NFC-Based Wearable Optoelectronics Working with Smartphone Application for Untact Healthcare
Abstract
:1. Introduction
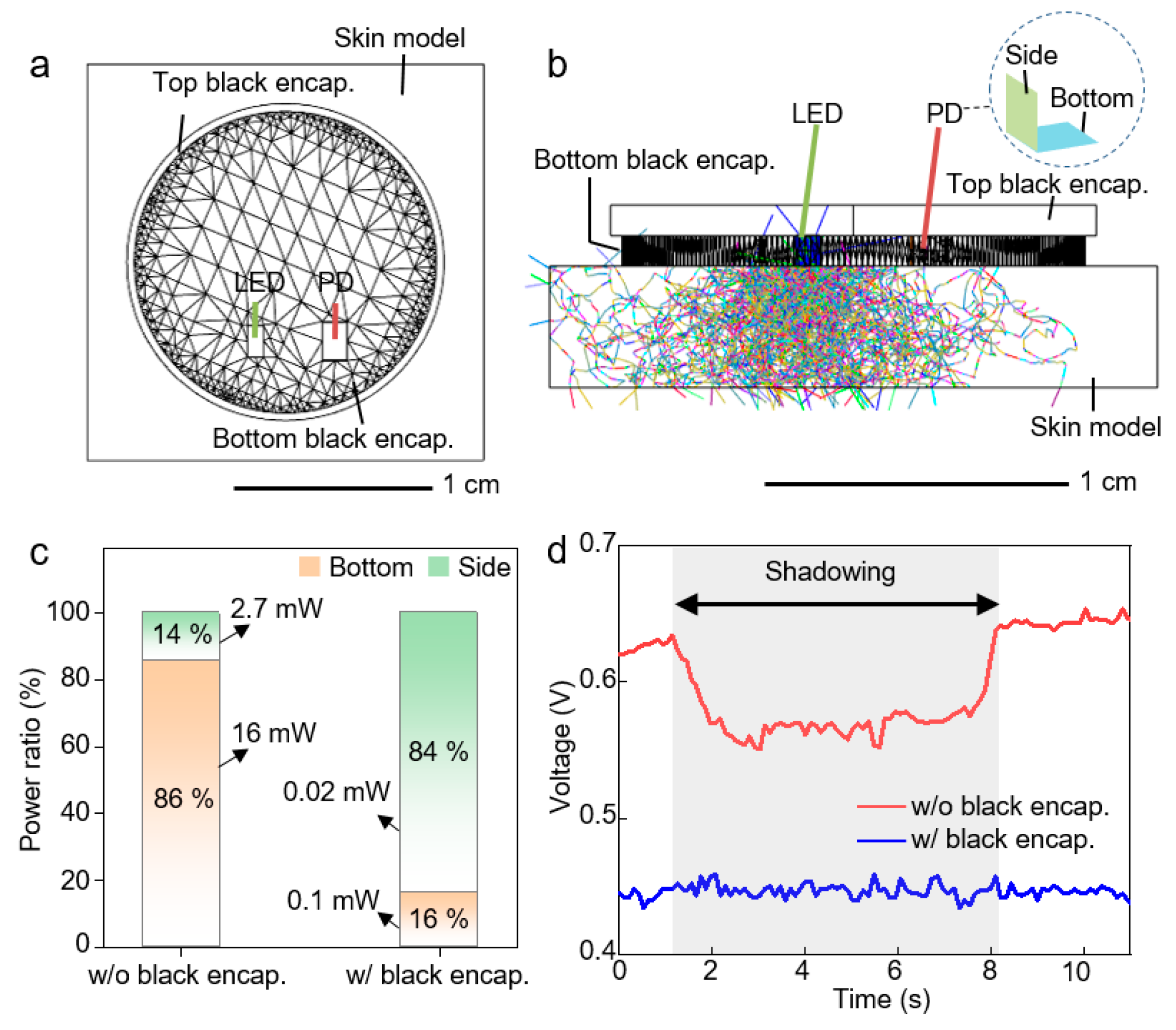
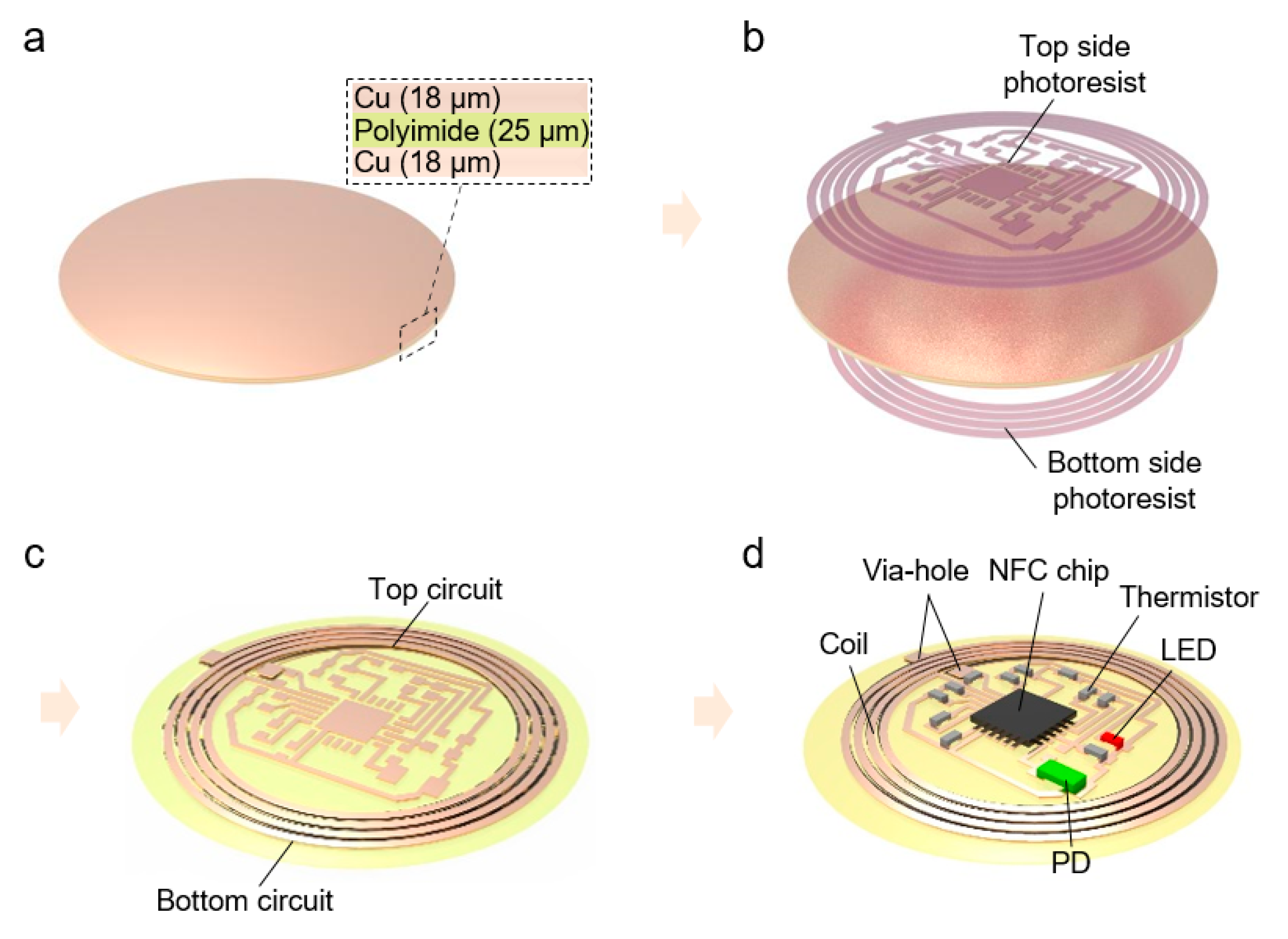
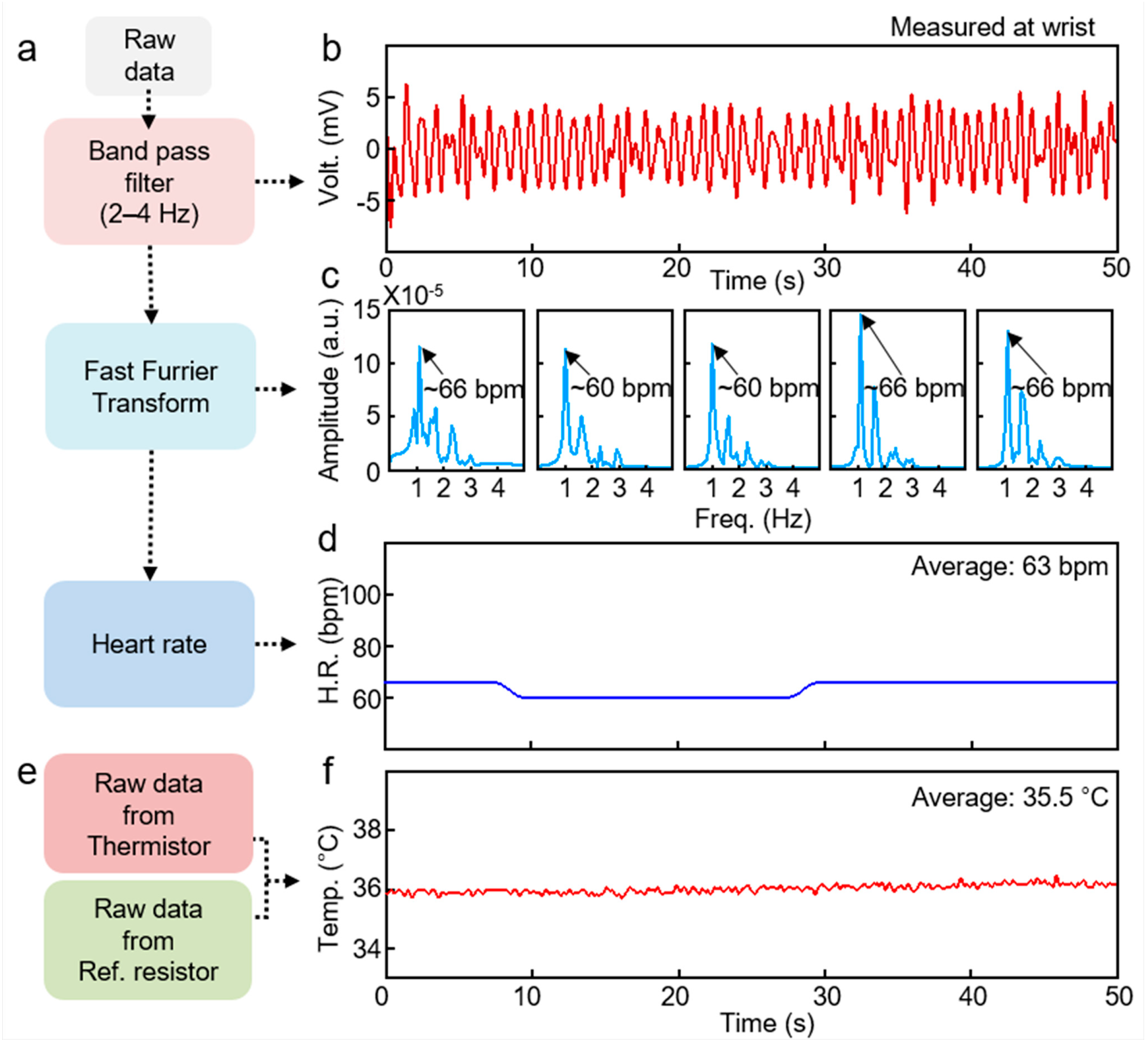
2. Result and Discussion
3. Conclusions
4. Experimental Section
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeong, H.; Rogers, J.A.; Xu, S. Continuous on-body sensing for the COVID-19 pandemic: Gaps and opportunities. Sci. Adv. 2020, 6, eabd4794. [Google Scholar] [CrossRef] [PubMed]
- Douglas, M.; Katikireddi, S.V.; Taulbut, M.; McKee, M.; McCartney, G. Mitigating the wider health effects of covid-19 pandemic response. Br. Med. J. 2020, 369, M1557. [Google Scholar] [CrossRef]
- Rutherford, J.J. Wearable technology. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2010, 29, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Lisa, C.-B.; Rosenberg, D.; Buman, M.P.; Lynch, B.M. Wearable technology and physical activity in chronic disease: Opportunities and challenges. Am. J. Prev. Med. 2018, 54, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Strong, K.; Mathers, C.; Leeder, S.; & Beaglehole, R. Preventing chronic diseases: How many lives can we save? Lancet 2005, 366, 1578–1582. [Google Scholar] [CrossRef]
- Ronco, C.; Mason, G.; Nayak Karopadi, A.; Milburn, A.; Hegbrant, J. Healthcare systems and chronic kidney disease: Putting the patient in control. Nephrol. Dial. Transplant. 2014, 29, 958–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodenheimer, T.; Chen, E.; Bennett, H.D. Confronting the growing burden of chronic disease: Can the US health care workforce do the job? Health Aff. 2009, 28, 64–74. [Google Scholar] [CrossRef]
- Kummitha, R.K.R. Smart technologies for fighting pandemics: The techno-and human-driven approaches in controlling the virus transmission. Gov. Inf. Q. 2020, 37, 101481. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef]
- Choi, S.; Han, S.I.; Jung, D.; Hwang, H.J.; Lim, C.; Bae, S.; Park, O.K.; Tschabrunn, C.M.; Lee, M.; Bae, S.Y.; et al. Highly conductive, stretchable and biocompatible Ag–Au core–sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 2018, 13, 1048–1056. [Google Scholar] [CrossRef]
- Byun, S.H.; Sim, J.Y.; Zhou, Z.; Lee, J.; Qazi, R.; Walicki, M.C.; Parker, K.E.; Haney, M.P.; Choi, S.H.; Shon, A.; et al. Mechanically transformative electronics, sensors, and implantable devices. Sci. Adv. 2019, 5, eaay0418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.S.; Mahmood, M.; Kwon, S.; Zavanelli, N.; Kim, H.S.; Rim, Y.S.; Epps, F.; Yeo, W.H. Fully Integrated, Stretchable, Wireless Skin-Conformal Bioelectronics for Continuous Stress Monitoring in Daily Life. Adv. Sci. 2020, 7, 2000810. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, J.; Won, S.M.; Ma, Y.; Kang, D.; Xie, Z.; Lee, K.-T.; Chung, H.U.; Banks, A.; Min, S.; et al. Battery-free, wireless sensors for full-body pressure and temperature mapping. Sci. Transl. Med. 2018, 10, eaan4950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.U.; Kim, B.H.; Lee, J.Y.; Lee, J.; Xie, Z.; Ibler, E.M.; Lee, K.; Banks, A.; Jeong, J.Y.; Kim, J.; et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 2019, 363, eaau0780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, K.-I.; Han, S.Y.; Xu, S.; Mathewson, K.E.; Zhang, Y.; Jeong, J.-W.; Kim, G.-T.; Webb, R.C.; Lee, J.W.; Dawidczyk, T.J.; et al. Rugged and breathable forms of stretchable electronics with adherent composite substrates for transcutaneous monitoring. Nat. Commun. 2014, 5, 4779. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.H.; Song, J.; Nie, S.; Jung, H.N.; Kim, M.S.; Jeong, J.-W.; Song, Y.M.; Song, J.; Jang, K.-I. Thin Metallic Heat Sink for Interfacial Thermal Management in Biointegrated Optoelectronic Devices. Adv. Mater. Technol. 2018, 3, 1800159. [Google Scholar] [CrossRef]
- Jeong, H.; Wang, L.; Ha, T.; Mitbander, R.; Yang, X.; Dai, Z.; Qiao, S.; Shen, L.; Sun, N.; Lu, N. Modular and Reconfigurable Wireless E-Tattoos for Personalized Sensing. Adv. Mater. Technol. 2019, 4, 1900117. [Google Scholar] [CrossRef]
- Jacob Rodrigues, M.; Postolache, O.; Cercas, F. Physiological and Behavior Monitoring Systems for Smart Healthcare Environments: A Review. Sensors 2020, 20, 2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.K.; Jung, J. Evolution of wearable devices with real-time disease monitoring for personalized healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef] [Green Version]
- Ha, M.; Lim, S.; Ko, H. Wearable and flexible sensors for user-interactive health-monitoring devices. J. Mat. Chem. B 2018, 6, 4043–4064. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.J.; Segundo, D.B.D.R.; Junqueira, H.A.; Sabino, M.H.; Prince, R.M.; Al-Muhtadi, J.; De Albuquerque, V.H.C. Enabling technologies for the internet of health things. IEEE Access 2018, 6, 13129–13141. [Google Scholar] [CrossRef]
- Lin, R.; Kim, H.J.; Achavananthadith, S.; Kurt, S.A.; Tan, S.C.; Yao, H.; Tee, B.C.K.; Lee, J.K.W.; Ho, J.S. Wireless battery-free body sensor networks using near-field-enabled clothing. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Salvatore, G.A.; Araki, H.; Chiarelli, A.M.; Xie, Z.; Banks, A.; Sheng, X.; Liu, Y.; Lee, J.W.; Jang, K.-I.; et al. Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv. 2016, 2, e1600418. [Google Scholar] [CrossRef] [Green Version]
- Araki, H.; Kim, J.; Zhang, S.; Banks, A.; Crawford, K.E.; Sheng, X.; Gutruf, P.; Shi, Y.; Pielak, R.M.; Rogers, J.A. Materials and device designs for an epidermal UV colorimetric dosimeter with near field communication capabilities. Adv. Funct. Mater. 2017, 27, 1604465. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Kim, S.-Y.; Cheong, W.H.; Jang, J.; Park, Y.-G.; Na, K.; Kim, Y.-T.; Heo, J.H.; Lee, C.Y.; et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 2018, 4, eaap9841. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Gutruf, P.; Chiarelli, A.M.; Heo, S.Y.; Cho, K.; Xie, Z.; Banks, A.; Han, S.; Jang, K.-I.; Lee, J.W.; et al. Miniaturized battery-free wireless systems for wearable pulse oximetry. Adv. Funct. Mater. 2017, 27, 1604373. [Google Scholar] [CrossRef] [Green Version]
- Lazaro, A.; Villarino, R.; Girbau, D. A survey of NFC sensors based on energy harvesting for IoT applications. Sensors 2018, 18, 3746. [Google Scholar] [CrossRef] [Green Version]
- Coskun, V.; Ozdenizci, B.; Ok, K. The survey on near field communication. Sensors 2015, 15, 13348–13405. [Google Scholar] [CrossRef]
- Polu, S.K.; Polu, S.K. NFC based Smart Healthcare Services System. Int. J. Innov. Res. Sci. Eng. Technol. 2018, 5, 45–48. [Google Scholar]
- Yang, S.; Chen, Y.C.; Nicolini, L.; Pasupathy, P.; Sacks, J.; Su, B.; Yang, R.; Sanchez, D.; Chang, Y.-F.; Wang, P.; et al. “Cut-and-paste” manufacture of multiparametric epidermal sensor systems. Adv. Mater. 2015, 27, 6423–6430. [Google Scholar] [CrossRef] [PubMed]
- Kinnen, E. Electrical impedance of human skin. Med. Biol. Eng. Comput. 1965, 3, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.H.; Brocker, D.E.; Sieber, P.E.; Werner, D.H. A compact, low-profile metasurface-enabled antenna for wearable medical body-area network devices. IEEE Trans. Antennas Propag. 2014, 62, 4021–4030. [Google Scholar] [CrossRef]
- RF430FRL15xH NFC ISO 15693 Sensor Transponder Datasheet (Rev. C). Available online: https://www.ti.com/lit/ds/symlink/rf430frl153h.pdf?ts=1611729164876&ref_url=https%253A%252F%252Fwww.google.com%252F (accessed on 27 December 2020).
- RF430FRL15xH NFC and ISO/IEC 15693 Sensor Transponder Practical Antenna Design. Available online: https://www.ti.com/lit/an/sloa217a/sloa217a.pdf (accessed on 15 January 2021).
- Fish, R.M.; Geddes, L.A. Conduction of electrical current to and through the human body: A review. Eplasty 2019, 9, e44. [Google Scholar]
- Q1-4010 Material Safety Data Sheet. Available online: https://www.dow.com/en-us/pdp.dowsil-q1-4010-conformal-coating.02112744z.html (accessed on 15 January 2021).
- Fever Scout, VIVALNK. Available online: https://www.vivalnk.com/products/medical-wearable-sensors/continuous-temperature-monitor (accessed on 20 December 2020).
- Temp TRAQ, BlueSparkTech. Available online: https://www.temptraq.com/Home (accessed on 20 December 2020).
- C5, Meilan. Available online: https://www.meilancycling.com/Product/59.html (accessed on 20 December 2020).
- Nearbebe. Available online: https://nearbebe.com/main?l=ko-KR (accessed on 20 December 2020).
- Temppal, iWEECARE. Available online: https://www.iweecare.com/ (accessed on 20 December 2020).
- VisualBeat, Wellue. Available online: https://getwellue.com/pages/visualbeat-heart-rate-monitor (accessed on 20 December 2020).
- Dzidek, B.M.; Adams, M.J.; Andrews, J.W.; Zhang, Z.; Johnson, S.A. Contact mechanics of the human finger pad under compressive loads. J. R. Soc. Interface 2017, 14, 20160935. [Google Scholar] [CrossRef] [PubMed]
- Konttila, A.; Maattala, M.; Alasaarela, E. Pulse Oximeter Signal Amplitudes in Different Body Parts for Wireless Solutions. Int. J. Future Gener. Commun. Netw. 2007, 1, 494–498. [Google Scholar]
- Longmore, S.K.; Lui, G.Y.; Naik, G.; Breen, P.P.; Jalaludin, B.; Gargiulo, G.D. A comparison of reflective photoplethysmography for detection of heart rate, blood oxygen saturation, and respiration rate at various anatomical locations. Sensors 2019, 19, 1874. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Kumar, K.S.; He, H.; Cai, C.J.; He, X.; Gao, H.; Yue, S.; Li, C.; Sheet, R.C.-S.; Ren, H.; et al. Fully organic compliant dry electrodes self-adhesive to skin for long-term motion-robust epidermal biopotential monitoring. Nat. commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Kim, J.; Kim, N.; Kwon, M.; Lee, J. Attachable pulse sensors integrated with inorganic optoelectronic devices for monitoring heart rates at various body locations. ACS Appl. Mater. Interfaces 2017, 9, 25700–25705. [Google Scholar] [CrossRef]




| Product | Operating System | Power Supply | Biological Signals | Weight | Size (mm3) |
|---|---|---|---|---|---|
| This work | Wireless (NFC) | Battery-free | Heart rate, temperature | 1 g | 20 × 20 × 2 |
| [39] | Wireless (Bluetooth) | Battery | Temperature | 3 g | 64 × 35 × 4.5 |
| [40] | Wireless (Bluetooth) | Battery | Temperature | 45 g | 99 × 25 × 48 |
| [41] | Wireless (Bluetooth) | Battery | Heart rate | 60 g | 200 × 80 × 3 |
| [42] | Wireless (Bluetooth) | Battery | Temperature | 15 g | 43 × 43 × 16 |
| [43] | Wireless (Bluetooth) | Battery | Temperature | 3 g | 28 × 26 × 3.5 |
| [44] | Wireless (Bluetooth) | Battery | Heart rate | 18 g | 100 × 23.1 × 8.3 |
| Mean Heart Rate (bpm) | STD | Mean Skin Temp. (°C) | STD | |
|---|---|---|---|---|
| Subject 1 | 63 | 2.7 | 35.5 | 0.18 |
| Subject 2 | 64 | 2.96 | 35.1 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.H.; Lee, G.J.; Yun, J.H.; Song, Y.M. NFC-Based Wearable Optoelectronics Working with Smartphone Application for Untact Healthcare. Sensors 2021, 21, 878. https://doi.org/10.3390/s21030878
Kang MH, Lee GJ, Yun JH, Song YM. NFC-Based Wearable Optoelectronics Working with Smartphone Application for Untact Healthcare. Sensors. 2021; 21(3):878. https://doi.org/10.3390/s21030878
Chicago/Turabian StyleKang, Min Hyung, Gil Ju Lee, Joo Ho Yun, and Young Min Song. 2021. "NFC-Based Wearable Optoelectronics Working with Smartphone Application for Untact Healthcare" Sensors 21, no. 3: 878. https://doi.org/10.3390/s21030878







