Multi-Channel Bioimpedance System for Detecting Vascular Tone in Human Limbs: An Approach
Abstract
1. Introduction
1.1. Vascular Tone Measurement
1.2. Problem Statement
1.3. Impedance Plethysmography for Detecting Vascular Tone
2. Materials and Methods
2.1. Subjects
2.2. Experimental Procedure
2.3. Equipment
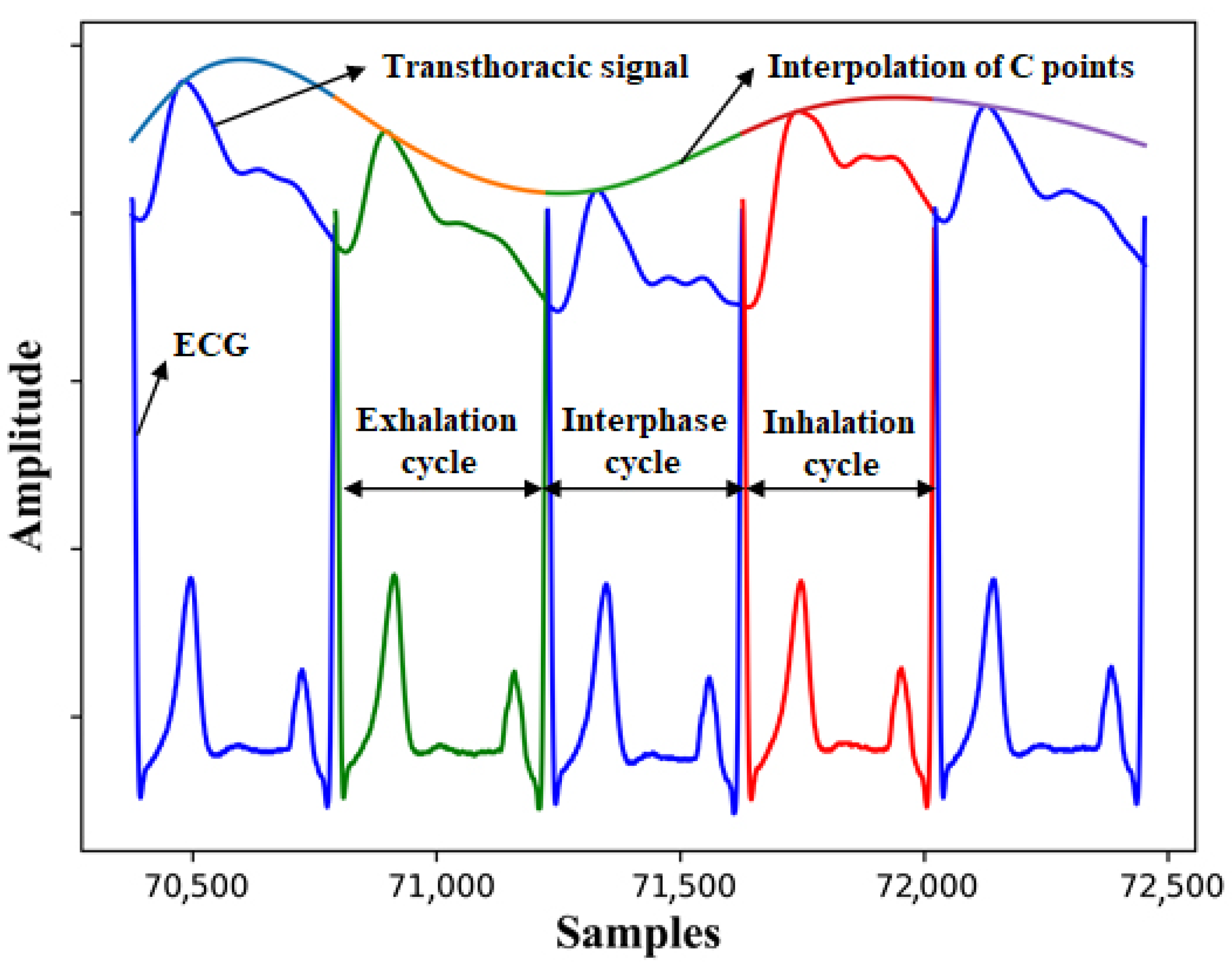
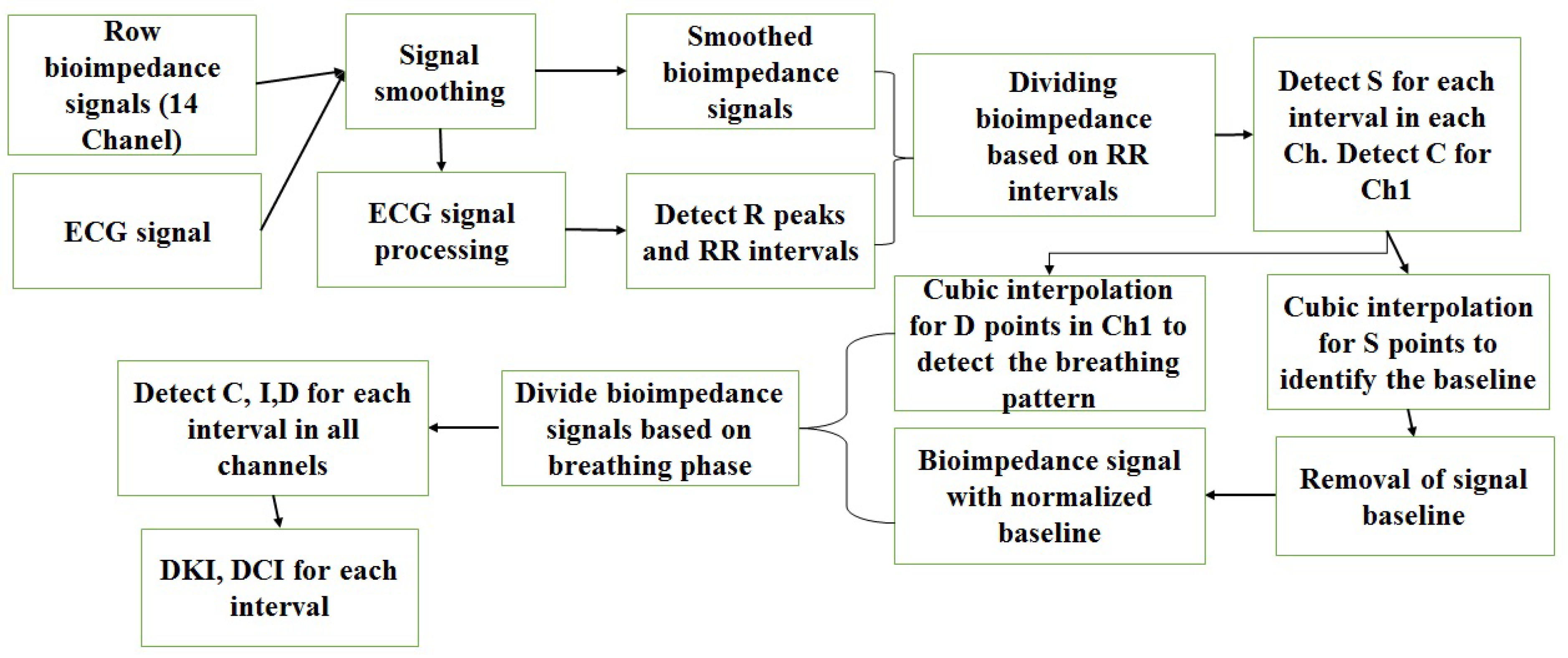
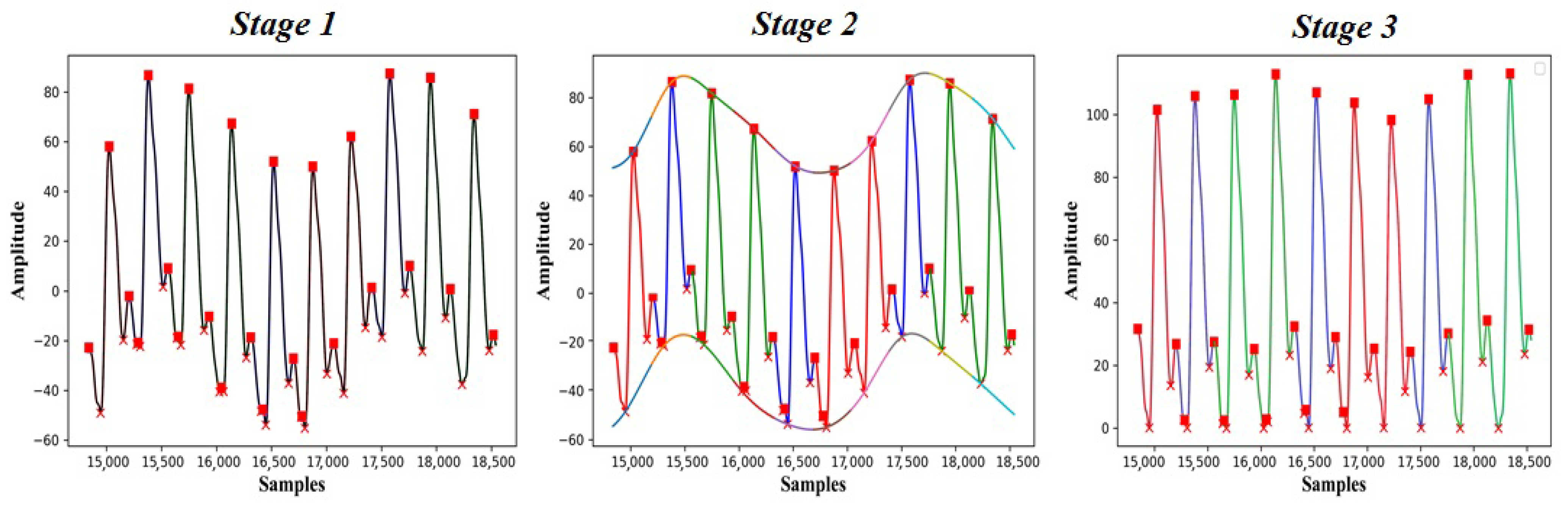
2.4. Signal Processing
3. Results
4. Discussion
4.1. Method Performance
4.2. Vascular Tone Type
4.3. Future Work
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, S.M.; Purnat, T.D.; Phuong, N.T.; Mwingira, U.; Schacht, K.; Fröschl, G. Non-Communicable Diseases (NCDs) in developing countries: A symposium report. Glob. Health 2014, 10, 81. [Google Scholar] [CrossRef]
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2021, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Orshal, J.M.; Khalil, R.A. Gender, sex hormones, and vascular tone. Am. J.-Physiol.-Regul. Integr. Comp. Physiol. 2004, 286, R233–R249. [Google Scholar] [CrossRef]
- Davis, M.J.; Hill, M.A. Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 1999, 79, 387–423. [Google Scholar] [CrossRef]
- Davis, M.J.; Hill, M.A.; Kuo, L. Local regulation of microvascular perfusion. In Microcirculation; Academic Press: Cambridge, MA, USA, 2008; pp. 161–284. [Google Scholar]
- D’Angelo, G.E.; Davis, M.J.; Meininger, G.A. Calcium and mechanotransduction of the myogenic response. Am. J.-Physiol.-Heart Circ. Physiol. 1997, 273, H175–H182. [Google Scholar] [CrossRef]
- Davis, M.J. Perspective: Physiological role (s) of the vascular myogenic response. Microcirculation 2012, 19, 99–114. [Google Scholar] [CrossRef]
- Borgström, P.; Grände, P.O.; Mellander, S. A mathematical description of the myogenic response in the microcirculation. Acta Physiol. Scand. 1982, 116, 363–376. [Google Scholar] [CrossRef] [PubMed]
- AGonzales, A.L. A PLCY1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci. Signal. 2014, 7, ra49. [Google Scholar]
- Hill, M.A.; Davis, M.J. Coupling a change in intraluminal pressure to vascular smooth muscle depolarization: Still stretching for an explanation. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2570–H2572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mufti, R.E.; Zechariah, A.; Sancho, M.; Mazumdar, N.; Brett, S.E.; Welsh, D.G. Implications of alphavbeta3 integrin signaling in the regulation of Ca2+ waves and myogenic tone in cerebral arteries. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Tykocki, N.R.; Boerman, E.M.; Jackson, W.F. Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles. Compr. Physiol. 2007, 7, 485–581. [Google Scholar]
- Ralevic, V.; Burnstock, G. Neural-Endothelial Interactions in the Control of Local Vascular Tone; RG Landes Company: Georgetown, TX, USA, 1993. [Google Scholar]
- Burnstock, G.; Ralevic, V. New insights into the local regulation of blood flow by perivascular nerves and endothelium. Br. J. Plast. Surg. 1994, 47, 527–543. [Google Scholar] [CrossRef]
- Saito, T.; Mori, S.; Arakawa, M.; Ohba, S.; Kobayashi, K.; Kanai, H. Estimation of viscoelasticity of radial artery via simultaneous measurement of changes in pressure and diameter using a single ultrasound probe. Jpn. J. Appl. Phys. 2020, 59, SKKE04. [Google Scholar] [CrossRef]
- Zlepko, S.M.; Sander, S.V.; Kozlovska, T.I.; Pavlov, V.; Wójcik, W.; Yesmakhanova, L.; Zhirnova, O. Analysis of the vascular tone and character of the local blood flow to assess the viability of the body using the photoplethysmographic device. Prz. Elektrotechniczny 2017, 93, 92–95. [Google Scholar] [CrossRef][Green Version]
- Tusman, G.; Acosta, C.M.; Pulletz, S.; Böhm, S.H.; Scandurra, A.; Arca, J.M.; Madorno, M.; Sipmann, F.S. Photoplethysmographic characterization of vascular tone mediated changes in arterial pressure: An observational study. J. Clin. Monit. Comput. 2019, 33, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, D.L., Jr. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J. Appl. Physiol. 2006, 100.5, 1709–1718. [Google Scholar] [CrossRef]
- Wang, L.; Ansari, S.; Slavin, D.; Ward, K.; Najarian, K.; Oldham, K.R. Non-invasive vascular resistance monitoring with a piezoelectric sensor and photoplethysmogram. Sens. Actuators A Phys. 2017, 263, 198–208. [Google Scholar] [CrossRef]
- Krupatkin, A.I.; Sidorov, V.V. Laser Doppler Flowmetry of Blood Microcirculation; Medicine: Moscow, Russia, 2005; pp. 32–58. [Google Scholar]
- Naumenko, A.I.; Skotnikov, V.V. Fundamentals of Electroplethysmography; Meditsina: Moscow, Russia, 1975; pp. 32–58. [Google Scholar]
- Ronkin, M.A.; Ivanov, L.B. Rheography in Clinical Practice; Meditsina: Moscow, Russia, 1997; p. 403. [Google Scholar]
- Anderson, F.A. Impedance plethysmography in the diagnosis of arterial and venous disease. Ann. Biomed. Eng. 1984, 12, 79–102. [Google Scholar] [CrossRef]
- Antle, D.M.; Cormier, L.; Findlay, M.; Miller, L.L.; Côté, J.N. Lower limb blood flow and mean arterial pressure during standing and seated work: Implications for workplace posture recommendations. Prev. Med. Rep. 2018, 10, 117–122. [Google Scholar] [CrossRef]
- Haynes, W.G.; Ferro, C.J.; O’Kane, K.P.; Somerville, D.; Lomax, C.C.; Webb, D.J. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation 1996, 93, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Luzhnov, P.V. Development of a Computer System for Biosynchronized Electromagnetic Impact. Ph.D. Thesis, Bauman Moscow State Technical University, Moscow, Russia, 2005. [Google Scholar]
- Shamkina, L.A. Biotechnical System of Electromagnetic Therapy of Lower Limb Blood Circulation Disorders. Ph.D. Thesis, Bauman Moscow State Technical University, Moscow, Russia, 2009. [Google Scholar]
- Hammoud, A.; Tikhomirov, A.N.; Shaheen, Z. Automatic Bio-impedance Signal Analysis: Smoothing Processes Efficacy Evaluation in Determining the Vascular Tone Type. In Proceedings of the 2021 Ural Symposium on Biomedical Engineering, Radioelectronics and Information Technology (USBEREIT), Yekaterinburg, Russia, 13–14 May 2021. [Google Scholar]
- Van Gent, P.; Farah, H.; van Nes, N.; van Arem, B. HeartPy: A novel heart rate algorithm for the analysis of noisy signals. Transp. Res. Part F Traffic Psychol. Behav. 2019, 66, 368–378. [Google Scholar] [CrossRef]
- Xakimjon, Z.; Bunyod, A. Biomedical signals interpolation spline models. In Proceedings of the 2019 International Conference on Information Science and Communications Technologies (ICISCT), Tashkent, Uzbekistan, 4–6 November 2019; pp. 1–3. [Google Scholar]
- Grossman, P.; Taylor, E.W. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biol. Psychol. 2007, 74, 263–285. [Google Scholar] [CrossRef] [PubMed]
- Karlen, W.; Raman, S.; Ansermino, J.M.; Dumont, G.A. Multiparameter respiratory rate estimation from the photoplethysmogram. IEEE Trans. Biomed. Eng. 2013, 60, 1946–1953. [Google Scholar] [CrossRef]
- Luzhnov, P.V.; Shamkina, L.A.; Shchukin, S.I. Multilevel biofeedback technology for electromagnetic therapy of vascular diseases. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009; pp. 392–395. [Google Scholar]

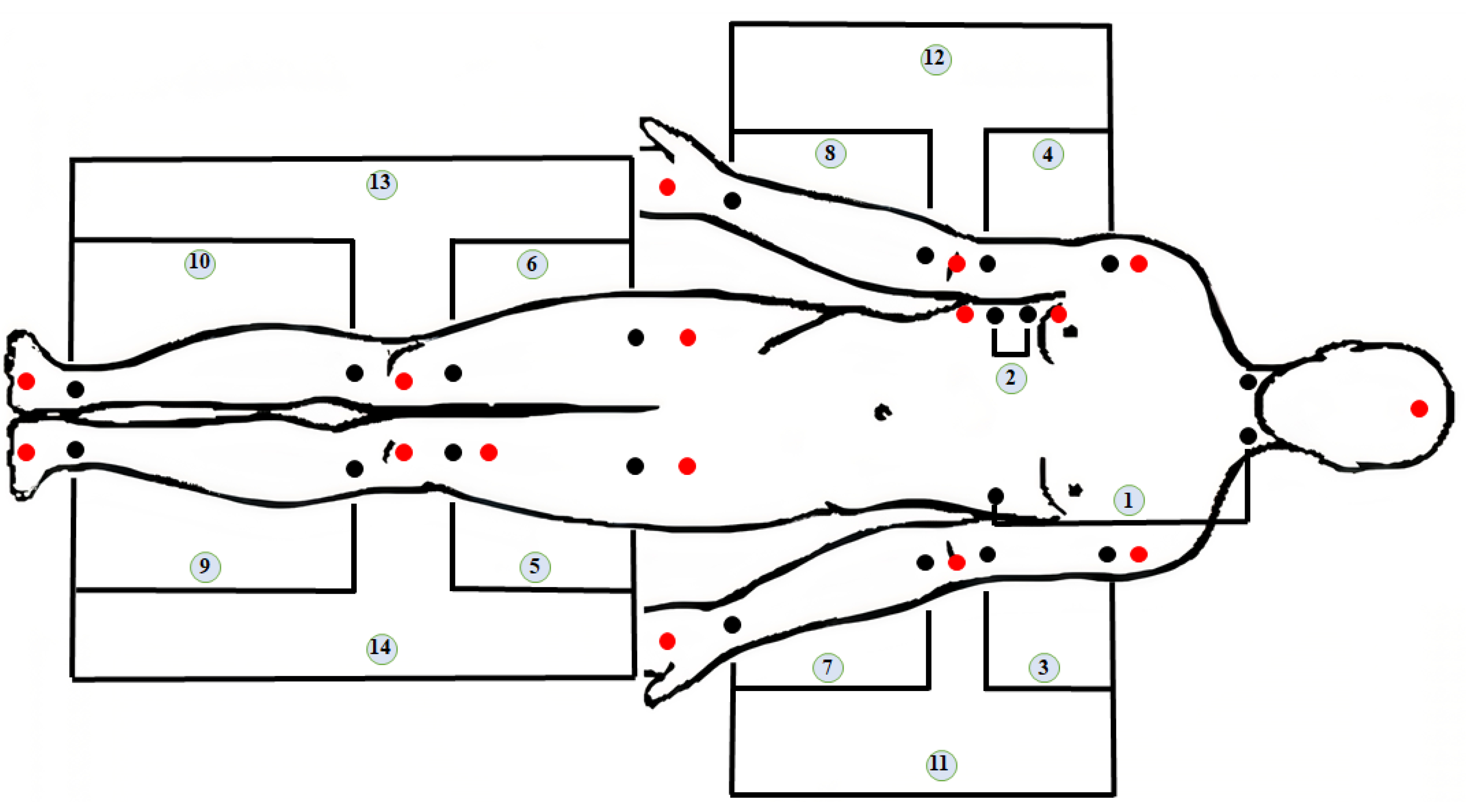

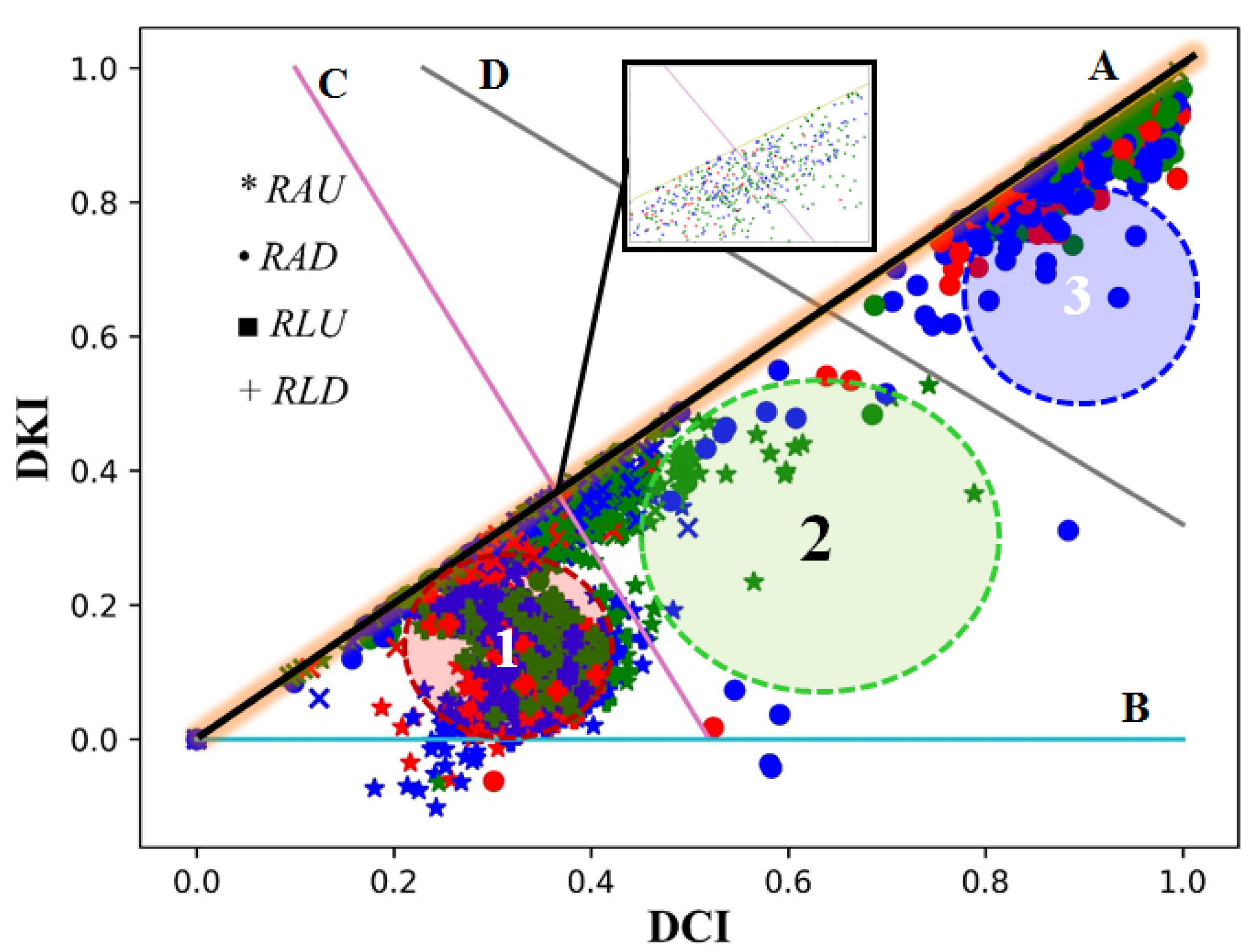

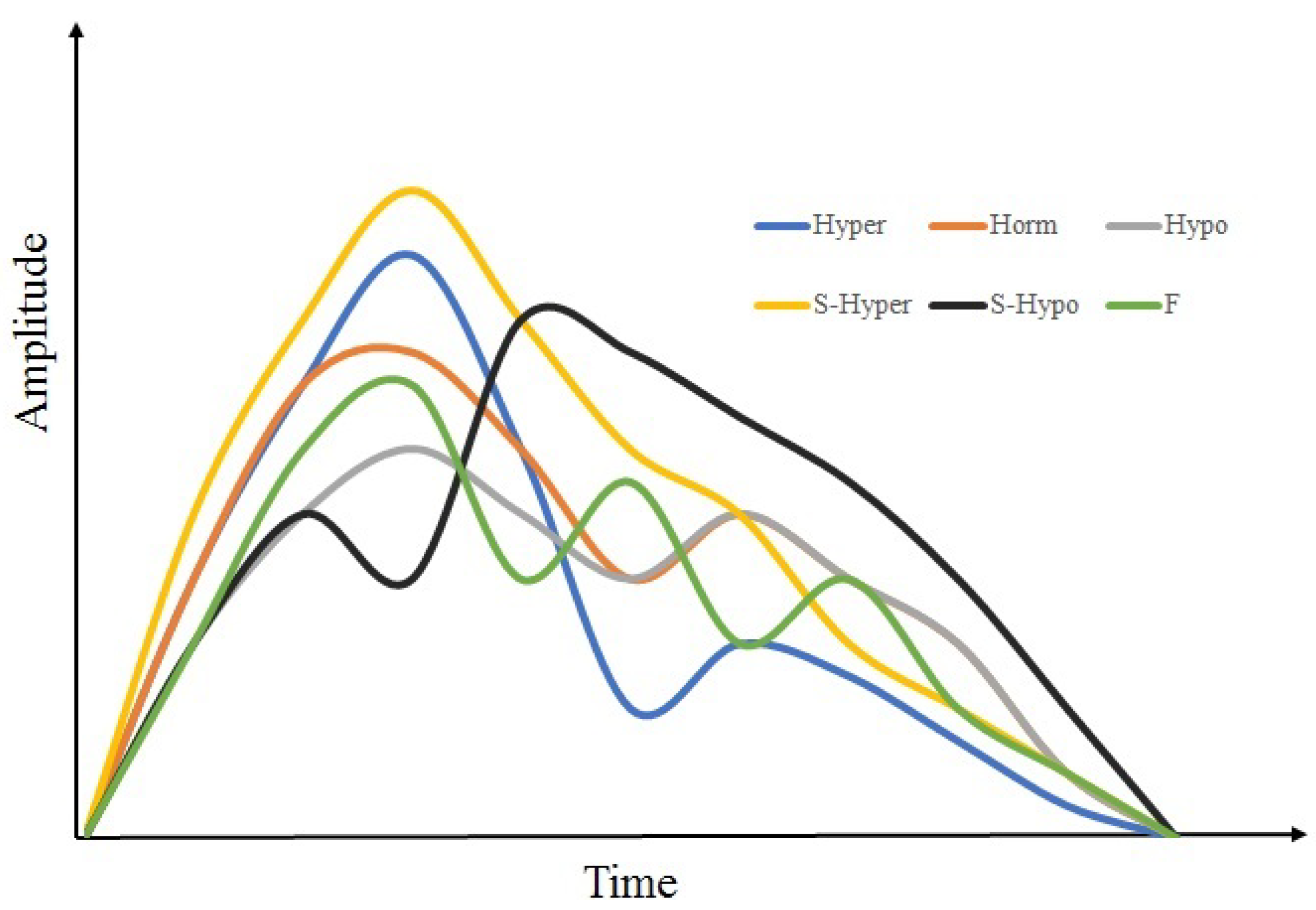
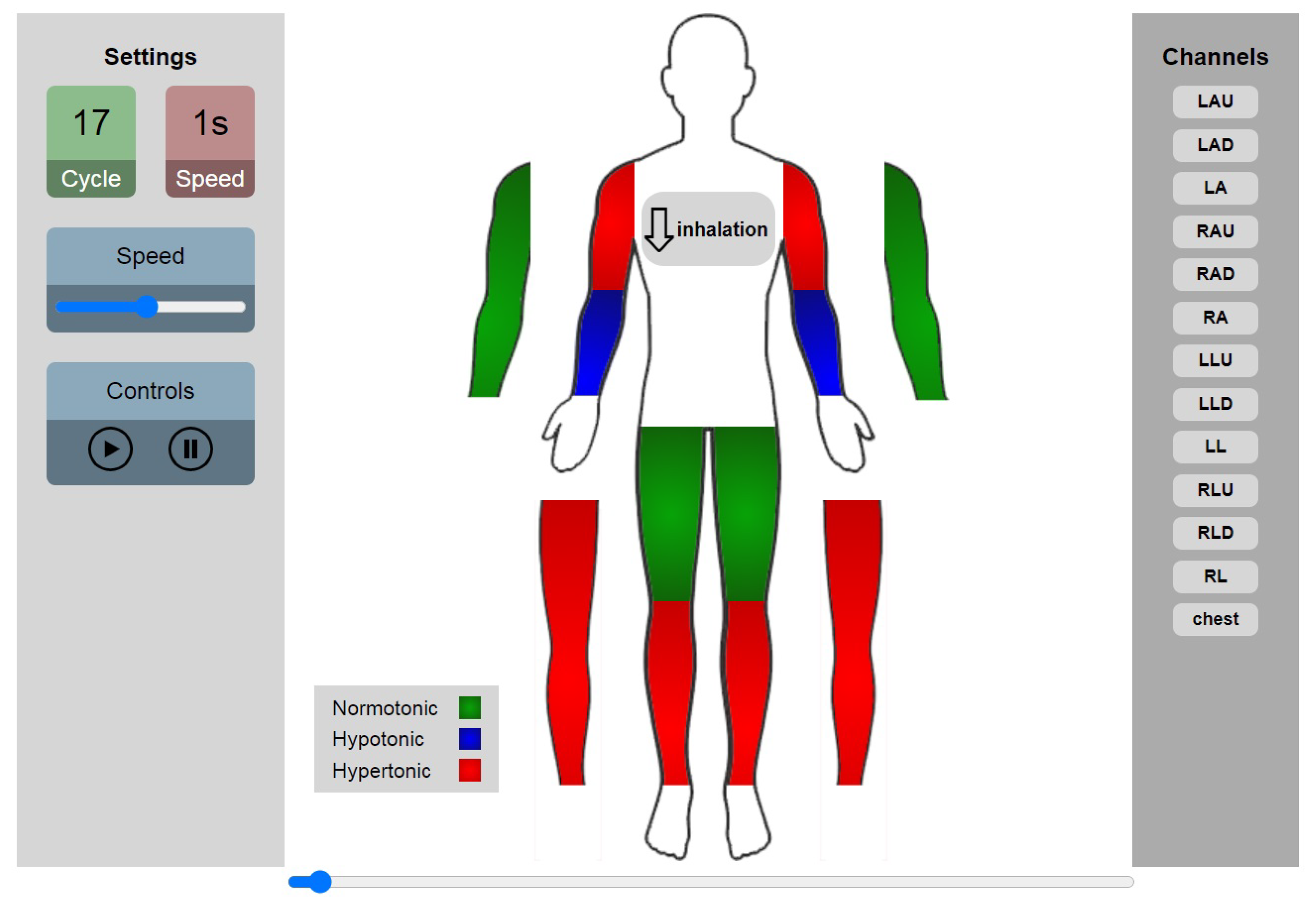
| ID | Abbr | Segment | Arteries |
|---|---|---|---|
| 1 | - | Transthoracic | - |
| 2 | - | Thoracic | - |
| 3 | LAU | Upper part of Left Arm | Left Brachial artery |
| 4 | RAU | Upper part of Right Arm | Right brachial artery |
| 5 | LLU | Left Thigh | Left femoral artery |
| 6 | RLU | Right Thigh | Right femoral artery |
| 7 | LAD | Lower part of Left Arm | Left radial and ulnar arteries |
| 8 | RAD | Lower part of Right Arm | Right radial and ulnar arteries |
| 9 | LLD | Lower part of the Left Leg | Left anterior tibial and Posterior tibial |
| 10 | RLD | Lower part of the Right Leg | Right anterior tibial and Posterior tibial |
| 11 | LA | Left Arm | All arteries of left arm |
| 12 | RA | Right Arm | All arteries of right arm |
| 13 | LL | Left Leg | All arteries of left leg |
| 14 | RL | Right Leg | All arteries of right leg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammoud, A.; Tikhomirov, A.; Myasishcheva, G.; Shaheen, Z.; Volkov, A.; Briko, A.; Shchukin, S. Multi-Channel Bioimpedance System for Detecting Vascular Tone in Human Limbs: An Approach. Sensors 2022, 22, 138. https://doi.org/10.3390/s22010138
Hammoud A, Tikhomirov A, Myasishcheva G, Shaheen Z, Volkov A, Briko A, Shchukin S. Multi-Channel Bioimpedance System for Detecting Vascular Tone in Human Limbs: An Approach. Sensors. 2022; 22(1):138. https://doi.org/10.3390/s22010138
Chicago/Turabian StyleHammoud, Ahmad, Alexey Tikhomirov, Galina Myasishcheva, Zein Shaheen, Alexander Volkov, Andrey Briko, and Sergey Shchukin. 2022. "Multi-Channel Bioimpedance System for Detecting Vascular Tone in Human Limbs: An Approach" Sensors 22, no. 1: 138. https://doi.org/10.3390/s22010138
APA StyleHammoud, A., Tikhomirov, A., Myasishcheva, G., Shaheen, Z., Volkov, A., Briko, A., & Shchukin, S. (2022). Multi-Channel Bioimpedance System for Detecting Vascular Tone in Human Limbs: An Approach. Sensors, 22(1), 138. https://doi.org/10.3390/s22010138







