophenol method for the determination of ammonium in natural waters using O-Phenylphenol. Talanta 2018, 179, 608–614. [Google Scholar] [CrossRef]
- Can, F.; Ozoner, S.K.; Ergenekon, P.; Erhan, E. Amperometric nitrate biosensor based on Carbon nanotube/Polypyrrole/Nitrate reductase biofilm electrode. Mater. Sci. Eng. C 2012, 32, 18–23. [Google Scholar] [CrossRef]
- Larsen, L.H.; Damgaard, L.R.; Kjær, T.; Stenstrøm, T.; Lynggaard-Jensen, A.; Revsbech, N.P. Fast responding biosensor for on-line determination of nitrate/nitrite in activated sludge. Water Res. 2000, 34, 2463–2468. [Google Scholar] [CrossRef]
- Sachdeva, V.; Hooda, V. A new immobilization and sensing platform for nitrate quantification. Talanta 2014, 124, 52–59. [Google Scholar] [CrossRef]
- Xuejiang, W.; Dzyadevych, S.V.; Chovelon, J.; Renault, N.; Ling, C.; Siqing, X.; Jianfu, Z. Conductometric nitrate biosensor based on methyl viologen/Nafion®/nitrate reductase interdigitated electrodes. Talanta 2006, 69, 450–455. [Google Scholar] [CrossRef]
- Akbari, A.; Derakhshesh, M.; Abrishami, M.E. Nitrate ion effect on gold nanorods plasmon resonance behavior: A potential chemical sensor. Inorg. Chem. Commun. 2018, 98, 159–164. [Google Scholar] [CrossRef]
- Miao, P.; Liu, Z.; Guo, J.; Yuan, M.; Zhong, R.; Wang, L.; Zhang, F. A novel ultrasensitive surface plasmon resonance-based nanosensor for nitrite detection. RSC Adv. 2019, 9, 17698–17705. [Google Scholar] [CrossRef] [Green Version]
- Daniel, W.L.; Han, M.S.; Lee, J.S.; Mirkin, C.A. Colorimetric nitrite and nitrate detection with gold nanoparticle probes and kinetic end points. J. Am. Chem. Soc. 2009, 131, 6362–6363. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.D.; Kant, R. Recent advances in surface plasmon resonance based fiber optic chemical and biosensors utilizing bulk and nanostructures. Opt. Laser Technol. 2018, 101, 144–161. [Google Scholar] [CrossRef]
- Parveen, S.; Pathak, A.; Gupta, B.D. Fiber optic SPR nanosensor based on synergistic effects of CNT/Cu-nanoparticles composite for ultratrace sensing of nitrate. Sens. Actuators B Chem. 2017, 246, 910–919. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Siyu, E.; Tao, B.; Wu, Q.; Han, B. Reflective SPR sensor for simultaneous measurement of nitrate concentration and temperature. IEEE Trans. Instrum. Meas. 2019, 68, 4566–4574. [Google Scholar] [CrossRef]
- Beaton, A.D.; Cardwell, C.L.; Thomas, R.S.; Sieben, V.J.; Legiret, F.-E.; Waugh, E.M.; Statham, P.J.; Mowlem, M.C.; Morgan, H. Lab-on-Chip measurement of Nitrate and Nitrite for in situ analysis of natural waters. Environ. Sci. Technol. 2012, 46, 9548–9556. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, Y.; Cho, J.; Lee, J.-Y.; Choi, M.-S.; Cho, Y.-K. Lab-on-a-Disc for simultaneous determination of nutrients in water. Anal. Chem. 2013, 85, 2954–2960. [Google Scholar] [CrossRef] [PubMed]
- Schierenbeck, T.M.; Smith, M.C. Path to impact for autonomous field deployable chemical sensors: A case study of in situ nitrite sensors. Environ. Sci. Technol. 2017, 51, 4755–4771. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Roche, P.; Briet, M.; Moore, B.; Morrin, A.; Diamond, D.; Paull, B. Fully automated, low-cost ion chromatography system for in-situ analysis of nitrite and nitrate in natural waters. Talanta 2020, 216, 120955. [Google Scholar] [CrossRef]
- FAO. Definition of Aquaculture; FAO: Bangkok, Thailand, 1988; pp. 1–3. [Google Scholar]
- Diver, S. Aquaponics—Integration of Hydroponics with Aquaculture; ATTRA—National Sustainable Agriculture Information Service: Butte, MT, USA, 2006; pp. 1–28. [Google Scholar]
- Lennard, W.A. Aquaponics: A nutrient dynamic process and the relationship to fish feeds. World Aquac. 2015, 46, 20–23. [Google Scholar]
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Goddek, S.; Strauch, S.M.; Vermeulen, T.; Jijakli, M.H.; Kotzen, B. Towards commercial aquaponics: A review of systems, designs, scales and nomenclature. Aquac. Int. 2018, 26, 813–842. [Google Scholar] [CrossRef]
- Yep, B.; Zheng, Y. Aquaponic trends and challenges—A review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Hu, Z.; Lee, J.-W.; Chandran, K.; Kim, S.; Brotto, A.C.; Khanal, S.K. Effect of plant species on nitrogen recovery in aquaponics. Bioresour. Technol. 2015, 188, 92–98. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Z.; Zhang, J.; Xie, H.; Liang, S.; Wang, J.; Yan, R. Attempts to improve nitrogen utilization efficiency of aquaponics through nitrifies addition and filler gradation. Environ. Sci. Pollut. Res. 2016, 23, 6671–6679. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Comparisons of nitrogen and phosphorus mass balance for tomato-, basil-, and lettuce-based aquaponic and hydroponic systems. J. Clean. Prod. 2020, 274, 122619. [Google Scholar] [CrossRef]
- Sachdeva, V.; Hooda, V. Immobilization of nitrate reductase onto epoxy affixed silver nanoparticles for determination of soil nitrates. Int. J. Biol. Macromol. 2015, 79, 240–247. [Google Scholar] [CrossRef]
- Amaya, K.R.; Kocherginskaya, S.A.; Mackie, R.I.; Cann, I.K.O. Biochemical and mutational analysis of Glutamine Synthetase Type III from the Rumen Anaerobe Ruminococcus albus 8. J. Bacteriol. 2005, 187, 7481–7491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilliam, M.; Sherman, M.; Griscavage, J.; Ignarro, L. A spectrophotometric assay for Nitrate using NADPH Oxidation by Aspergillus Nitrate reductase. Anal. Biochem. 1993, 212, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Colanduoni, J.; Nissan, R.; Villafranca, J.J. Studies of the mechanism of Glutamine Synthetase utilizing pH-dependent behavior in catalysis and binding. J. Biol. Chem. 1987, 262, 3037–3043. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface plasmon resonance: A versatile technique for biosensor applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Naimushin, A.N.; Soelberg, S.D.; Nguyen, D.K.; Dunlap, L.; Bartholomew, D.; Elkind, J.; Melendez, J.; Furlong, C.E. Detection of Staphylococcus aureus enterotoxin B at femtomolar levels with a miniature integrated two-channel surface plasmon resonance (SPR) sensor. Biosens. Bioelectron. 2002, 17, 573–584. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Scarano, S.; Scuffi, C.; Mascini, M.; Minunni, M.E. Surface plasmon resonance imaging (SPRi)-based sensing: A new approach in signal sampling and management. Biosens. Bioelectron. 2010, 26, 1380–1385. [Google Scholar] [CrossRef]
- Piliarik, M.; Párová, L.; Homola, J. High-throughput SPR sensor for food safety. Biosens. Bioelectron. 2009, 24, 1399–1404. [Google Scholar] [CrossRef]
- Chinowsky, T.M.; Grow, M.S.; Johnston, K.S.; Nelson, K.; Edwards, T.; Fu, E.; Yager, P. Compact, high performance surface plasmon resonance imaging system. Biosens. Bioelectron. 2007, 22, 2208–2215. [Google Scholar] [CrossRef] [Green Version]
- Mélaïne, F.; Roupioz, Y.; Buhot, A. Gold nanoparticles surface plasmon resonance enhanced signal for the detection of small molecules on split-aptamer microarrays (small molecules detection from split-aptamers). Microarrays 2015, 4, 41–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mélaïne, F.; Roupioz, Y.; Buhot, A. Small molecule SPR imaging detection from split aptamer microarrays. Procedia Technol. 2017, 27, 6–7. [Google Scholar] [CrossRef]
- Pillet, F.; Romera, C.; Trevisiol, E.; Bellon, S.; Teulade-Fichou, M.-P.; François, J.-M.; Pratviel, G.; Leberre, V.A. Surface plasmon resonance imaging (SPRi) as an alternative technique for rapid and quantitative screening of small molecules, useful in drug discovery. Sens. Actuators B Chem. 2011, 157, 304–309. [Google Scholar] [CrossRef]
- Fan, Z.-B.; Gong, X.-Q.; Lu, D.-F.; Gao, R.; Qi, Z.-M. Benzo[a]pyrene sensing properties of surface plasmon resonance imaging sensor based on the Hue Algorithm. Acta Physico-Chimica Sin. 2017, 33, 1001–1009. [Google Scholar] [CrossRef]
- Hausler, P.; Wunderlich, L.; Fischer, J.; Pfab, C.; Heckscher, S.; Hirsch, T.; Bierl, R. Surface plasmon resonance imaging for detection of drug metabolites in water. In Optical Sensors 2019; Baldini, F., Homola, J., Lieberman, R.A., Eds.; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2019; Volume 11028. [Google Scholar]
- Liang, S.; Cai, C.; Gao, R.; Zhang, M.; Xue, N.; Qi, Z.-M. AuAg alloy film-based colorful SPR imaging sensor for highly sensitive immunodetection of benzo[a]pyrene in water. Appl. Opt. 2019, 58, 6942–6948. [Google Scholar] [CrossRef]
- Genua, M.; Garçon, L.-A.; Mounier, V.; Wehry, H.; Buhot, A.; Billon, M.; Calemczuk, R.; Bonnaffe, D.; Hou, Y.; Livache, T. SPR imaging based electronic tongue via landscape images for complex mixture analysis. Talanta 2014, 130, 49–54. [Google Scholar] [CrossRef]
- Ru, D.; Liu, J.; Hu, Z.; Zou, Y.; Jiang, L.; Cheng, X.; Lv, Z. Improvement of aquaponic performance through micro- and macro-nutrient addition. Environ. Sci. Pollut. Res. 2017, 24, 16328–16335. [Google Scholar] [CrossRef]
- Timmons, M.B.; Ebeling, J.M. Recirculating Aquaculture; Cayuga Aqua Ventures, LLC: Ithaca, NY, USA, 2010. [Google Scholar]
- Gräber, A.; Junge, R. Aquaponic systems: Nutrient recycling from fish wastewater by vegetable production. Desalination 2009, 246, 147–156. [Google Scholar] [CrossRef]
- Kyaw, T.Y.; Ng, A.K. Smart Aquaponics System for Urban Farming. Energy Procedia 2017, 143, 342–347. [Google Scholar] [CrossRef]
- Murad, S.A.Z.; Harun, A.; Mohyar, S.N.; Sapawi, R.; Ten, S.Y. Design of aquaponics water monitoring system using Arduino microcontroller. AIP Conf. Proc. 2017, 1885, 020248. [Google Scholar] [CrossRef]
- Huang, J.; Bennett, W.W.; Teasdale, P.R.; Kankanamge, N.R.; Welsh, D.T. A modified DGT technique for the simultaneous measurement of dissolved inorganic nitrogen and phosphorus in freshwaters. Anal. Chim. Acta 2017, 988, 17–26. [Google Scholar] [CrossRef] [PubMed]




| Sample No. | c (NO3−) mg·L−1 | c (NH4+) mg·L−1 |
|---|---|---|
| 1 | 24.0 | 0.45 |
| 2 | 25.1 | 0.40 |
| 3 | 25.2 | 0.52 |
| 4 | 88.7 | 11.34 |
| 5 | 251.0 | 122.29 |
| 6 | 772.4 | 218.14 |
| 7 | 32.2 | 0.26 |
| 8 | 108.3 | 0.57 |
| 9 | 136.3 | 1.27 |
| 10 | 307.0 | 9.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vráblová, M.; Koutník, I.; Smutná, K.; Marková, D.; Veverková, N. Combined SPRi Sensor for Simultaneous Detection of Nitrate and Ammonium in Wastewater. Sensors 2021, 21, 725. https://doi.org/10.3390/s21030725
Vráblová M, Koutník I, Smutná K, Marková D, Veverková N. Combined SPRi Sensor for Simultaneous Detection of Nitrate and Ammonium in Wastewater. Sensors. 2021; 21(3):725. https://doi.org/10.3390/s21030725
Chicago/Turabian StyleVráblová, Martina, Ivan Koutník, Kateřina Smutná, Dominika Marková, and Nikola Veverková. 2021. "Combined SPRi Sensor for Simultaneous Detection of Nitrate and Ammonium in Wastewater" Sensors 21, no. 3: 725. https://doi.org/10.3390/s21030725






