Comparing of Frequency Shift and Impedance Analysis Method Based on QCM Sensor for Measuring the Blood Viscosity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method 1: Oscillating Circuit Analysis Method
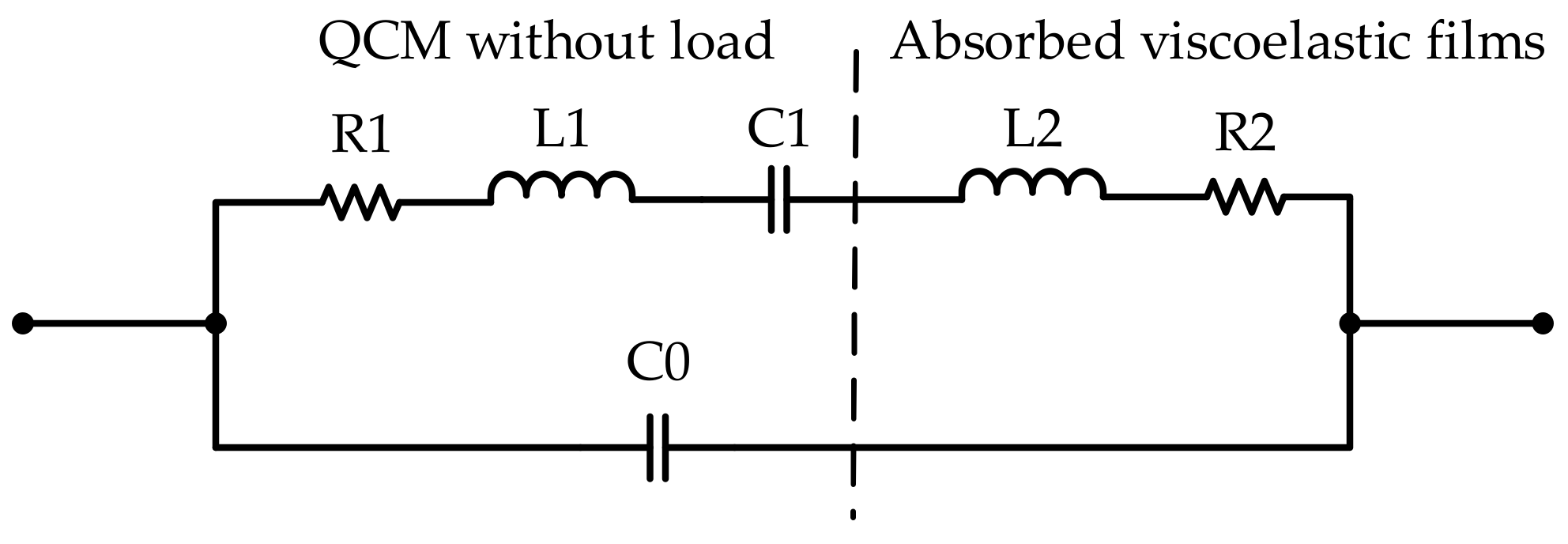
2.3. Method 2: Impedance Analysis Method
3. Results and Discussion
3.1. Oscillating Circuit Analysis Method
3.1.1. Measurement of Frequency Coefficient
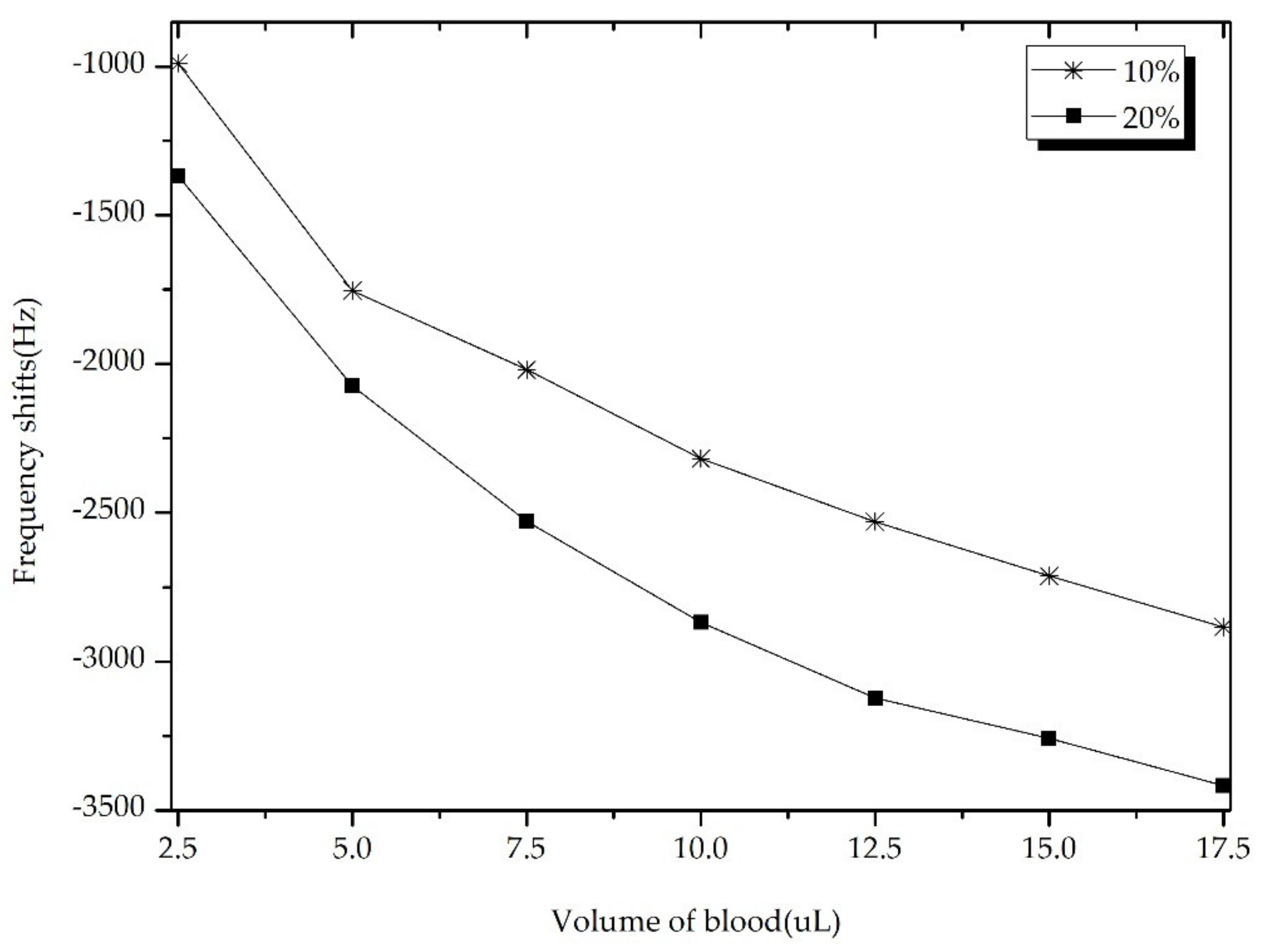
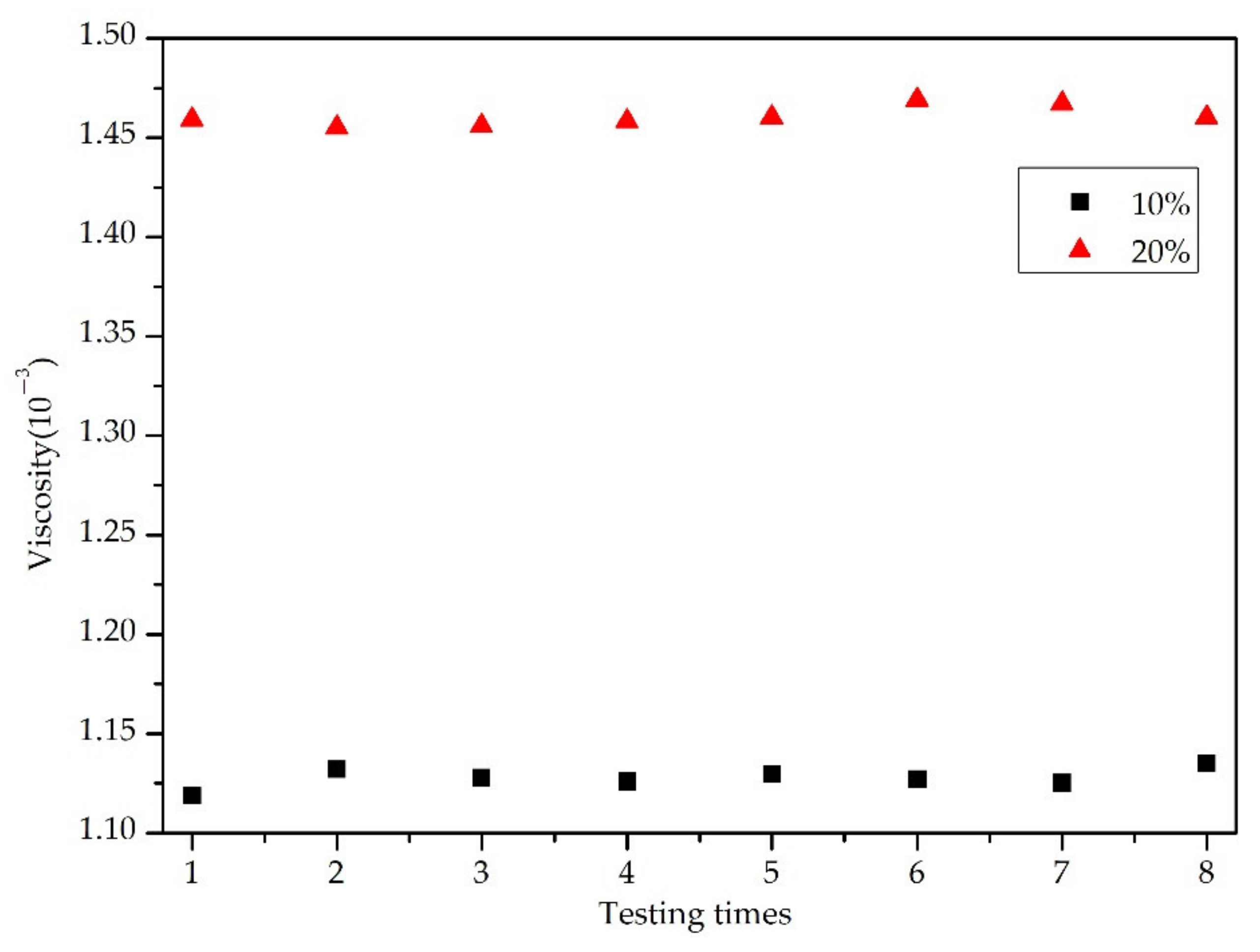
3.1.2. Measurement Blood Viscosity
3.2. Impedance Analysis Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lowe, G.D.; Lee, A.J.; Rumley, A.; Price, J.F.; Fowkes, F.G. Blood viscosity and risk of cardiovascular events: The Edinburgh Artery Study. Br. J. Haematol. 1997, 96, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Katus, H.A.; Hoberg, E.; Roebruck, P.; Braun, M.; Haupt, H.M.; Tillmanns, H.; Kübler, W. Increased plasma viscosity and erythrocyte aggregation: Indicators of an unfavourable clinical outcome in patients with unstable angina pectoris. Heart 1991, 66, 425–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cekirdekci, E.I.; Bugan, B. Whole blood viscosity in microvascular angina and coronary artery disease: Significance and utility. Rev. Port. Cardiol. 2020, 39, 17–23. [Google Scholar] [CrossRef]
- Cetin, E.H.; Cetin, M.S.; Canpolat, U.; Kalender, E.; Topaloglu, S.; Aras, D.; Aydogdu, S. The forgotten variable of shear stress in mitral annular calcification: Whole blood viscosity. Med. Princ. Pract. 2015, 24, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Nwose, E.U. Cardiovascular risk assessment and support techniques: Whole blood viscosity assessment issues I: Extrapolationchart and reference values. N. Am. J. Med. Sci. 2010, 2, 65. [Google Scholar]
- Antonova, N.M.; Paskova, V.K.; Velcheva, I.V. Blood rheological and electrical properties and relationships with the microvascular tone regulation in patients with diabetes mellitus type 2. Reg. Blood Circ. Microcirc. 2021, 20, 25–33. [Google Scholar] [CrossRef]
- Koltai, K. Hemorheological Parameters in Diabetic Patients: Role of Glucose Lowering Therapies. Metabolites 2021, 11, 806. [Google Scholar]
- Oh, S.; Kim, B.; Lee, J.K.; Choi, S. 3D-printed capillary circuits for rapid, low-cost, portable analysis of blood viscosity. Sens. Actuators B Chem. 2018, 259, 106–113. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, Y.S.; Zhbanov, A.; Yang, S. A physiometer for simultaneous measurement of whole blood viscosity and its determinants: Hematocrit and red blood cell deformabilit. Analyst 2019, 144, 3144–3157. [Google Scholar] [CrossRef]
- Kim, G.; Jeong, S.; Yang, J.K. Ultrasound Standing Wave-Based Cell-to-liquid Separation for Measuring Viscosity and Aggregation of Blood Sample. Sensors 2020, 20, 2284. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Lee, S.Y.; Jee, S.; Atajanov, A.; Yang, S. Micro-Viscometer for Measuring Shear-Varying Blood Viscosity over a Wide-Ranging Shear Rate. Sensors 2017, 17, 1442. [Google Scholar] [CrossRef] [Green Version]
- Elenkov, M.; Ecker, P.; Lukitsch, B.; Janeczek, C.; Harasek, M.; Gföhler, M. Estimation Methods for Viscosity, Flow Rate and Pressure from Pump-Motor Assembly Parameters. Sensors 2020, 20, 1451. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.K.; Yang, S. Integrated microfluidic viscometer equipped with fluid temperature controller for measurement of viscosity in complex fluids. Microfluid. Nanofluid. 2013, 14, 657–668. [Google Scholar]
- Choi, S.; Park, J.K. Microfluidic Rheometer for Characterization of Protein Unfolding and Aggregation in Microflows. Small 2010, 6, 1306–1310. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yeom, E.; Lee, S.J. Microfluidic Biosensor for Monitoring Temporal Variations of Hemorheological and Hemodynamic Properties Using an Extracorporeal Rat Bypass Loop. Anal. Chem. 2013, 85, 10503–10511. [Google Scholar] [CrossRef]
- Kang, Y.J. Simultaneous measurement of erythrocyte deformability and blood viscoelasticity using micropillars and co-flowing streams under pulsatile blood flows. Biomicrofluidics 2017, 11, 014102. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Cho, Y.I.; Lee, D.H.; Park, C.M.; Moon, H.W.; Hur, M.; Kim, J.Q.; Yun, Y.M. Analytical performance evaluation of the scanning capillary tube viscometer for measurement of whole blood viscosity. Clin. Biochem. 2013, 46, 139–142. [Google Scholar] [CrossRef]
- Mujahid, A.; Dickert, F.L. Blood Group Typing: From Classical Strategies to the Application of Synthetic Antibodies Generated by Molecular Imprinting. Sensors 2016, 16, 51. [Google Scholar] [CrossRef] [Green Version]
- Mujahid, A.; Aigner, S.; Dickert, F.L. Micro-structured interdigital capacitors with synthetic antibody receptors for ABO blood-group typing. Sens. Actuators B Chem. 2017, 242, 378–383. [Google Scholar] [CrossRef]
- Kanazawa, K.K.; Gordon, J.G. Frequency of a quartz microbalance in contact with liquid. Anal. Chem. 1985, 57, 1770–1771. [Google Scholar] [CrossRef]
- Reed, C.E.; Kanazawa, K.K.; Kaufmann, J.H. Physical description of a viscoelastically loaded AT-cut quartz resonator. J. Appl. Phys. 1990, 68, 1993–2001. [Google Scholar] [CrossRef]
- Wang, D.; Mousavi, P.; Hauser, P.J.; Oxenham, W.; Grant, C.S. Quartz crystal microbalance in elevated temperature viscous liquids: Temperature effect compensation and lubricant degradation monitoring. Colloids Surf. A 2005, 268, 30–39. [Google Scholar] [CrossRef]
- Rodahl, M.; Höök, F.; Kasemo, B. QCM operation in liquids: An explanation of measured variations in frequency and Q factor with liquid conductivity. Anal. Chem. 1996, 68, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Chen, Y.; Ma, H. Solidified liquid layer model expands the application fields of quartz crystal microbalance Macromol. Rapid Commun. 2012, 33, 735–741. [Google Scholar] [CrossRef]
- Han, T.; Lin, W.; Lin, J. Errors of phases and group delays in SAW RFID tags with phase modulation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 2565. [Google Scholar] [CrossRef]
- Bruckenstein, S.; Michalski, M.; Fensore, A.; Li, Z.; Hillman, A.R. Dual quartz crystal microbalance oscillator circuit. Minimizing effects due to liquid viscosity, density, and temperature. Anal. Chem. 1994, 66, 1847–1852. [Google Scholar] [CrossRef]
- Dunham, G.C.; Benson, N.H.; Petelenz, D.; Janata, J. Dual quartz crystal microbalance. Anal. Chem. 1995, 67, 267–272. [Google Scholar] [CrossRef]
- Johannsmann, D.; Bucking, W.; Bode, B.; Petri, J. Simple frequency-based sensing of viscosity and dielectric properties of a liquid using acoustic resonators. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010, 57, 677–683. [Google Scholar] [CrossRef]
- Martin, S.J.; Wessendorf, K.O.; Gebert, C.T.; Frye, G.C.; Cernosek, R.W.; Casaus, L.; Mitchell, M.A. Measuring liquid proper ties with smooth-and textured-surface resonators. In Proceedings of the 1993 IEEE International Frequency Control Symposium, Salt Lake City, UT, USA, 2–4 June 1993; pp. 603–608. [Google Scholar]
- Itoh, A.; Ichihashi, M. A frequency of the quartz crystal microbalance (QCM) that is not affected by the viscosity of a liquid. Meas. Sci. Technol. 2008, 19, 075205. [Google Scholar] [CrossRef]
- Itoh, A.; Ichihashi, M. Separate measurement of the density and viscosity of a liquid using a quartz crystal microbalance based on admittance analysis (QCM-A). Meas. Sci. Technol. 2011, 22, 015402. [Google Scholar] [CrossRef]
- Tan, F.; Ye, P.; Qiu, D.; Guo, L.; Huang, W.; Zeng, H.; Hou, D. A new method for measuring properties of liquid by using a single quartz crystal microbalance. In Proceedings of the Joint Conference of the European Frequency and Time Forum and IEEE International Frequency Control Symposium, Besancon, France, 9–13 July 2017; pp. 649–651. [Google Scholar]
- Tan, F.; Qiu, D.; Ye, P.; Zeng, H.; Zhao, Y.; Jiang, J.; Pan, H.; Guo, L.; Wu, S. Method for Measuring the Properties of Liquid Based on a Quartz Crystal Microbalance Sensor. U.S. Patent US10145819B2, 4 December 2016. [Google Scholar]
- Liao, S.; Ge, C.; Qiu, D.; Tang, J.; Tan, F.; Chen, C.; Xu, L. BVD model for QCM loaded by viscoelastic film in gas phase application. AIP Adv. 2020, 10, 075119. [Google Scholar] [CrossRef]
- Tiersten, H.F. Linear Piezoelectric Plate Vibrations: Elements of the Linear Theory of Piezoelectricity and the Vibrations Piezoelectric Plate; Springer: Berlin/Heidelberg, Germany, 1969. [Google Scholar]
- Song, S.H.; Kim, J.H.; Lee, J.H.; Yun, Y.M.; Choi, D.H.; Kim, H.Y. Elevated blood viscosity is associated with cerebral small vessel disease in patients with acute ischemic stroke. BMC Neurol. 2017, 17, 20. [Google Scholar] [CrossRef] [Green Version]

| Parameters | Values | Units |
|---|---|---|
| 2651 | Kg·m−3 | |
| 9.27 × 10−3 | Pa·s | |
| 2.92 × 10−5 | m2 | |
| 1.66 × 10−4 | m | |
| 7.44 × 10−3 | ||
| 2.947 × 1010 | N·m−2 | |
| 3.32 × 103 | N1/2·Kg−1/2·m5/2 | |
| 9.657 × 10−2 | A·s·m−2 | |
| 3.982 × 10−11 | A2·s4·Kg−1·m−3 |
| 1.39 × 105 | −5.704 × 106 |
| Concentration (wt%) | Viscosity (mPa·s) | ||||||
|---|---|---|---|---|---|---|---|
| Reference Value | Method 1 Measured Value | Standard Deviation | |||||
| 1 | 2 | 3 | 4 | 5 | |||
| 10 | 1.14 | 1.13 | 1.127 | 1.12 | 1.20 | 1.135 | 5.5% |
| 20 | 1.48 | 1.44 | 1.455 | 1.456 | 1.458 | 1.41 | 2.1% |
| Testing Times | Concentration (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10% | 20% | |||||||||
| R (Ω) | Measured Viscosity (mPa·s) | Reference Viscosity (mPa·s) | Standard Deviation | R (Ω) | Measured Viscosity (mPa·s) | Reference Viscosity (mPa·s) | Standard Deviation | |||
| 1 | 83.56 | 1167.8 | 1.119 | 1.14 | 0.9% | 102.05 | 1195.5 | 1.459 | 1.48 | 0.5% |
| 2 | 84.03 | 1193.2 | 1.14 | 101.79 | 1189.9 | 1.465 | ||||
| 3 | 83.69 | 1186.7 | 1.12 | 101.9 | 1182.9 | 1.47 | ||||
| 4 | 83.72 | 1185.4 | 1.121 | 101.79 | 1200.1 | 1.458 | ||||
| 5 | 83.98 | 1189.1 | 1.13 | 101.59 | 1171.7 | 1.46 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, S.; Ye, P.; Chen, C.; Zhang, J.; Xu, L.; Tan, F. Comparing of Frequency Shift and Impedance Analysis Method Based on QCM Sensor for Measuring the Blood Viscosity. Sensors 2022, 22, 3804. https://doi.org/10.3390/s22103804
Liao S, Ye P, Chen C, Zhang J, Xu L, Tan F. Comparing of Frequency Shift and Impedance Analysis Method Based on QCM Sensor for Measuring the Blood Viscosity. Sensors. 2022; 22(10):3804. https://doi.org/10.3390/s22103804
Chicago/Turabian StyleLiao, Shuang, Peng Ye, Cheng Chen, Jie Zhang, Lin Xu, and Feng Tan. 2022. "Comparing of Frequency Shift and Impedance Analysis Method Based on QCM Sensor for Measuring the Blood Viscosity" Sensors 22, no. 10: 3804. https://doi.org/10.3390/s22103804





